Highlights
-
•
Cortical networks relevant for OCD and MD (reward, affect, cognitive control) can be extended to subcortical fiber pathways.
-
•
Tractographic methods can be used to define these networks.
-
•
Description of subcortical network extensions helps to understand effects of stereotactic procedures (DBS, lesion surgery) in OCD and MD.
-
•
A corticopetal ordering systematics for those networks follows evolutionary concepts.
Keywords: Anterior limb of internal capsule; DBS; Stereotactic lesion surgery; Depression; MD; OCD; Projection pathways; Hyperdirect pathway, Midbrain; Neocortex; Functional networks; Prefrontal cortex
Abstract
Background
Major depression (MD) and obsessive-compulsive disorder (OCD) are psychiatric diseases with a huge impact on individual well-being. Despite optimal treatment regiments a subgroup of patients remains treatment resistant and stereotactic surgery (stereotactic lesion surgery, SLS or Deep Brain Stimulation, DBS) might be an option. Recent research has described four networks related to MD and OCD (affect, reward, cognitive control, default network) but only on a cortical and the adjacent sub-cortical level. Despite the enormous impact of comparative neuroanatomy, animal science and stereotactic approaches a holistic theory of subcortical and cortical network interactions is elusive. Because of the dominant hierarchical rank of the neocortex, corticofugal approaches have been used to identify connections in subcortical anatomy without anatomical priors and in part confusing results. We here propose a different corticopetal approach by identifying subcortical networks and search for neocortical convergences thereby following the principle of phylogenetic and ontogenetic network development.
Material and methods
This work used a diffusion tensor imaging data from a normative cohort (Human Connectome Project, HCP; n = 200) to describe eight subcortical fiber projection pathways (PPs) from subthalamic nucleus (STN), substantia nigra (SNR), red nucleus (RN), ventral tegmental area (VTA), ventrolateral thalamus (VLT) and mediodorsal thalamus (MDT) in a normative space (MNI). Subcortical and cortical convergences were described including an assignment of the specific pathways to MD/OCD-related networks. Volumes of activated tissue for different stereotactic stimulation sites and procedures were simulated to understand the role of the distinct networks, with respect to symptoms and treatment of OCD and MD.
Results
The detailed course of eight subcortical PPs (stnPP, snrPP, rnPP, vlATR, vlATRc, mdATR, mdATRc, vtaPP/slMFB) were described together with their subcortical and cortical convergences. The anterior limb of the internal capsule can be subdivided with respect to network occurrences in ventral-dorsal and medio-lateral gradients. Simulation of stereotactic procedures for OCD and MD showed dominant involvement of mdATR/mdATRc (affect network) and vtaPP/slMFB (reward network).
Discussion
Corticofugal search strategies for the evaluation of stereotactic approaches without anatomical priors often lead to confusing results which do not allow for a clear assignment of a procedure to an involved network. According to our simulation of stereotactic procedures in the treatment of OCD and MD, most of the target regions directly involve the reward (and affect) networks, while side-effects can in part be explained with a co-modulation of the control network.
Conclusion
The here proposed corticopetal approach of a hierarchical description of 8 subcortical PPs with subcortical and cortical convergences represents a new systematics of networks found in all different evolutionary and distinct parts of the human brain.
- ALIC,
anterior limb of internal capsule (DBS target)
- am STN,
anteromedial subthalamic nucleus (DBS target)
- BNST,
bed nucleus of stria terminalis
- DBS,
deep brain stimulation
- DTI,
diffusion tensor imaging
- FT,
fiber tractography
- HDP,
hyperdirect pathway
- ICa,
anterior limb of the internal capsule (anatomical)
- ITP,
inferior thalamic peduncle (DBS target)
- lHDP,
limbic hyperdirect pathway (macaque anatomy)
- MD,
major depression
- mdATR,
anterior thalamic radiation from dorsomedial thalamus
- mdATRc,
mdATR with extension to cerebellum
- mfb,
medial forebrain bundle (rodent anatomy)
- MRI,
magnetic resonance imaging
- MDT,
mediodorsal thalamus
- NAc,
nucleus accumbens septi
- OCD,
obsessive-compulsive disorder
- OFC,
orbitofrontal cortex
- PAG,
periaqueductal grey
- PFC,
prefrontal cortex
- PP,
projection pathway
- RN,
red nucleus
- rnPP,
projection pathway from red nucleus
- SLS,
stereotactic lesion surgery
- slMFB,
see vtaPP
- SNR,
substantia nigra
- snrPP,
projection pathway from substantia nigra
- STN,
subthalamic nucleus
- stnPP,
projection pathway from subthalamic nucleus (analogous to hyperdirect pathway)
- TMS,
transcranial magnetic stimulation
- TPT,
target point superolateral medial forebrain bundle (DBS target)
- VAT,
volume of activated tissue
- VC/VS,
ventral capsule ventral striatum (DBS target)
- VC,
ventral capsule (DBS target)
- vlATR,
anterior thalamic radiation from ventrolateral thalamus
- vlATRc,
vlATR with extension to cerebellum
- VLT,
ventrolateral thalamus
- VTA,
ventral tegmental area
- vtaPP,
projection pathway of the ventral tegmental area (= slMFB)
Introduction
Both major depression (MD) and obsessive-compulsive disorder (OCD) (Karas et al., 2019; Pittenger et al., 2005) are psychiatric diseases sharing certain clinical symptoms such as anxiety, low mood and social withdrawal. They are are addressed by partially congruent pharmacological treatments – these facts taken together point to the disorders having overlapping structural and/or/functional disease correlates. MD is clinically characterized by key behavioral symptoms which extend into emotional, motivational, physiological and also cognitive domains of daily living. Anhedonia and hopelessness are key symptoms and might point to a deficiency of the reward system (Nestler et al., 2002; Russo and Nestler, 2013; Schlaepfer et al., 2014) and other networks (Li et al., 2018). OCD has a life-time prevalence of 2-3% and like MD can be a significantly disabling disorder. Patients typically suffer from recurrent ego-dystonic thoughts (obsessions) of various topics (e.g. contamination, religious content, harming others) leading to repetitive behaviours (compulsions), which are typically stereotyped like hand washing, checking, mental rituals, a need to repeat activities or the concern about the own appearance. OCD can occur comorbidly to depression and other psychiatric diseases (Pittenger et al., 2005). OCD, MD and other psychiatric disorders are now widely accepted as network and more precisely as white matter diseases (Alves-Pinto et al., 2019; Apergis-Schoute et al., 2018; Bai et al., 2018; Cao et al., 2018; Li et al., 2018). Effective medical treatments for MD and OCD have been established, typically a combination of medication and psychotherapy (Gaynes et al., 2009; Pittenger et al., 2005). In advanced stages they can be treated with non-invasive stimulation approaches (e.g. transcranial magnetic stimulation = TMS) (Carmi et al., 2017; Johnson et al., 2013). In treatment resistant stages deep brain stimulation (DBS) (Bergfeld et al., 2016; Jiménez et al., 2013; Mayberg et al., 2005; Naesström et al., 2016; Riva-Posse et al., 2017; Tyagi et al., 2019) or stereotactic lesion surgery (SLS) (Hurwitz et al., 2012; Hurwitz et al., 2006a; Kisely et al., 2018; Rasmussen et al., 2018a; Rück et al., 2008; Schoene-Bake et al., 2010; Volpini et al., 2017) might be further options.
Recent research has identified four relevant networks which contribute to our understanding of OCD and MD: reward, affect, control and default mode (Li et al., 2018). The reward network is a major driver of motivation, behavior and learning. Panksepp has coined the term “SEEKING system”, related to the motivational drive which characterizes this system more than the reward itself (Panksepp, 2012). It is therefore conceivable to use the name “reward / SEEKING network”. Upon dysregulation it plays and important role for diseases of emotion and affect. Deficiencies in the reward system have been discussed in the context of MD and OCD (Alves-Pinto et al., 2019; Coenen et al., 2016; Keren et al., 2018). In MD the key symptoms anhedonia and hopelessness have been attributed to a dysfunctional reward system (Anisman and Matheson, 2005; Blood et al., 2010; Li et al., 2018; Nestler et al., 2002; Russo and Nestler, 2013; Schlaepfer et al., 2014). Overactivation of the reward system on the contrary can be observed during mania (Abler et al., 2008; Coenen et al., 2009) and is a hallmark in remitted depression (Dichter et al., 2012). For OCD neuroscientists conclude that the disease is a reward and affect (network) related disease (Alves-Pinto et al., 2019; Pallanti and Grassi, 2015).
The affect network serves the purpose of processing and regulating the emotions (Li et al., 2018). The affect network is the main system that deals with conscious and unconscious human fear (Öhman et al., 2007) (Gross et al., 2012; Motta et al., 2017). In affective neuroscience terms, this system is further concerned with separation distress, sadness (“feeling the pain of social loss”) (Panksepp, 2003), anxiety, mourning and grief. Especially the effect of rejection has elegantly been substantiated in fMRI experiments on social isolation (Eisenberger et al., 2003). Along these lines an overactivity of this system can be closely linked to the symptoms of depression - which also includes feelings of isolation and rejection – anxiety and OCD. The cognitive control network is important for motor program regulation but also plays and important role in the top down control of emotion regulation (Alexander et al., 1986; Li et al., 2018). Patients suffering from MD often suffer from cognitive impairment which is closely associated with the degree of emotional suffering. Patients suffering from OCD are known to show cognitive inflexibility related to a dysfunction of the control network. Moreover, the consequences of effective (subcortical) treatments for OCD and MD need to be regulated by the cognitive control network in a top down manner. This is potentially the reason why successful stereotactic interventions for OCD and MD should be accompanied by subsequent psychotherapy (Greenberg et al., 2006) while in turn DBS and SLS should not directly affect the control network. The default mode network is the task negative network as it only is functional if the subject is not performing any kind of action (Li et al., 2018). This network has been associated with increased ruminations e.g in MD.
Upon pathological dysregulation these four networks play distinct roles in the specific disorder leading to a disbalance of the entire system whose functioning is based on delicate network interactions. Depending on the specific clinical disease phenotypes, a differential involvement of the four networks has been discussed (Li et al., 2018).
The neocortex as the highest hierarchical level controls most parts of the brain and by this regulates human behavior. However, a top-down organization of brain networks is not necessarily the result of this hierarchy and an evolutionary driven bottom-up organization principle might actually be more likely. Some approaches have been made to dissect and understand subcortical fiber pathways in the context of brain networks which are involved in psychiatric disorders like OCD and MD. These dissection methods commonly used the top-down or corticofugal approach (Fig. 1) with can lead to in part confusing results (Frankle et al., 2006; Greenberg et al., 2010; Haynes and Haber, 2013; Nanda et al., 2017; Safadi et al., 2018a). The reasons are probably related to brain evolution and its consequences for the brain's composition in conjunction with methodical considerations: Neocortical functional regions are network hubs (with task specific changing function) and as such receive fiber connections from many (certainly more than one) subcortical networks, which are phylogenetically much older (Fig. 1). In typical corticofugal dissection approaches fiber pathways have been identified, which connect orbitofrontal cortex (OFC, or Brodmann's area 11) with the thalamus and the brainstem (Nanda et al., 2017; Safadi et al., 2018b; Lehman et al., 2011; Makris et al., 2016). The same fibers are found when seeding (top-down) in the anterior limb of the internal capsule (Baldermann et al., 2019) . However, fibers from brainstem and thalamus will most likely belong to different (albeit interconnected) functional units (networks). Under such circumstances an analysis without anatomical prior knowledge of involved PPs/networks (Fig. 1) and their function is not easy to comprehend (see discussion on search strategies).
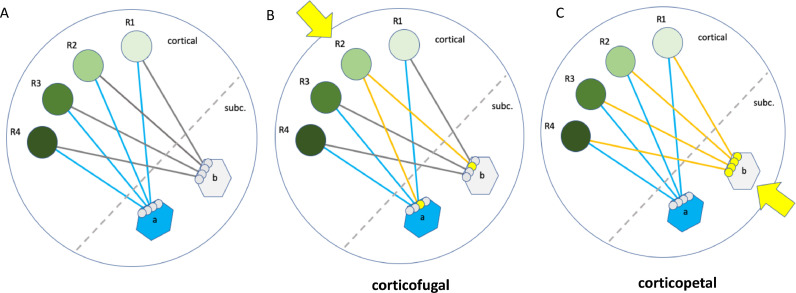
Fig. 1.
Identification of cortical / subcortical networks with different tractographic approaches. Schematic representation. A, anatomical situation: Cortical functional regions (R1-R4, green spheres) are connected with distinct subcortical hub regions (a, b). These hub regions are subcortical nuclei. Hub regions with distinct functions converge onto the same cortical functional regions. A hub together with its fiber connections and the cortical functional regions constitutes a network. B, corticofugal tractographic approach: Seeding from a single cortical functional region (R2, yellow arrow) leads to an only partial identification of the involved hubs (a,b) and their attached network but shows the overall connectedness of the cortical region with subcortical structures. The network as a whole cannot be appreciated, nor can the convergence of subcortical networks (as a whole) onto cortical regions be understood. C, corticopetal approach (as used in this paper): Seeding from a subcortical hub region (b, yellow arrow) identifies the entire network which consists of the hub region (b), the fiber connections (yellow lines) and the cortical projection fields (R1-R4). The other hub (a) is not part of this network (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
To overcome the shortcomings, here we propose a corticopetal approach (Fig. 1) based on the following rationales: 1. Networks that regulate distinctive emotional behavior as affected in MD and OCD have developed in parallel and have been present – albeit in simpler versions - in all evolutionary levels in ancestral species in phylogenesis (Panksepp, 2012; Panksepp, 2011). The brains of our phylogenetic ancestors have evolved from simpler entities to more complicated ones and the human brain with its neocortex is the latest evolutionary step. Ancient development steps can be found in comparison with simpler species (Kaiser, 2015;Panksepp, 2003) and it has been proposed that evolutionary development steps and functions are retained in the human brain in sub-cortical anatomy (brain stem, diencephalon, basal ganglia). Evolutionary newer developments (neocortex) functionally rely on these older brain parts, although there is a dominating top down control. 2. Subcortical networks have significantly lesser cells (as compared to the neocortex) and their construction are simpler (Nieuwenhuys et al., 2008). As such an identification of the subcortical network including its interconnections might be more straightforward if looking corticopetal (bottom-up) as opposed to seeding an algorithm in a neocortical region which task specifically and frequently changes its function.
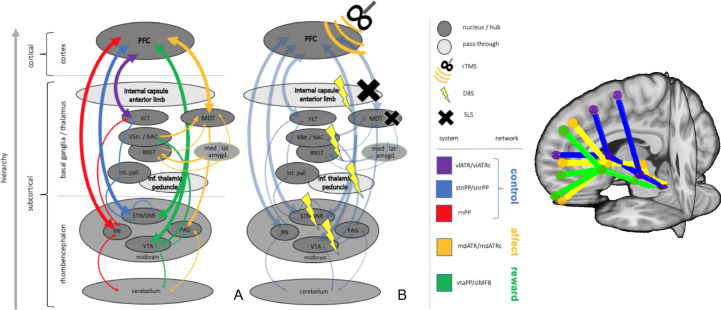
Specific networks serve specific purposes in behavior and for an effective functioning they are interconnected through hubs. These interconnections need to be realized in convergences on all hierarchical levels and inter-modular hubs (realized in subcortical nuclei) - are still present in all evolutionary conserved parts of the human brain and can also be found in less developed species. Despite the top-down neocortical control, some of these hubs resemble the very intervention points that have been addressed in subcortical stereotactic procedures like deep brain stimulation (DBS) and stereotactic lesion surgery (SLS) (Fig. 2).
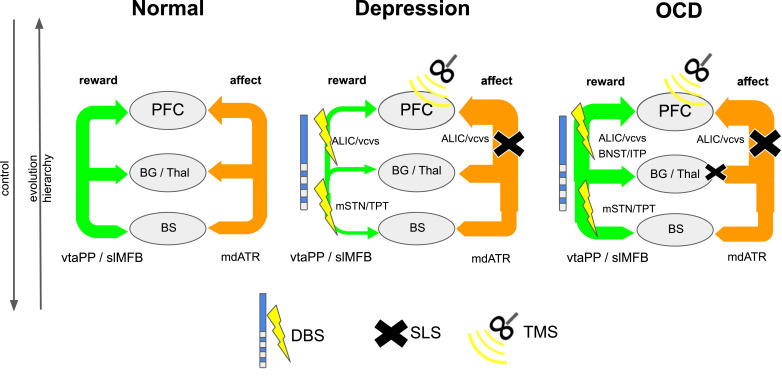
Fig. 2.
Interaction of two networks (exemplarily: reward, affect) in an evolutionary and hierarchical context (normal, depression, OCD). Legend: DBS, deep brain stimulation; SLS, stereotactic lesion surgery; rTMS, repetitive transcranial magnetic stimulation; PFC, prefrontal cortex; Thal, thalamus; BG, basal ganglia; BS, brain stem; slMFB, superolateral medial forebrain bundle; mdATR, anterior thalamic radiation from dorsomedial thalamus; ALIC, anterior limb of internal capsule (DBS target); VC, ventral capsule (DBS target); mSTN, medial subthalamic nucleus; TPT, target point for slMFB DBS.
Projection pathways (PPs) bi-directionally connect cortical with subcortical structures including basal ganglia, thalamus, midbrain, brainstem and cerebellum (Nieuwenhuys et al., 2008). PPs establish immediate and typically bidirectional functional connections between cortical functional regions and subcortical and evolutionary older structures. Upon deconstruction of PPs one finds hyperdirect, direct and indirect connections (Haynes and Haber, 2013; Nambu et al., 1996; 2002). Projection pathways from the prefrontal cortices have a particular importance for psychiatric disorders (Mega and Cummings, 1994). DBS and SLS are effective because of their effects on these PPs (Karas et al., 2019). Orthodromic (downstream, synaptic silencing) and antidromic (upstream) effects on a network level have been offered as explanations for the clinical effectiveness of DBS (Kang, 2014) (Gradinaru et al., 2009; McIntyre et al., 2004). SLS constitutes an inactivation of subcortical white matter tracts with disconnecting effects from hierarchically higher network parts. Although DBS can be more activating on the axonal level and SLS respectively is always inactivating the clinical similarity of their effectiveness is so far not sufficiently explained. Network descriptions in OCD and MD typically focus on function and there is a lack of topographical (and surgical) anatomy beyond naming of in principle involved structures (e.g. OCD, cortico-striato - thalamo-cortical = CSTC loop (Mega and Cummings, 1994) which essentially is an application of the proposed scheme by Alexander and DeLong (Alexander et al., 1986). Especially a clear assignment of PPs to specific cortical networks such as reward, affect, control and default mode is lacking. Such an assignment, however, would be important for our understanding of subcortical anatomy in relation to cortical function and disease pathophysiology. Moreover, the application of this knowledge could also lead to better informed therapeutic interventions.
Based on the corticopetal approach we here present a detailed description of PP anatomy based on a large sample (n=200) from the human connectome project cohort (HCP). The aims of the here presented work are:
-
•
To describe the detailed subcortical PPs from brainstem, basal ganglia, and thalamus towards cerebral and cerebellar cortex including their cortical convergences in a common atlas space (MNI) and with a special focus on the anterior limb of the internal capsule (ICa).
-
•
To attribute the established PPs to cortical networks associated to MD and OCD
-
•
To elaborate on subcortical evolutionary network connection hubs of some involved networks (reward, affect, control) with respect to already used stereotactic targets (DBS, SLS).
-
•
To simulate distinct stereotactic targets in a common space to shed light on their possible effectiveness with respect to subcortical network parts despite their presumably contradictory mode of action (DBS vs. SLS).
We will focus on the corticopetal description of PPs to/from subthalamic nucleus (STN), substantia nigra (SNr), red nucleus (RN), ventrolateral thalamus (VLT), mediodorsal thalamus (MDT) tand ventral tegmental area (VTA). An integration of a detailed anatomical description into the neuroscientific context of three large networks is then discussed and put into perspective with the interpretation of results of simulated stereotactic interventions in OCD and MD with a special focus on the anterior limb of the internal capsule.
Methods and material
The principle idea was to tractographically define subcortical projection pathways (PPs). Following a corticopetal approach defined subcortical key structures (RN, SNr, STN, VTA, MDT, VLT) served as anatomical priors for the specification of eight subcortical PPs in a common atlas space (MNI) in a large normative cohort of subjects (HCP). Based on their anatomical functioning these PPs were assigned to three networks which are known to be relevant in OCD and MD (reward, affect, control). The default mode network will only implicitly and not explicitly be addressed. Since direction, synapses and transmitters contained in these fiber pathways cannot be visualized with the DTI technology, we performed a selective literature analysis to interpret our findings, especially for the understanding of far-reaching connections between subcortical nuclei (hubs) and the cortex. Simulations of DBS and SLS targets for OCD and MD were then performed to understand the subcortical parts of networks interventions these target regions might affect.
Imaging: Analyses were based on diffusion tensor imaging (DTI) data from the Human Connectome Project (HCP) database (https://ida.loni.usc.edu/login.jsp) using the Q1: S3, S4 subsample (n = 200 subjects; 78 male; mean age ± SD, 29 ± 3.5 years). DTI was acquired with the following parameters: resolution 1.25 mm isotropic, three b-shells with 1000, 2000, 3000 (see Glasser et al., 2013 for more details on the protocol and preprocessing). Normalization to MNI space was performed based on the provided T1 images using CAT12 (http://dbm.neuro.uni-jena.de/cat12/CAT12-Manual.pdf) implemented in the Statistical Parametric Mapping software (SPM12, http:// www.fil.ion.ucl.ac.uk/spm/software/spm12).
Tractography: Tractography was performed using a global approach (Reisert et al., 2011). As opposed to local walker-based tractography, global fiber tracking tries to find a fiber configuration that best explains the acquired DTI data. Practically, the optimization process is similar to a polymerization process, where initially the streamlines are short and fuzzy, while during optimization connections proliferate and fibers become more and more congruent with the data. The algorithm proposed by Reisert et al. (2011) is implemented in a publicly available toolbox (http://www.uniklinik-freiburg.de/mr-en/research_groups/diffperf/fibertools.html) which provides two standard parameter sets. For the present analyses, we have applied the ‘dense’ parameter set and used ten re-iterations to optimize intra-individual reproducibility of estimated fiber bundles (Schumacher et al., 2018). As reconstruction area the white matter segmentation obtained from CAT12 at the loose threshold of 0.1 were used to ascertain the inclusion of the subcortical areas.
Bundle selection: The volumes of interest (VOIs) used for the selection of fibers and the specifications of projection pathways (PPs) were taken from different atlases. The prefrontal cortex was defined based on the atlas by Desikan et al. (2006) using the following VOIs: lateralorbitofrontal, medialorbitofrontal, rostralmiddlefrontal, superiorfrontal, caudalmiddlefrontal, frontalpole, parstriangularis, parsopercularis, parsorbitalis (the regions adjacent to the cortex were used, prefix wm-lh and wm-rh). The definition of the anterior limb of the internal capsule and the cerebellum was based on the John Hopkins atlas (JHU-WMPM-Type I; see Oishi et al., 2009) For the deep midbrain structures we employed the atlasses by Ilinsky et al. (2018) and Ewert et al. (2018): Definitions of the red nucleus and subthalamic nucleus were based on Ilinsky et al. (2018); definitions of the medial dorsal thalamus, the ventrolateral thalamus and the substantia nigra were taken from Ewert et al. (2018).
In total, eight PPs were specified in this study. All PPs had to pass through the anterior limb of the internal capsule and reach the prefrontal cortex. These fibers were then dissected into PPs of fibers visiting the red nucleus (rnPP), the substantia nigra (snrPP), the subthalamic nucleus (stnPP). The superolateral medial forebrain bundle (slMFB) was defined as fibers visiting a spherical seed previously specified by our group (MNI coordinates +-6,-12,-8, radius 3mm; see Coenen et al., 2018c; Hosp et al., 2019), in the following abbreviated by ppVTA/slMFB. Thalamic PPs were dissected by selecting fibers terminating in the medial dorsal thalamus and not visiting the ventrolateral thalamus (mdATR) and vice versa (vlATR). In addition, we specified further thalamic PPs as fibers passing through the thalamus (again, exclusively either medial dorsally or ventrolaterally) and reaching the cerebellum (mdATRc and vlATRc, respectively). See supplement Fig. A2 for definition of nuclei.
Aggregation and bundle specific tractography: All selected PPs were further analyzed by computing fiber density maps, terminal density maps, and directional fiber density maps, which were normalized to template space by the transformation obtained from CAT12 and aggregated to get the final density representation of the bundles in MNI standard space. The fiber densities and terminal densities were computed by means of trilinear interpolation on an isotropic matrix of resolution 1.5 mm. As the number of streamlines obtained from DTI is not truly able to quantitatively estimate the underlying neurite density but gives rather a qualitative picture of the underlying anatomical structure, we normalized the tract specific maps by their absolute streamline counts per individual. This enabled us to compare the tract specific maps by using one common threshold for all bundles. To understand the variability of the streamline counts we show in Fig. 3a the tract specific counts over the cohort (given relative to the total number of streamlines in the whole connectome). In Fig. 6, where we show sections of the considered PPs in a compound, we used a common threshold of 10^-2 for all PPs.
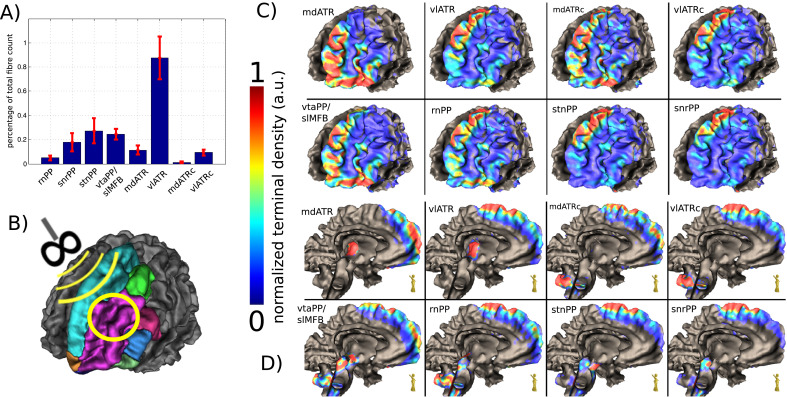
Fig. 3.
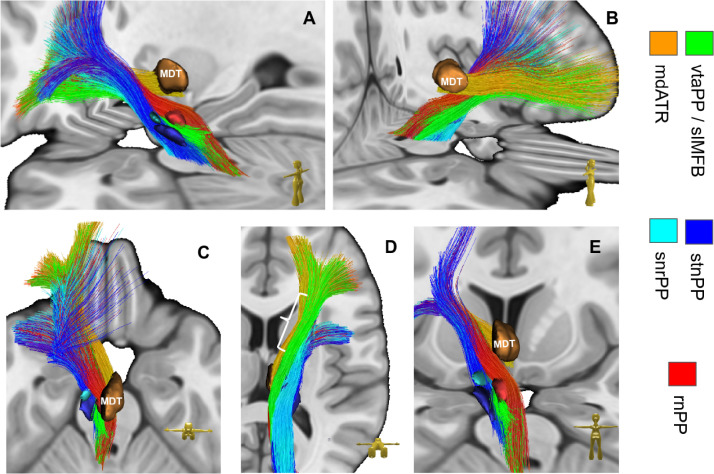
Terminals of subcortical projection pathways (PP) in streamline rendition. Right side shown only. The convergence of PP fibers can nicely be seen. Note how fibesr from mediodorsal thalamus (mdATR, copper) converge together with fibers from the ventral tegmental area (vtaPP/slMFB, green) on the same frontopolar and orbitofrontal regions. For quantification of terminals see Fig. 4 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Fig. 6.
Topographic positions of mdATR/mdATRC and vlATR/vl ATRc. Left side shown only.
The directional fiber density maps were obtained by rendering the rank-1 tensor formed by the tangent of the fibers. The tensor field representation allows to compute means in the common additive manner as for the scalar densities. The directional density maps were normalized in the same way as explained above. However, the tensorial nature of the field has to be taken into account for normalization to MNI standard space. We therefore used the Jacobian matrix of the associated template warp to map the tensor from subject space to MNI standard space. The so obtained tensor fields in MNI standard space were then used for deterministic bundle-specific tractography. They were obtained by randomly placing seeds in high density regions (threshold>10^-1) with a very loose stopping criterion (threshold > 10^-8) to also reach cortical areas. For better understanding the individual tractograms are partly cut at a certain y-coordinate in MNI space. For example, in Fig. 5 the mdAT, rnPP and vtaPP/slMFB are cut at y = −22. For visualization and bundle specific tractography the medical imaging platform NORA was used (www.nora-imaging.org).
Fig. 5.
Subcortical anatomical course of distinct subcortical projection pathways. Left side shown only. Note how fibers of reward network (green) and affect network (copper) run in the ventral half of the anterior limb of the internal capsule on their way to the OFC. Fibers of the control network (rnPP, stnPP, snrPP) are located more dorsal and head toward dlPFC. vlATR and vlATRc not shown. Left white parenthesis in D (view from ventral) shows anterior-posterior dimension of anterior limb of internal capsule. Legend: see Fig. 3; MDT, mediodorsal nucleus of thalamus (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
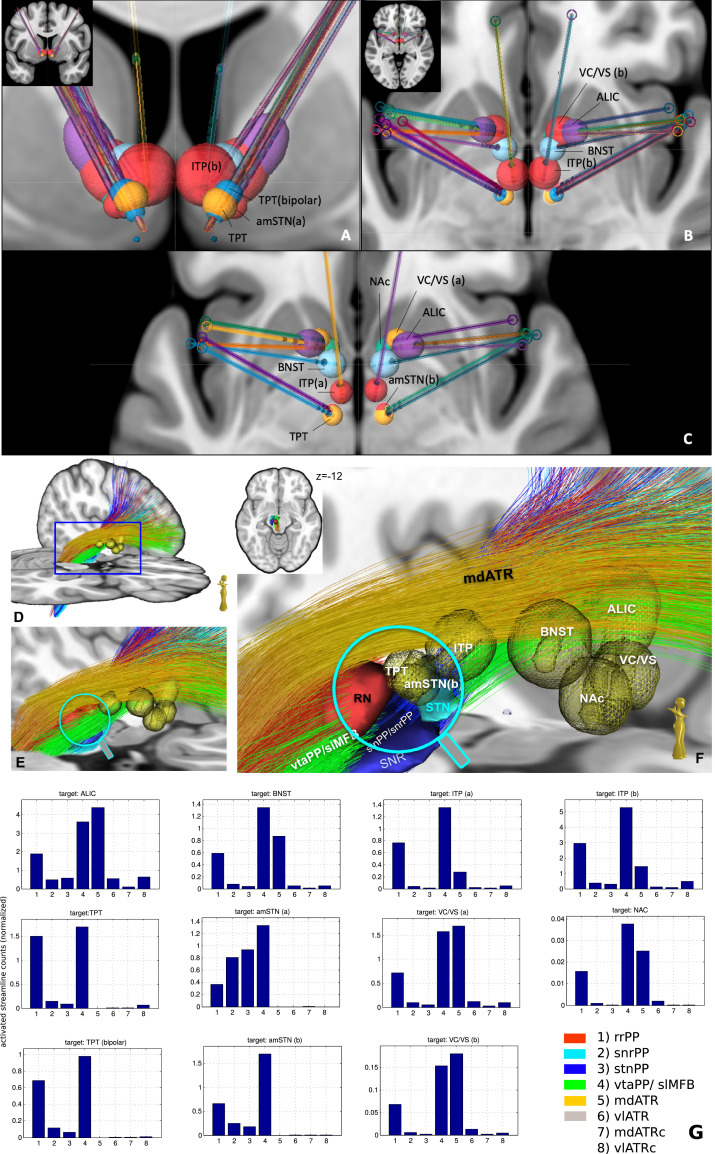
Simulation of selected DBS targets for OCD and MD: Stereotactic procedures and stimulation sites were simulated on an MNI template (Fonov et al., 2011, 0.5 mm isotropic, MNI152 asym.) serving as individual anatomical basis using an Elements® system (BrainLab, Munich, Germany) and electric field simulations using Guide XT® Elements (Boston Scientific, Valencia, CA, USA & BrainLab, Munich, Germany). Simulations were performed by an experienced stereotactic neurosurgeon without the use of fiber architecture according to the surgical approaches described by the authors of individual publications (Table 1). Trajectories respected the principles of safe trajectory planning (typically no trans-ventricular, trans-sulcal routes; as an exception the inferior thalamic peduncle target which needs to be trans-ventricular). Volumes of activated tissue (VAT) were simulated with coordinate base as described in Table 1. Electrode geometries could not directly be simulated but contacts of the Boston linear electrode (1.5 mm contact length, 0.5 mm spacing) were used to emulate stimulated regions in best comparison to distinct electrode geometries for the distinct targets. These targets were: ALIC, anterior limb of the internal capsule; amSTN(a)/amSTN(b), anteromedial (limbic) subthalamic nucleus in two definitions; BNST, bed nucleus of the stria terminais; NAc, nucleus accumbens septi; VC/VS(a)/VC/VS(b), ventral capsule ventral striatum (in two descriptions); ITP, inferior thalamic peduncle (simulated with two different simulated stimulation patterns); TPT, target point of superolateral medial forebrain bundle. VATs were extracted from the DICOM images by a simple thresholding operation. Then, fiber activations are computed as the sum within each VAT over the fiber densities of the individual PPs in MNI standard space (computed as described above).
Table. 1.
| Target | Source | MCP coordinate (in MNI template Fonov et al, 2011) [x,y,z] | MNI coordinate [x,y,z] | Simulation§ |
|---|---|---|---|---|
| ALIC | Nuttin et al. (2003) | 11, 5.5*, 0 | -14, 8, 0 | co 2,3 negative; 10mA |
| BNST | Nuttin et al. (2013) | 6, 0, 0 | -8, 2, -3 | co 1,2 negative; 6.5mA |
| ITP (a) | Jimenez et al. (2013) | 4.5, -10*, -2.5 | -5, -6, -3 | co 2,3 negative; 4mA |
| ITP (b) | Jimenez et al. (2013) | 4.5, -10*, -2.5 | -5, -6, -3 | co 2,3 negative; 4mA, (200us, 130Hz) |
| TPT | Coenen et al. (2018) | 6.5, -2.5, -5 | 7, –13, -6 | co 1 negative; 2.8mA |
| TPT (bipolar) | Coenen et al. (2018) | 6.5, -2.5, -5 | 7, -13, -6 | co 1 positive, 2,3 negative; 2.8mA |
| amSTN (a) | Mallet et al. (2008) | 8.5, 1, -5 | 9,-11,-8 | co 1 negative, 2mA |
| amSTN (b) | Tyagi et al. (2019) | 6.5, -13.5*, -5 | 7, -13, -7 | co 1 negative; 2mA |
| VC/VS (a) | Malone et al. (2009) | 7.5, 5*, -3 | (-11,7,-4) | co 1,2 negative; 6.7 mA (113us, 127Hz) |
| VC/VS (b) | Tyagi et al. (2019) | 8, 4*, -6 | (-11,8,-6) | co 1 negative; 4mA |
| NAc | Sturm et al. (2003) | 7.5, 2.5*, -4.5 | -9, 5, -8 | co 1,2 negative; 4.5 mA |
§ if not otherwise indicated simulated with case positive, single electrode contact (co) negative; logic: 1,2,3,4 (distal -> proximal), 130Hz, 60us; *reference AC. MCP, mid-commissural point, MNI (Montreal Neurological Institute standard brain); abbreviations of target regions: see material & methods.
Target region definitions in detail:
ALIC (Nuttin et al., 2003): Simulation of Pisces (Medtronic) electrode, 4 mm interspace, 3 mm contact length. Lowest contact at border to NAc, upper contact just above the anterior limb of the internal capsule, pre-coronal entry of electrode. In MNI/ACPC x = 11mm, y = 5.5 mm anterior of AC (posterior border), z = 0 (on ACPC horizontal plane). Simulation with 10 mA, contacts 5–8 (cathode, as of Boston linear electrode) monopolar stimulation, would be contacts 1-, 2- of the Pisces electrode used. In their paper, up to 10V stimulation (10 mA @ 1 kOhms impedance).
amSTN (a) (Mallet et al., 2008): Targeting 2 mm more anterior and 1 mm more medial than for patients with Parkinson's disease (Benabid et al., 2002). MNI T2-template used. In MNI/ACPC x = 8.5 mm, y = 1 mm ant MCP, 5 mm inferior MCP (adjusted to medial STN on T2 template). Stimulation 2.0V (2mA @ 1kOhms) mean stimulation (130 Hz, 60 us).
amSTN (b) (Tyagi et al., 2019): Medtronic electrode (model 3389). Anteromedial STN activated volume (VTA) was located at the border to the ventral tegmental area. Stimulation point was copied over from MNI-positions in publication (center of VTA in Fig. 2, Tyagi et al., 2019). MNI/ACPC (inferior most contact) x = 6.5 mm, y = -13.5 mm (posterior AC), z = -5 (below ACPC). Stimulation: 0 - 4 V, mean 2 V (2 mA @ 1kOhms impedance). Note, this is factually stimulation of the white matter medial to the nucleus.
BNST (Nuttin et al., 2013): Medtronic electrodes with different geometries (models 3387, 3391, 3389). We chose 3387 as best agreement (contact length 1.5 mm, 1.5 mm interspace, active tip total 10.5 mm). Target identified on ACPC horizontal plane. Posterior border (0–2 mm behind) of the AC 6 mm lateral to midline (BNST definition). Trajectory parallel and inside the anterior limb of the internal capsule. In MNI/ACPC x = 6 mm, y = 0, z = 0 (on ACPC horizontal plane). 6.5V median stimulation according to Luyten et al. (2015) on the lowest contacts were used (6.5 mA @ 1kOhms impedance).
NAc (Sturm et al., 2003): Medtronic electrode (model 3387). MNI/ACPC x = 7.5 mm, y = 4.5 mm (2.5 mm anterior to AC), z = -4.5 (below CACP). Stimulation 2 - 6.5 V, mean 4.5 V (4.5 mA @ 1kOhms impedance).
VC/VS (a) (Malone et al., 2009): Medtronic electrode (model 3387). Contact 0 in the ventral striatum, contact 1 at junction to anterior limb of internal capsule. Contacts 2,3 in the anterior limb of the internal capsule. Typical: 6-7 mm lateral to midline (X), 1-2 mm anterior to the posterior border of the anterior commissure (Y), and 3-4 mm inferior to the anterior commissure–posterior commissure line. MNI/ACPC 7.5 mm, 5mm anterior AC (posterior border), 3 mm inferior MCP. Stimulation: Distal contacts (0,1) negative, mean 6.7 V (6.7mA presumed 1kOhms), 113us pulse width, 127 Hz (frequency).
VC/VS (b) (Tyagi et al., 2019): Medtronic electrode (model 3387). 2 contacts in NAc shell and core, respectively, 2 contacts in the ventral part of the anterior limb of the internal capsule. MNI center of VTA respected in planning. MNI/ACPC (electrode tip) x=8 mm, y= 6.5mm (4 mm anterior to AC), z = -6 (below CACP). Stimulation 0-8V, here 4V taken (4 mA @1kOhms impedance).
ITP (Jiménez et al., 2013): Medtronic 3389 electrode used. Triangle between fornix, mammillo-thalamic tract and genu of posterior limb of internal capsule visually targeted at level of ACPC. Tip of electrode in MNI/ACPC system: x = 4.5 mm, y = -10 mm (posterior AC), z = -2.5mm (below ACPC). Stimulation: Contacts adjacent to ACPC plane, 3–5 V, 130 Hz, 450 us (4 mA @ 1kOhms impedance). Simulated with 60 us (and 200 us) only, otherwise VAT too big.
TPT (Coenen et al., 2018a): Medtronic 3389 electrode used. The typical individual tractographic planning cannot be applied in MNI T1 template. Triangle between mammillo-thalamic tract, STN and anterior circumference of red nucleus chosen as target. MNI/ACPC system x = 6.5 mm, y = -2.5 mm (posterior MCP), z = -5 mm (below ACPC). Stimulation: 2.86 mA, 130 Hz, 60 us.
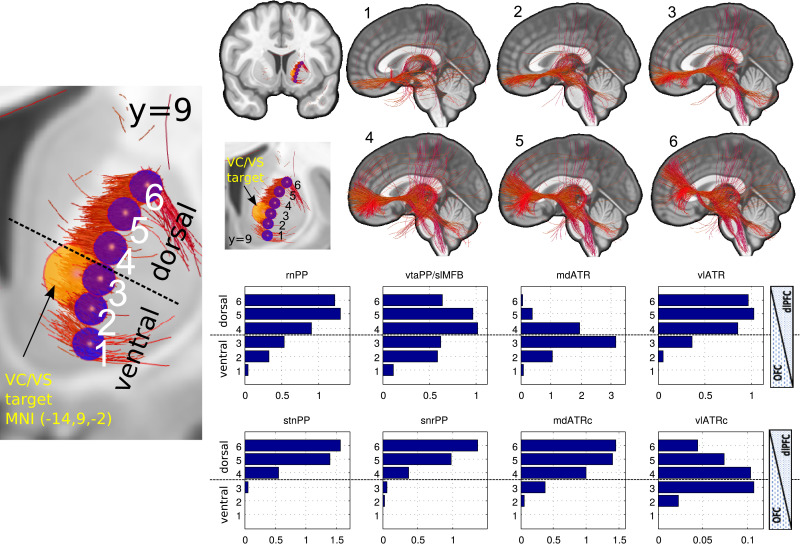
Decomposition of the anterior limb of internal capsule: To understand how the specified PPs traverse through the anterior limb of the internal capsule it was decomposed at y = 13 in MNI group space at six different combinations of x- and z-coordinates: {(-22,9),(-19.5,6),(-18,2),(-16.4,-2),(15.4,-6),(-15.2,-10)}. The aggregated fiber densities of all considered PPs were then evaluated at the location of the six resulting points as the mean of the densities within a radius of 2mm. For visualization purposes, the streamlines of the HCP group connectome (as described in Coenen et al., 2018c) were selected by interpreting the above points as spherical seeds.
Results
A coarse overview of the cortical terminals of the five main PPs (rrPP, snrPP, stnPP, vtaPP, mdATR) and their bundle specific tractographies is provided in Fig. 3. As becomes evident, PPs originating from the VTA and the mediodorsal thalamus mainly project towards orbitofrontal cortex, whereas the VLT, SNR, STN and RN mainly project towards the dorsolateral prefrontal cortex. The more fine-grained projection patterns in Fig. 4C and D further highlight the significant overlap between PPs: vtaPP/slMFB and mdATR project to identical cortical regions indicating a neocortical network-interplay. rnPP shows some overlap in the OFC with both PPs. mdATRc projects to the superior frontal gyrus and overlaps with stnPP, snrPP vltPP and rnPP. Its OFC extension is somewhat smaller than for mdATR. vlATR and vlATRc show almost the same projection pattern albeit it is located somewhat more lateral.
Fig. 4.
Cortical convergences of distinct projection pathways which belong to distinguishable networks. A, histogram showing the relative distribution of fibers over the considered cohort. error bars indicate the inter-individual deviations. B, Definition of the prefrontal cortex according to (Desikan et al., 2006). Left side shown only. C, density of cortical terminals in a view from anterolateral left. .D, view from midsagittal. Legend: PP, projection pathway; rnPP, red nucleus PP; snrPP, substantia nigra PP; stnPP, subthalamic nucleus PP (hyperdirect pathway); vtaPP, ventral tegmental area PP; slMFB, superolateral branch of the medial forebrain bundle; ATR, anterior thalamic radiation; mdATR, mediodorsal nucleus ATR; vlATR, ventrolateral nucleus ATR; mdATRc, mdATR with extension to cerebellum; vlATRc, vlATR with extension to cerebellum (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Tractographies of selected PPs are shown in Figs. 5–8 for understanding the subcortical trajectories. For perceivability we left out in Fig. 5 the bundles mdATRc and vlATRc which further extend to brainstem and cerebellum. Therefore, we show in Fig. 6 sagittal views of mdATRc and vlATRc. PPs take a quite intricate course during their ascension to the PFC. A view from inferior (cf. Fig. 5D) reveals that vtaPP/slMFB is spread out in the most inferior parts of frontal fiber system. On its way from the ventral midbrain (ventral tegmental area, VTA) the vtaPP/slMFB is followed by the rnPP dorsally in close proximity. vtaPP ascends via the inferior thalamic peduncle and under-crosses the mdATR/mdATRc to a lateral trajectory in the anterior limb of the internal capsule. Here it resides in the ventral portion more lateral while mdATR/mdATRc are located more medial on their way to the OFC. stnPP and snrPP ascend from the midbrain and follow a more lateral trajectory towards the dlPFC, leaving the previous fiber tracts in a medial position. They have no contact with the inferior thalamic peduncle but intersect and intermingle with fibers from vltATR/vlATRc which are almost strictly lateral to mdATR/mdATRc.
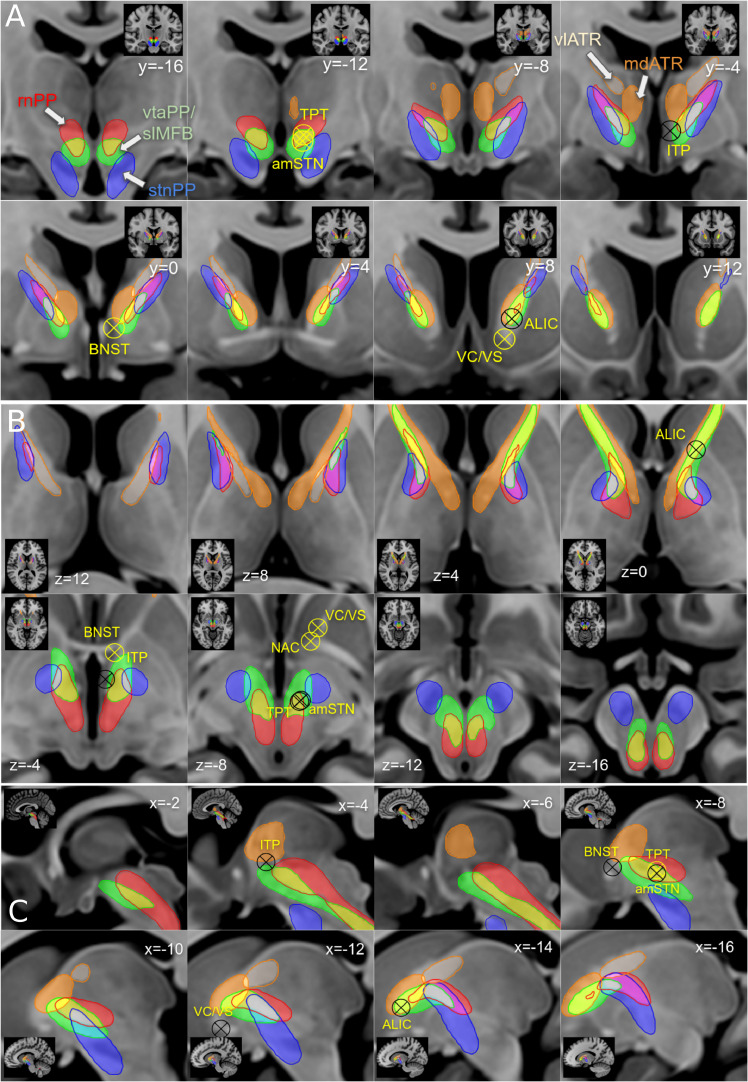
Fig. 8.
Approaching the anterior limb of the internal capsule with sub-segmentation based on distinct PPs. A, coronal; B, axial; C, sagittal. The anterior limb of the internal capsule is a fiber pass-through for different fiber pathways which run parallel and in part overlap. Targets for DBS and SLS are roughly indicated (sometimes the slice coordinate of the target does not perfectly match the imaging slice). Note that pathways assigned to reward and affect networks are located in the ventral/inferior anterior limb of the internal capsule, pathways assigned to control network are located dorsally. Stereotactic targets: ALIC, anterior limb of internal capsule; VC/VS, ventral capsule ventral striatum; NAc, nucleus accumbens; amSTN(b), medial subthalamic nucleus; ITP, inferior thalamic peduncle; BNST, bed-nucleus of stria terminalis; TPT, target point of vtaPP/slMFB DBS.
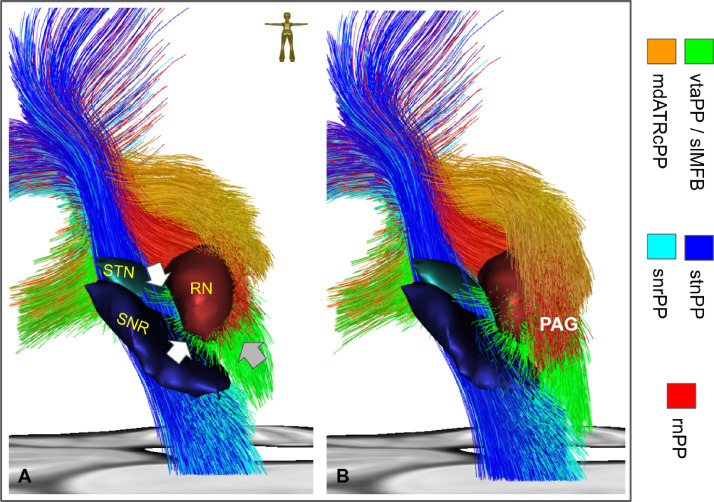
Because of its especially complicated origin vtaPP/slMFB was depicted in a closer view (Fig. 7). Mesocortical fibers conjoin from medial STN and SNr with mesolimbic fibers from VTA (not shown) and the dorsal raphe nuclear group (not shown) to ascend to PFC.
Fig. 7.
Midbrain region viewed from posterior. Left side shown only. A, fibers end in a coronal cut just posterior of the red nucleus (RN). White arrows indicate fiber which originate from antero-medial STN (upper) or from medial SNR (lower). Together they constitute the mesocortical projections of the vtaPP/slMFB. Grey arrow indicates fiber of the mesolimbic projections which extend into the dorsal raphe nucleus (not shown). B, coronal cut further posterior in the periaqueductal grey (PAG) shows intermingling/connection of mdATRC and vtaPP/slMFB fibers. For a better view streamlines of the vtaPP, rnPP and mdATRc are cut coronally at the levels of y = -22 (a) and y = -25 (b), respectively. The STN, SNR and RN are additionally shown for better orientation. Legend: STN, subthalamic nucleus; SNR, substantia nigra; PAG, periaqueductal grey (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Fig. 8 a–c) shows sections of thresholded fiber density maps together with selected target positions to display the anterior limb of the internal capsule. Stereotactic targets are identical to the ones shown in Fig. 9. Fig. 9 shows a closeup of the most relevant OCD and depression targets (see Table 1) and the corresponding PPs. In Fig. 9 D–F the rnPP, vtaPP and stnPP are coronally cut at the levels of y>-19 and y<-9 to allow an in-depth view of the ventral tegmental area. TPT (target point for slMFB DBS) and amSTN (b) are in a very similar (if not identical) position. Further, Fig. 9G shows plots for each target the distribution over the tract activations.
Fig. 9.
Simulation of DBS approaches for MD and OCD in different target regions. A-C; Definition of VATs (volumes of activated tissue) in MNI space, specific for distinct electrode geometries and significantly different stimulation amplitudes and settings (see methods). A, coronal overview; B, C axial views. Note: amSTN(b) and TPT are almost identical in coordinates and VAT size. D-F, simulation of same VATs in MNi152 space D, tractographic view of vtaPP, stnPP, snrPP, rnPP and mdATR; for the close-up view fibers of vtaPP, stnPP and rnPP are cut at levels y > -9 and y < -19. G, plots of all pairwise fiber activations as the sum of fiber visits within the simulated VATs. All target regions significantly recruit fibers from the reward network (vtaPP/slMFB system, column 4). ALIC (anterior limb of internal capsule DBS target) recruits almost 3-fold the fiber count from reward system than amSTN (b) and TPT (a) but needs 5-fold higher amplitude setting (10mA) and a larger electrode geometry (3 mm contact, 4 mm spacing).
3.1 Tractographic decomposition of the anterior limb of the internal capsule in a top-down approach
As a result of the tractographic analysis Fig. 10 shows the decomposition of the anterior limb of the internal capsule without (1–6) and with (histograms) anatomical priors. Every PP and by this network can be differentiated, and further evidence is found for a dorso-ventral / dlPFC-OFC gradient and a mediolateral gradient. Fibers belonging to reward and affect are located almost exclusively in the ventral part of the anterior limb (segments 1-3) while control network fibers are located in the dorsal aspect. A recently defined motor-pathway connecting OFC and motor cortex via the ventral tegmental area (Hosp et al., 2019) could additionally be reproduced here (2–3).
Fig. 10.
Connectomic assessment of the anterior limb of the internal capsule. Left overview, coronal (left side shown only); upper right, resultant fiber tracts from spherical seed regions (A) without anatomical priors projected on mid sagittal view; lower right, resulting histograms now including anatomical priors. Distinct fiber systems can be addressed in distinct parts of the anterior limb of the internal capsule. Note how maximal likelihood of mdATR perturbation is at level 3 while likelihood for vtaPP/slMFB is maximal in 4 and 5, for vlATRc maximal in 3 and 4. Fibers reaching the precentral region have recently been described (Hosp et al., 2019) (Legend: 1-6, ventral-dorsal gradient. PP, projection pathways compare Figs. 4–6)
Discussion
4.1 Corticopetal systematics of networks associated with psychiatric diseases
We have described in detail connection pathways to the prefrontal cortex, which ascend from thalamus, basal ganglia, midbrain and from the cerebellum. These connection pathways have been named projection pathways bearing in mind that some of them are hyperdirect projection pathways (which certainly applies to the stnPP). Despite the accepted significance of network dysregulation with respect to specific disease symptoms (Li et al., 2018) the link to deeply located (fiber) structures has not been made. Nevertheless, the role of deep seated anatomy for emotional states has long been discussed (Choi and McNally, 2017; LeDoux, 1995; Panksepp, 2003) and the founders of modern Stereotaxy had already detailed concepts where to distinctively treat diseases of the emotions (Gildenberg, 2002; Spiegel et al., 1947; Spiegel et al., 1951). Interestingly, researchers appear to relate the therapeutic concept of their doing to the very substrate they are dealing with. It is thus not surprising that modern neuropsychiatrists who deal with clinical non-invasive stimulation modalities like TMS will envision their network environment as being purely cortical. The same holds true for recent developments in DBS targets with cortical location (Johansen-Berg et al., 2007; Mayberg et al., 2005; Riva-Posse et al., 2014). Stereotactic neurosurgeons, who typically work in the subcortical part of the brain more likely follow a view in favor of deep-seated mechanisms of cognition, motor control and emotion (Spiegel et al., 1951). This view is certainly closer to the view of animal scientists or comparative neuroscientists with respect to evolutionary concepts, possibly allowing some careful comparison between the emotional life of the different species (Loonen and Ivanova, 2016; Panksepp, 2012; Panksepp et al., 1997; Slavich et al., 2010).
However, for the sake of evolutionary differences, animal scientists typically deal with much simpler emotional systems which are less differentiated, and which do not converge on a complicated and evolutionary far developed neocortex. These researchers have paved the path towards a more genuine and evolutionary understanding of emotional systems, and this is largely a role that has been taken by affective neuroscience (Panksepp, 2005; 2003). In order to come to a broader understanding concerning networks of emotions, it might be useful to combine the different views. Advanced imaging technologies like dMRI and fMRI today bridge a cleft that is left open by classical neuroanatomical techniques and now allows to describe far reaching connections and to describe the whole brain connectome in three-dimensional space. Especially dMRI takes the role of a non-invasive and modern analog to degeneration and tract tracing studies (BECK, 1950; Frankle et al., 2006; Haynes and Haber, 2013) with certain limitations of DTI (see limitations section).
We will in the following assign tractographically defined projection pathways of this contribution to networks relevant for MD and OCD (Fig. 11). We will then stress the use of anatomical priors for tract identification studies in a corticopetal fashion. This is especially important as there is a certain lack of ground truth (e.g. histological staining in human specimen) in the results of this contribution and in the light of somewhat different results found in publications on primate and human anatomy when analyzed in a corticofugal fashion. We will further extrapolate to some extent to stereotactic procedures (SLS, DBS) in MD and OCD and their possible mode of action.
Fig. 11.
Detailed subcortical projection pathways in a hierarchical network perspective. A, Hub-regions represent network connections or stations in networks, pass-through -regions allow anatomical proximity of networks but no direct physiological functional connection and interaction. Default network not shown. Legend: STN=subthalamic nucleus; SNR=substantia nigra, VTA=ventral tegmental area, RN = red nucleus, PAG = peri-aquaeductal grey, MDT=mediodorso nucleus of thalamus, VS/NAC = ventral striatum/nucleus accumbens, BNST=bed nucleus of stria terminalis, ITP=inferior thalamic peduncle, VLT = ventrolateral thalamus; PFC = prefrontal cortex) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Attribution of subcortical anatomical projection pathways to established networks relevant for MD and OCD
4.2 Reward network
On the cortical level prefrontal (dlPFC, OFC) regions have been assigned as being reward or reward-learning associated (Neubert et al., 2015). Caudate, putamen and nucleus accumbens (VS, ventral striatum) anterior cingulum and ventromedial PFC are further anatomical components (Li et al., 2018). According to the results of this contribution the subcortical portion of this network is largely confluent with a projection pathway arising from the ventral tegmental area (VTA) the vtaPP. The vtaPP is a massive fiber structure which connects the VTA with distinct subcortical (nuclear) parts of the reward system (NAc, septal region) but at the same time reaches reward associated regions of the prefrontal cortex (Brodmann's areae BA8, 9, 10, 11, 11 m, 47; Fig. 3; Coenen et al., 2018c; Neubert et al., 2015). It is thus concerned with the experience of reward (OFC) and the further computation of reward-related choices, behavior (dlPFC) and learning. Stimulation of the structure in swine shows activation in cortical and subcortical regions analog to the ones described in this contribution (Settell et al., 2017). We have in our previous work used the term slMFB (superolateral branch of the medial forebrain bundle) for this structure (Coenen et al., 2009; 2012; 2011). The slMFB is in a stricter sense not identical to the mfb (medial forebrain bundle) with its trans-hypothalamic route (Coenen et al., 2012; Nieuwenhuys et al., 1982) but in our view comprises a projection out of the VTA (just like the mfb) but with a topographically distinctive course (hence “slMFB”) while functionally assuming some similarity in conjunction with an evolutionary newly developed reward associated neocortex. It is here – for the sake of a uniform terminology – also named vtaPP (projection pathway from the ventral tegmental area). White matter alterations have been found within the vtaPP/slMFB and were correlated to hedonic tone, effective treatment or subtypes of depression (Bracht et al., 2014a; Bracht et al., 2014b; Bracht et al.,2015). Zacharopoulos et al. (2016) described vtaPP/slMFB as a hedonism hub of the human brain. Recently, a correlation was found between microstructural measures (increased structural connectivity) in the same structure and correlated to feelings of grandiosity and paranoia in schizophrenia (Bracht et al., 2019) which might reflect on the vtaPP/slMFB's importance in the experience of SEEKING and magnitude of the motivational drive.
4.3 Affect network
The modern (and cortical description) of the anatomical substrate of the affect network include OFC, PFC, insula, amygdala and anterior cingulate cortex. Spiegel and Wycis, the founders of modern stereotactic surgery, developed their surgical approaches to the mediodorsal nucleus of thalamus (MDT) as a direct consequence of the unnecessary extensive lesions to the frontal lobe applied in the lobotomy era. They intended to apply lesions more focally for the treatment of the emotional diseases (Gildenberg, 2002; Spiegel et al., 1947) and developed according stereotactic techniques. At that time, postmortem studies were used to reveal the degeneration of projection pathways affected by the destructive lesion through lobotomy to the prefrontal cortices (MEYER, 1949; Safadi et al., 2018b). Degeneration studies were first described more than 70 years earlier by the German psychiatrist and neuroanatomist Johan Bernhard Aloys van Gudden (Sarikcioglu, 2007). Based on similar investigations and with the somewhat limited techniques of their time, Spiegel and Wycis developed an already remarkably detailed concept of the central mechanisms of emotion grouped around MDT (Spiegel et al., 1951) which we today assign to the affect network (Fig. 11). Choi and co-workers in rodents identified the MDT as an important decision maker to evaluate the individual cost of obtaining rewards (Choi and McNally, 2017). They highlight the role of the MDT (ventricular thalamus in the rodent) and the general affect system for reward evaluation and decision making. The MDT has further an important role in the retrieval of fearful memories (Maren et al., 2004) and as such is clinically intimately wired to the OCD and MD circuitry. Affective neuroscience – which uses animal studies to develop a framework for the emotional self of humans - also attributed subcortical parts of the brain to this network and integrated the MDT, the periaqueductal grey (PAG) as well as the bed nucleus of the stria terminalis (BNST) and the amygdala into the same system (Panksepp, 2003). It is of note that in anterior nucleus of thalamus (ANT) DBS a significant rate of depression occurred during the seminal trial (SANTE). The number of 14.8% newly diagnosed depression (albeit self-rating, BDI) might be related to a co-stimulation of the MDT just adjacent and below ANT (Fisher et al., 2010) and by this an activation of the mdATR. It is moreover possible, that in a case report for NAc DBS in OCD that reported fear and panic during acute stimulation, the affect pathway in its outflow to the OFC was effectively stimulated (Shapira, 2005). Our tractographic description here does not include the temporo-mesial connection to the amygdala (because of a restriction to the PFC) but we have previously described this connection in a similar context in humans (Coenen et al., 2012) also including the PAG. The PAG was recently investigated concerning prediction error with regards to pain perception (Roy et al., 2014). These researchers also found an overlap of reward associated regions and regions promoting affect (Figs. 2 and 3). The somatic/emotional pain pathway from the PAG to the PFC has previously been mapped with DWI MRI in conjunction with DBS for neuropathic somatic pain (Sillery et al., 2005).
4.4 Control network
We have here assigned PPs from STN, RN, vlThal and SNr to the control network. Based on the previous work by other groups, this appears to be justified and it in part replicates classical work on parallel prefrontal circuits, especially the association circuit (Alexander et al., 1986). We were here able to show that PPs from our candidate structures actually traverse the dorsal (superior) part of the anterior limb of the internal capsule. VL thalamus, SNR: The ventrolateral thalamus is part of the dorsal thalamus (Nieuwenhuys et al., 2008) and is constituted of the ventral anterior nucleus which receives input from the basal ganglia and the globus pallidus and projects to the premotor cortex. The ventrolateral posterior nucleus receives main input from the contralateral cerebellum and is referred to the cerebellar receiving part of the thalamus. The role of the motor thalamus was exhaustively described in the seminal work of Alexander and de Long (Alexander et al., 1986). We here found evidence that the main projection of the VLT as a whole (realized via vlATR or vlATRc if including the cerebellar outflow) has a very similar distribution with cortical convergences like stnPP and snrPP thus mainly the premotor and supplementary motor regions (including BA6, BA8). The here defined stnPP has been described in other work as hyperdirect pathway (Nambu et al., 2002; 1996; Miocinovic et al., 2018) and can – tractographically – be regarded analogous. We have here found similar projection patterns to the prefrontal cortex as has been described by Aron et al. (2007). The STN is probably the most important subcortical structure in regulating the frontal lobe with respect to cognitive control (Aron et al., 2007). The often-discussed tri-partite structure (motor, limbic and associative) is very convenient with respect to stereotactic surgery. However, some authors have debated a strictly tripartite division. See Keuken et al. for a further discussion (Keuken et al., 2012). The role of the STN and its involvement in frontal lobe control was especially discovered and then described in the context of increased impulsivity during STN DBS (Ballanger et al., 2009; Frank et al., 2007). Impulsivity clinically is characterized as premature decision making with low quality decisions which cannot be revised. The snrPP cannot completely be separated from our stnPP. Red nucleus (RN): The results of our cortical projection pattern for rnPP are in keeping with previous results including some projection to OFC (Fig. 3). RN has previously been shown to have extensive connections to the prefrontal cortex. In the macaque the magnocellular part, which is associated to the rubro-spinal system receives most projections from the precentral regions. Parvicellular parts receive projections from pre and supplementary motor regions (Kuypers et al., 1967). In another macaque study, Monakow et al. (1979) were not able to demonstrate far-reaching premotor connections besides some from BA6. Milardi et al. (2016) found very similar connections to the superior frontal gyrus. Again, their results were in part replicated by us but the projection to OFC was not seen by them. In an elegant study using resting state MRI to scrutinize functional connectivity of RN the authors found evidence for the RN participating in the cognitive circuits involved in executive control but also in the interpretation of salience and with a clear involvement of the OFC (Nioche et al., 2009). In keeping with these results, the rnPP is closely linked to the reward network and helps to interpret saliency or aversiveness of signals.
4.5 Default mode network
Despite its role in the neurocircuitry of MD and OCD (Buckner et al., 2008; Koch et al., 2018; Li et al., 2018) we have not reflected on the default mode network in this work. Other authors have found and described subcortical connectivity which certainly touches on this network especially with respect to the cingulum bundle (Riva-Posse et al., 2017; 2014).
4.6 Comparison with corticofugal human and primate anatomy and additional methodological considerations
Previous research in primate anatomy is not entirely congruent with our results (Frankle et al., 2006; Haynes and Haber, 2013) - especially with respect to the connecting fibers between VTA and OFC and dlPFC (reward network). These differences need to be discussed: Despite the massive connection between VTA and frontal lobe structures in this and previous contributions, in primates some dissociative results have been reported. Injection studies in a standard text of descriptive macaque anatomy shows connection pathways between precentral cortex, motor cortex and the midbrain (Schmahmann and Pandya, 2006) (eg case 30–33). These results are in keeping with our results and with publications in other monkey species (owl monkey and rhesus monkey) (Gaspar and Neurology, 1992; Porrino and Neurology, 1982) which also include corticopetal dopaminergic projection pathways. It is of note that the true route of dopaminergic projections (especially mesocortical) in humans has not fully been cleared up and reports are contradictory reporting lateral (over ICa) (Taber et al., 2012) and other trajectories (Ciliax et al., 1999). In the context of macaque standard fiber anatomy (Schmahmann and Pandya, 2006) a report of sparse midbrain connections (especially to the VTA) has been somewhat surprising (Frankle et al., 2006). On the contrary, this group reported a rich connection of the prefrontal cortex to the subthalamic nucleus (STN) (Haynes and Haber, 2013) and defined in the macaque a “limbic hyperdirect pathway” (lHDP) which realizes a connection to the medial and anterior portion of the STN in the macaque while – according to their results - not reaching the VTA. We have previously discussed that the vtaPP/slMFB is likely the human analog of this pathway (Coenen et al., 2018c). In a recent publication of comparatively derived human anatomy (comparison to macaque tract tracing studies) (Petersen et al., 2019) according to the authors fiber tracts of the hyperdirect pathway reach the (medial) STN only, despite the presence of fibers medial and outside the STN (including the VTA) also present in the referenced macaque fiber injection studies (Haynes and Haber, 2013). These fibers outside the STN reach further down into midbrain and pons (Fig. 4 A, B of this study, Petersen et al., 2019). It is likely that these streamlines are congruent with vtaPP/slMFB of this and previous contributions and it might be regarded as problematic in this context, that displayed streamlines of a final atlas are in part the results of manual and subjective alterations based on an anatomical peer consensus during holographic inspection (Petersen et al., 2019) without interpreting own results of tract tracing studies to their full extent (Haynes and Haber, 2013).
As a general principle we observe that corticofugal tractographic approaches in humans, which actually mimic primate tract tracing studies with cortical injection strategies (cf. corticofugal approaches, above) find in general similar fiber trajectories as compared to this and previous corticopetal contributions, which serve different models of interpretation depending on the specific viewpoint (corticofugal vs. corticopetal). Thus, further fiber pathways found in this contribution (stnPP, mdATR and vlATR) can easily be found in the prefrontal cortex distribution of a resultant atlas (Petersen et al., 2019), only that there is no consequent assignment to subcortical networks as we have tried in this contribution. In this context it is important to note that the DTI technology cannot differentiate between fibers arising from the VTA and heading towards the PFC/OFC region and others descending from PFC/OFC to the ventral tegmentum. Therefore, it is important to discuss that for our contribution here – and to some extent this holds true for other tractographic work - there is a certain lack of ground truth (e.g. histological tract tracing in the human) which serves as comparison. However, for our case Hurwitz and co-workers have reported a ponto-frontal pathway which they suspected to be antidromic to Arnold's bundle and which they observed in three of their five patients after anterior capsulotomies with a novel T1 MRI signal (Hurwitz et al., 2006b). Moreover, dopaminergic projections have been described to reach the prefrontal and motor cortices from the ventral midbrain in other primate studies (Gaspar and Neurology, 1992) despite an as yet unsolved discussion of fiber routes. Based on this research and in conjunction with psychotropic side effects in Parkinson's disease (DTI Coenen et al., 2009) and antidepressant effects in MD (Bewernick et al., 2017; Coenen et al., 2019a; Schlaepfer et al., 2013) of DBS in the region medial to the STN the vtaPP/slMFB was described with DTI (Coenen et al., 2012) and now reconfirmed in the present contribution. Clinical effects clearly add ground truth supporting the here described anatomical course of corticopetal fiber projections out of the VTA.
4.7 Network interplay and the influence of some stereotactic procedures affecting subcortical PPs
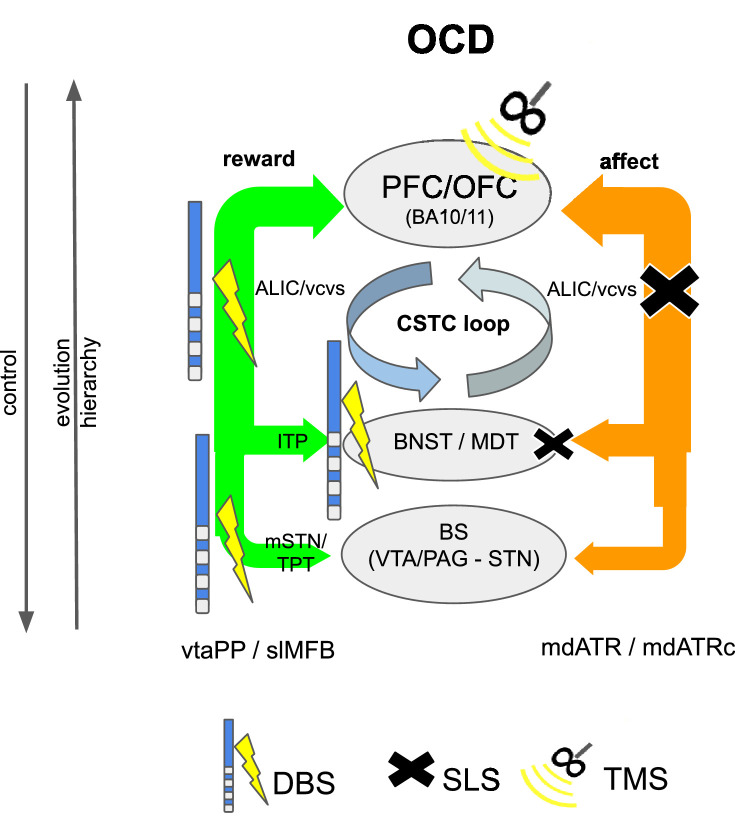
Dysregulation in the cortico-striato-thalamo-cortical (CSTC) loop is typically used to explain the pathophysiology of OCD and the mode of action of stereotactic interventions (Greenberg et al., 2010; Mega and Cummings, 1994). We have here found evidence that this loop is in fact spread out over two interacting network systems (reward -> CS, affect -> TC; Fig. 11) and have thereby found further evidence that OCD (and its therapy) involves both systems (Coenen et al., 2016). Involvement of the reward network has been proposed by other researchers (Alves-Pinto et al., 2019; van Westen et al., 2015). Van den Munckhoff and co-workers showed that the effective contacts of their DBS electrode in the nucleus accumbens (NAc) in OCD were located more dorsal than the nucleus and in the ICa (van den Munckhof et al., 2013). On the highest hierarchical cortical level, deep TMS to the ACC and mPFC have proven to be valid targets for OCD (Carmi et al., 2017) and at least in part affect and reward network converge in these regions.
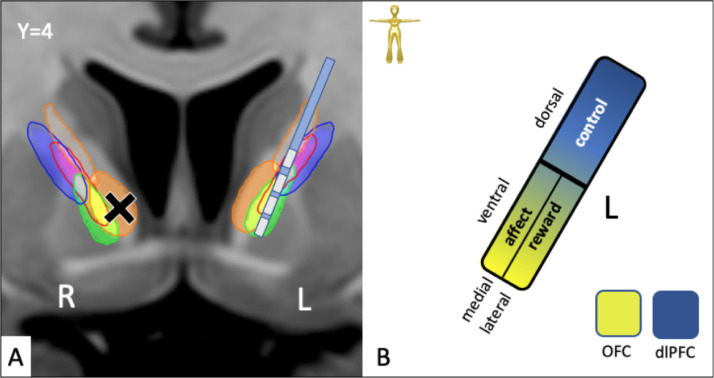
Our streamline model in normative space predicts that PPs for reward network (vtaPP/slMFB) and affect network (mdATR) are located in the ventral part of the ICa as opposed to the control network, which is located further dorsal (Figs. 8 and 13). On a closer look, mdATR fibers are more ventral and medial while vtaPP/slMFB fibers are located slightly more lateral in the ICa. This potentially explains why lesions are more effective if applied more ventral (Rasmussen et al., 2018b) while DBS electrode in tendency are more effective if located slightly more dorsal, therewith affecting the reward network (Liebrand et al., 2019a) (Liebrand et al., 2019b) (Fig. 13). On another note, fatigue can be the result of anterior capsulotomy in up to 30% of cases (Hurwitz et al., 2012), potentially reflection severing vtaPP/slMFB fibers and by this reducing motivative drive (SEEKING) while still improving depression (sadness) by lesioning mdATR. In a recent study directly comparing anteromedial STN (amSTN) DBS with ventral capsule (VC/VS) DBS in a crossover design (n = 6 patients), the latter has shown to improve OCD but not cognitive flexibility while both targets effectively treated OCD. This study is of particular interest since it reports neuropsychological outcomes along with MNI coordinates of the VC/VS and mSTN contact locations. In this series cognitive flexibility (as the neuropsychological hallmark of OCD) improved better with anteromedial STN DBS only (Tyagi et al., 2019) while VC/VS DBS showed lesser improvement. They discuss that amSTN retrogradely activate part of the hyperdirect projection to the STN which – in original function – suppresses the activity in the target region (Nambu et al., 2002; 2017) and by this attenuates cognitive inflexibility in OCD.
Fig. 13.
Proposed mode of action for SLS (cross) and DBS of the ICa in OCD and MD. A, overview applying the network model. B, schematic. DBS and SLS are most effective in the ventral part. Further dorsal application will likely result in cognitive effects (confusion and deficiency of cognitive control of emotions in SLS, changes in decision making and cognitive emotion control in DBS). DBS (electrode) in tendency more lateral, SLS (cross) more medial.
Applying our corticopetal PP network model, we find an additional explanation: DBS to VC/VS modulates vtaPP/slMFB (reward network) and even more mdATR (affect network) (Fig. 9G) which in our simulations are included in the effective stimulation. Since there is already a hyper-connectivity in these pathways it is like stepping on brake (affect network) and the gas pedal (reward network) at the same time with secondary effect on cognitive control and decision making (more dorsal in the ICa). Moreover, the cognitive control network (including the stnPP/hyperdirect pathway) might be directly co-modulated if current reaches up higher in the ICa (Fig. 13). Both mechanisms might explain the neuropsychological effect of persistently reduced cognitive flexibility in VC/VS DBS in this report (Tyagi et al., 2019).
Interestingly, according to our own MNI-based simulations, the reported amSTN-stimulation (Tyagi et al., 2019) actually modulates vtaPP/slMFB fibers serving this region (Figs. 8 and 9) and is close – if not identical - to direct stimulation of the vtaPP/slMFB (TPT) (Coenen et al., 2016; Coenen et al., 2018b; Schlaepfer et al., 2013). Since in MD the same regions for SLS and DBS are used, the same considerations on network interplay can be applied. In MD a deficient reward network is presumed. Therefore, the effect in MD is likely an enhancement of the reward system. At the same time the affect system is influenced similarly.
When looking at the results of the analysis of PFC terminals of the PPs (Fig. 3) it becomes clear that the affect network and the reward network show a dlPFC convergence at the anterior part of the middle frontal gyrus (BA46). According to the most recent literature this region coincides with typical application regions of rTMS in MD and OCD (Johnson et al., 2013; Kisely et al., 2018) and also epidural cortical stimulation in MD (Kopell et al., 2011; Williams et al., 2016). Du et al. found that early responders after left dlPFC rTMS had their TMS location in BA9 and 46 (Du et al., 2018) that the efficacy of stimulation coincided with the strength of resting state connectivity (FC) between left dlPFC and NAc. This could be interpreted as again a pathological connection in the CSTC loop but here for patient suffering from depression. It is important to point out, that we scrutinized a normative cohort and that the salient functional and anatomical connectivity – corresponding to BA46 – more markedly shows up in diseased populations for which it has been described. We have recently found the same region as volume altered with respect to antidepressant efficacy of DBS (Coenen et al., 2019b).
4.8 The anterior limb of the internal capsule and a newly proposed corticopetal systematics
The anterior limb of the internal capsule (ICa) is classically described as a macro-anatomical structure which contains fibers from different brain regions. These fibers bidirectionally connect the PFC to thalamus, basal ganglia (including striatum) and brainstem. Moreover, there are fibers which interconnect parts of the basal ganglia, which we have not regarded here. In the literature the ICa is viewed as a fiber pass through without any connections between these fibers (Nieuwenhuys et al., 2008). Despite the view of some authors that ICa is a single large fiber bundle which might be microstructurally altered in diseases like diabetes, depression, and bipolar disorder (Nanda et al., 2017; Safadi et al., 2018c; Zhang et al., 2013), we find evidence, that this structure is a rather heterogeneous white matter region which carries at least 8 subcortical module systems (vlATR, vlATRc, mdATR, mdATRc, stnPP, snrPP, rnPP, vtaPP/slMFB) with distinct functions which can be grouped in a corticopetal systematics. The modular systems can be allocated to distinct parts of ICa allowing for an explanation of distinct effects of stereotactic interventions. ICa contains information from thalamic, basal ganglia, brain stem regions as well as cerebellum and connects them to the prefrontal cortex. Using the heuristic applied here these systems can be assigned to distinct networks (affect, reward, control) and since they converge onto similar neocortical parts allow for a network interaction on the highest hierarchical level. Fiber systems related to emotion and affect are located in the ventral part of the internal capsule (vtaPP/slMFB, mdATR, mdATRc) and sub serve very similar parts of the PFC and OFC. It is a new finding of this contribution that these PPs - which reach up from different subcortical regions (mediodorsal thalamus and ventral tegmental midbrain) - have similar cortical terminal regions. In our interpretation this indicates that these fibers constitute modular parts of larger networks and cortically even of networks between networks which are important for flexible control of emotion and behavior. These connections to the OFC/vmPFC are congruently found in previously reported corticofugal systematics (Nanda et al., 2017; Safadi et al., 2018a) and here these fiber connections also reside in the most ventral part of the anterior limb. Our further differentiation helps to explain previous finding of a CSTC loop dysregulation which we find to spread over the two systems (see previous section and Fig. 8, 12 and 13). Moreover, fibers of vtaPP/slMFB and mdATR wich are located higher up in the ventral anterior limb will reach dlPFC (BA8, 9,10) and overlap with the position of the control networks (rnPP, stnPP, snrPP) while the most ventral fibers rather address OFC regions (BA10, 11,11 m,47). It is likely that fibers to the dlPFC are the very part of the emotional system that deals with emotional control and as such with the consequences of emotional feelings (and of successful DBS/SLS). This systematics might explain, why SLS is effectively performed in ventral parts of the anterior limb (Santos et al., 2019) and why further dorsal reaching lesions lead to (transient) confusion and (if even further dorsal and encroaching on the control network) to decreased verbal fluency (Hurwitz et al., 2012).
Fig. 12.
Detailed Integration of subcortical projection pathways and the cortico-striato-thalamo-cortical (CSTC) loop theory in OCD. DBS potentially works largely over a modulation of the reward system (vtaPP/slMFB) and changes a top down pathological synchrony while SLS will largely inhibit affect system fibers (mdATR). DBS and SLS will have effect on both systems and act on both arms of the same CSTC loop. Both systems in combination have also been named the “salience network” (Peters et al., 2016). Legend: BS, brainstem; STN = subthalamic nucleus; VTA = ventral tegmental area; MDT=mediodorsal nucleus of thalamus; BNST = bed nucleus of stria terminalis, ITP=inferior thalamic peduncle, ALIC=anterior limb of internal capsule (DBS target), vc, ventral capsule (DBS target); PFC=prefrontal cortex; mdATR=medial anterior thalamic radiation; mdATRc= mdATR with cerebellar extension; vtaPP= VTA projection pathway; slMFB=superolateral medial forebrain bundle).
As a final note, fiber systems ascending from the brainstem and on their way to the PFC pass the ICa can be addressed with electrical stimulation at different and evolutionary distinct target points of their anatomical course (Figs. 8 and 9) (Coenen et al., 2016; 2011). Based on the subcortical differentiation of PPs the notion that any stimulated region (like TPT or amSTN in the midbrain) therefore should be named ICa (VC/VS or ALIC) – as has been discussed - is a truncation and oversimplification of the discussion. The implications of stimulating a network at different (and evolutionary distinct) points has been addressed in recent work (Tyagi et al., 2019).
4.9 Search strategies for effective networks with and without anatomical priors
Based on previously published work (Baldermann et al., 2019; Nanda et al., 2017; Safadi et al., 2018c) we have in our contribution performed a pure topographical parcellation of the anterior limb of the internal capsule (Fig. 9). We have first performed an analysis without any anatomical priors which appear to replicate previous results by Baldermann et al. who analyzed a cohort of patients with DBS to VC/VS for the treatment of OCD. Just as these authors we found fibers in the ventral part of the internal capsule and (Fig. 9 (1–3)) which pass through and at the same time address OFC, thalamus and brain stem. This is in keeping with the corticofugal model suggested by Safadi et al. (2018a) and their proposed dorsal/ventral axis. By applying our corticopetal network model we find that the majority of the fibers addressed in the ventral ICa (our segments 1–3) belong to the affect (mdATR/mdATRc) and reward networks (vtaPP/slMFB) which are likely co-modulated at this position (Figs. 10 and 13) (Liebrand et al., 2019b;Liebrand et al., 2019a).
Limitations
Anatomical descriptions based on normative data sets like used here share certain limitations which have to be addressed especially when extended to interpretation of surgical results in specific diseased populations like patients suffering from MD and OCD. Tractographic techniques based on dMRI by themselves share several limitations. The tractographic technique suffers from a determination of direction, it does not show synapses or any transmitter specifity. The fiber densities considered here (and the bundle specific tractograms) are not real quantitative estimates of the true underlying neurite density, they are rather a coarse depiction of the underlying anatomy. The low signal anisotropy in midbrain regions (and the low signal strength itself) makes it difficult to make quantitative estimates. Tractographic approaches based on local, streamlining approaches have significant problems there (either deterministic or probabilistic): errors accumulate and lead to either completely erroneous in case of deterministic algorithms, or, for probabilistic approaches, result in very fuzzy tracts with limited anatomical information. Therefore, we used here the technique of global tractography, which is known to be rather robust (it has shown superior performance on the FiberCup phantom Fillard et al., 2011). Results suggest the anatomical plausibility (see Fig. A1) even on subject level, however, the cortical projection patterns might also not fully represent the true picture, because, in particular the anterior corona radiata as one of the major crossing areas on the way of the PPs towards the cortex, makes reliable streamline reconstruction difficult. The correlation between simulation of VATs needs to be interpreted with care since white matter anatomy does not necessarily resemble altered tracts under disease conditions. However, we are looking for general descriptions of network interactions, therefore we think the approach is valid in its interpretation. The estimated activations of tracts by the simulation of VATs need also to be interpreted with care since white matter anatomy does not necessarily resemble altered tracts under disease conditions. Simulations of DBS in MNI-space need to be regarded with care and can only serve as basis for discussion of principle mechanisms. No real patient data and clinical outcomes were corelated with these simulations. However, we think in order to show the principle involvement of specific networks the strategy used is valid when cautiously interpreted. Finally, our viewpoint of a phylogenetically driven network ordering principle in itself might not be the only way of interpretation. Therefore, the results of this work need to be interpreted with caution.
7. Conclusion
This is a largely synthetic paper which tries to combine views from different neuroscientific specialties (psychiatry, affective neuroscience, neuroanatomy) with respect to subcortical extensions of networks relevant for OCD and MD in a corticopetal neurotopological view. We have here described PPs, which constitute main trajectories between subcortical structures and distinctive parts of the prefrontal cortex. These PPs contribute to most of the pre-described networks (reward, affect, control) relevant for OCD and MD. Based on their hierarchically grouped convergences (midbrain, thalamus, basal ganglia and prefrontal cortex) these networks potentially interact on multiple evolutionary conserved levels in different nuclei or cortical projection fields which we here have grouped together as “hub"-regions. The here described “hub vs. pass-through “- concept allows for an understanding of the plethora of effective stereotactic targets which have been described for DBS or SLS in OCD and MD. Some of these targets are hub regions (e.g. BNST, NAC) others are simple pass-throughs (e.g. ALIC, ITP) that allow for an anatomical proximity of networks with distinct functions, while physiologically not enabling network interactions. Stereotactic interventions at the same time might modulate different PPs and by this several networks at once. Influencing several networks at one target point might explain the clinically quite similar effects of the more activating DBS and the inhibiting SLS. We have explained this in detail for the CSTC loop and OCD, but this explanation likely holds true for MD as well (Fig. 1). Moreover, influence on one subcortical network might be sufficient to rebalance the entire network system (e.g. lesioning overactive affect network or stimulating the underactive reward system might have quite similar effects on the entire system level). Outbalancing between networks then happens on different hierarchical levels. According to our analysis, target regions for OCD and MD mostly group around (but not exclusively address) fibers that are confluent with subcortical parts of the reward network. These results support our earlier hypotheses of dynamic network balances between reward and affect system relevant for major depression and OCD (Coenen et al., 2016; Coenen et al., 2012;Coenen et al., 2011).
With respect to phylogenetic brain development and despite of the highest hierarchical functional rank of the neocortex with its top down control, this rank does not automatically imply a neocortical organization principle of holistic brain networks. It is conceivable that the functional organization principle of behavioral networks (and the connection of networks between networks) can be identified in an evolutionary driven “bottom up” order. Hence it seems reasonable to use a corticopetal analysis to gain wider understanding of network organization. Besides a horizontal organization (between hierarchically identical networks) we have here proposed a “corticopetal and vertical” organization principle that might help to understand network interactions. The vertical organization principle might explain, how subcortical interventions on a hierarchically inferior level lead to altered network balances on hierarchically higher ones. In the future, this detailed knowledge on the distinct PP anatomy in conjunction with their phylogenetic development might help to investigate specific network effects of psychiatric and other diseases, including distinct disease phenotypes that might find their expression in network hyper- or hypo-connectivity (Li et al., 2018). These strategies might allow far more focused and personalized interventions including non-invasive and invasive treatment strategies.
8. Disclaimer
This paper represents a largely conceptual view which is not (entirely) based on our own patients treated with DBS or SLS but on the evaluation of a normative cohort (HCP). While we invite other neuroscientists and clinicians to look for similar systematics in their patient cohorts (esp. for SLS and DBS), the imaging information is not intended for surgical approaches, stimulation planning or steering which should always be based on individual imaging.
CRediT authorship contribution statement
Volker A. Coenen: Conceptualization, Methodology, Visualization, Writing - original draft. Thomas E. Schlaepfer: . Bastian Sajonz: Writing - review & editing. Máté Döbrössy: Writing - review & editing. Christoph P. Kaller: Writing - review & editing. Horst Urbach: Writing - review & editing. Marco Reisert: Visualization, Investigation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Normative and probabilistic masks of the PPs / networks can be obtained from the authors upon reasonable request. We would like to thank Dr. Balint Varkuti (Cortics, Munich) for his critical input on network interactions especially in the planning phase of this paper. Dr. Coenen has received honoraria for talks from Boston Scientific, USA. He has received grants for clinical trials (IITs) from Boston Scientific, USA and Medtronic, USA. He receives an ongoing collaborative grant from BrainLab (Munich, Germany). Dr. Coenen is scientific advisor for Cortics (Munich) and Cortec (Freiburg). No specific external funding was received for this work which is based on departmental funds only.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102165.
Appendix. Supplementary materials
References
- Abler B., Greenhouse I., Ongur D., Walter H., Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alves-Pinto A., Rus O.G., Reess T.J., Wohlschläger A., Wagner G., Berberich G., Koch K. Altered reward-related effective connectivity in obsessive-compulsive disorder: an fMRI study. J. Psychiatry Neurosci. 2019;44:1–12. doi: 10.1503/jpn.180195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H., Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute A.M., Bijleveld B., Gillan C.M., Fineberg N.A., Sahakian B.J., Robbins T.W. Hyperconnectivity of the ventromedial prefrontal cortex in obsessive-compulsive disorder. Brain Neurosci. Adv. 2018;2 doi: 10.1177/2398212818808710. 239821281880871–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Behrens T.E., Smith S., Frank M.J., Poldrack R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Zu M., Chen Y., Xie W., Cai C., Wei Q., Ji G.-J., Tian Y., Wang K. Decreased Connection Between Reward Systems and Paralimbic Cortex in Depressive Patients. Front Neurosci. 2018;12:2087–2089. doi: 10.3389/fnins.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldermann J.C., Melzer C., Zapf A., Kohl S., Timmermann L., Tittgemeyer M., Huys D., Visser-Vandewalle V., Kühn A.A., Horn A., Kuhn J. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol. Psychiatry. 2019;85:735–743. doi: 10.1016/j.biopsych.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Ballanger B., van Eimeren T., Moro E., Lozano A.M., Hamani C., Boulinguez P., Pellecchia G., Houle S., Poon Y.Y., Lang A.E., Strafella A.P. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann. Neurol. 2009;66:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK E. The origin, course and termination of the prefronto-pontine tract in the human brain. Brain. 1950;73:368–391. doi: 10.1093/brain/73.3.368. [DOI] [PubMed] [Google Scholar]
- Benabid A.L., Koudsie A., Benazzouz A., Le Bas J.-F.O., Pollak P. Imaging of subthalamic nucleus and ventralis intermedius of the thalamus. Mov. Disord. 2002;17:S123–S129. doi: 10.1002/mds.10153. [DOI] [PubMed] [Google Scholar]
- Bergfeld I.O., Mantione M., Hoogendoorn M.L.C., Ruhé H.G., Notten P., van Laarhoven J., Visser I., Figee M., de Kwaasteniet B.P., Horst F., Schene A.H., van den Munckhof P., Beute G., Schuurman R., Denys D. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psych. 2016;73:456–464. doi: 10.1001/jamapsychiatry.2016.0152. [DOI] [PubMed] [Google Scholar]
- Bewernick B.H., Kayser S., Gippert S.M., Switala C., Coenen V.A., Schlaepfer T.E. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimulat. 2017;10:664–671. doi: 10.1016/j.brs.2017.01.581. [DOI] [PubMed] [Google Scholar]
- Blood A.J., Iosifescu D.V., Makris N., Perlis R.H., Kennedy D.N., Dougherty D.D., Kim B.W., Lee M.J., Wu S., Lee S., Calhoun J., Hodge S.M., Fava M., Rosen B.R., Smoller J.W., Gasic G.P., Breiter H.C., Phenotype Genotype Project on Addiction and Mood Disorders Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS ONE. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Doidge A.N., Keedwell P.A., Jones D.K. Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol. Med. 2014:1–10. doi: 10.1017/S0033291714001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Schnell S., Höfle O., Stegmayer K., Wiest R., Dierks T., Müller T.J., Walther S. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J. Affect. Disord. 2014;155:186–193. doi: 10.1016/j.jad.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Bracht T., Jones D.K., Müller T.J., Wiest R., Walther S. Limbic white matter microstructure plasticity reflects recovery from depression. J. Affect. Disorders. 2015;170:143–149. doi: 10.1016/j.jad.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Bracht T., Viher P.V., Stegmayer K., Strik W., Federspiel A., Wiest R., Walther S. Increased structural connectivity of the medial forebrain bundle in schizophrenia spectrum disorders is associated with delusions of paranoid threat and grandiosity. NeuroImage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes Bradley N, Warden Diane, H Trivedi Madhukar, Wisniewski Stephen R, Fava Maurizio, John Rush A. What did star*d teach us? results from a large-scale, practical, clinical trial for patients with depression. Psych. Serv. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- Buckner, R.L., Academy, J.A.H.O.T.N.Y., 2008, 2008. The brain's default network: anatomy, function, and relevance to disease. nslc.wustl.edu1124, 1–38. 10.1196/annals.1440.011. [DOI] [PubMed]
- Cao H., Chén O.Y., Chung Y., Forsyth J.K., McEwen S.C., Gee D.G., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Carrión R.E., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Thermenos H., Tsuang M.T., van Erp T.G.M., Walker E.F., Hamann S., Anticevic A., Woods S.W., Cannon T.D. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nature Commun. 2018;9(1):1–9. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi L., Alyagon U., Barnea-Ygael N., Zohar J., Dar R., Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimulat. 2017;11:158–165. doi: 10.1016/j.brs.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Choi E.A., McNally G.P. Paraventricular thalamus balances danger and reward. J. Neurosci. 2017;37:3018–3029. doi: 10.1523/JNEUROSCI.3320-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax B.J., Drash G.W., Staley J.K., Haber S., Mobley C.J., Miller G.W., Mufson E.J., Mash D.C., Levey A.I. Immunocytochemical localization of the dopamine transporter in human brain. J. Comp. Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Bewernick B.H., Kayser S., Kilian H., Boström J., Greschus S., Hurlemann R., Klein M.E., Spanier S., Sajonz B., Urbach H., Schlaepfer T.E. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;26:587. doi: 10.1038/s41386-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Honey C.R., Hurwitz T., Rahman A.A., Mcmaster J., Bürgel U., Mädler B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson's disease. Neurosurgery. 2009;64:1106–1114. doi: 10.1227/01.NEU.0000345631.54446.06. discussion 1114–5. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Panksepp J., Hurwitz T.A., Urbach H., Mädler B. Human medial forebrain bundle (mfb) and anterior thalamic radiation (atr): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J. Neuropsych. Clin. Neurosci. 2012;24:223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Sajonz B., Reisert M., Bostroem J., Bewernick B., Urbach H., Jenkner C., Reinacher P.C., Schlaepfer T.E., Mädler B. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. NeuroImage Clin. 2018;20:580–593. doi: 10.1016/j.nicl.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Sajonz B., Reisert M., Bostroem J., Bewernick B., Urbach H., Jenkner C., Reinacher P.C., Schlaepfer T.E., Mädler B. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. NeuroImage Clin. 2018;20:580–593. doi: 10.1016/j.nicl.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Goll P., Reinacher P.C., Voderholzer U., Tebartz van Elst L., Urbach H., Freyer T. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 2016;493:1–8. doi: 10.1017/S1092852916000286. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Maedler B., Panksepp J. Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neuroscience and Biobehavioral Reviews. 2011;35:1971–1981. doi: 10.1016/j.neubiorev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Coenen, V.A., Schlaepfer, T.E., Translational, B.B., 2019, 2019b. Frontal white matter architecture predicts efficacy of deep brain stimulation in major depression. nature.com 9, 1905. 10.1038/s41398-019-0540-4. [DOI] [PMC free article] [PubMed]
- Coenen V.A., Schumacher L.V., Kaller C., Schlaepfer T.E., Reinacher P.C., Egger K., Urbach H., Reisert M. The anatomy of the human medial forebrain bundle_ Ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. NeuroImage Clin. 2018;18:770–783. doi: 10.1016/j.nicl.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into Gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Kozink R.V., affective F.M.J.O. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Elsevier. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Liu H., Du W., Chao F., Zhang L., Wang K., Huang C., Gao Y., Tang Y. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psych. 2018;7:3. doi: 10.1038/s41398-017-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ewert S., Plettig P., Li N., Chakravarty M.M., Collins D.L., Herrington T.M., Kühn A.A., Horn A. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. NeuroImage. 2018;170:271–282. doi: 10.1016/j.neuroimage.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Fillard P., Descoteaux M., Goh A., Gouttard S., Jeurissen B., Malcolm J., Ramirez-Manzanares A., Reisert M., Sakaie K., Tensaouti F., Yo T., Mangin J.-F., Poupon C. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. NeuroImage. 2011;56:220–234. doi: 10.1016/j.neuroimage.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R., Oommen K., Osorio I., Nazzaro J., Labar D., Kaplitt M., Sperling M., Sandok E., Neal J., Handforth A., Stern J., Desalles A., Chung S., Shetter A., Bergen D., Bakay R., Henderson J., French J., Baltuch G., Rosenfeld W., Youkilis A., Marks W., Garcia P., Barbaro N., Fountain N., Bazil C., Goodman R., Mckhann G., Krishnamurthy Babu, K. Papavassiliou, S. Epstein, C. Pollard, J. Tonder, L. Grebin, J. Coffey, R. Graves, N. SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.J., Samanta J., Moustafa A.A., Sherman S.J. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frankle W.G., Laruelle M., Haber S.N. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Neurology I.S.O.C. Wiley Online Library; 1992. Topography and Collateralization of the Dopaminergic Projections to Motor and Lateral Prefrontal Cortex in Owl Monkeys. n.d. [DOI] [PubMed] [Google Scholar]
- Gildenberg P.L. Spiegel and Wycis – the early years. Stereotact Funct Neurosurg. 2002;77:11–16. doi: 10.1159/000064587. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., van Essen D.C., Jenkinson M., Consortium F.T.W.-M.H. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V., Mogri M., Thompson K.R., Henderson J.M., Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B.D., Malone D.A., Friehs G.M., Rezai A.R., Kubu C.S., Malloy P.F., Salloway S.P., Okun M.S., Goodman W.K., Rasmussen S.A. Three-Year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Greenberg B.D., Rauch S.L., Haber S.N. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, C.T., Neuroscience, N.C.N.R., 2012, 2012. The many paths to fear. nature.com13, 651–658. 10.1038/nrn3301. [DOI] [PubMed]
- Haynes W.I.A., Haber S.N. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J. Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp J.A., Coenen V.A., Rijntjes M., and K.E.B.S. Vol. 44. Springer; 2019. p. 125. (Ventral Tegmental Area Connections to Motor and Sensory Cortical Fields in Humans). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz T.A., Honey C.R., Allen J., Gosselin C., Hewko R., Martzke J., Bogod N., Taylor P. Bilateral anterior capsulotomy for intractable depression. J. Neuropsych. Clin. Neurosci. 2012;24:176–182. doi: 10.1176/appi.neuropsych.11080189. [DOI] [PubMed] [Google Scholar]
- Hurwitz T.A., Mandat T., Forster B., Honey C. Tract identification by novel MRI signal changes following stereotactic anterior capsulotomy. Stereotact. Funct. Neurosurg. 2006;84:228–235. doi: 10.1159/000096496. [DOI] [PubMed] [Google Scholar]
- Hurwitz T.A., Mandat T., Forster B., Honey C. Tract identification by novel MRI signal changes following stereotactic anterior capsulotomy. Stereotact Funct Neurosurg. 2006;84:228–235. doi: 10.1159/000096496. [DOI] [PubMed] [Google Scholar]
- Ilinsky I., Horn A., Paul-Gilloteaux P., Gressens P., Verney C., Kultas-Ilinsky K. Human motor thalamus reconstructed in 3d from continuous sagittal sections with identified subcortical afferent territories. eNeuro. 2018;5 doi: 10.1523/ENEURO.0060-18.2018. ENEURO.0060–18.2018–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez F., Nicolini H., Lozano A.M., Piedimonte F., Salín R., Velasco F. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80:S30.e17–S30.e25. doi: 10.1016/j.wneu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Gutman D., Behrens T., Matthews P., Rushworth M., Katz E., Lozano A., Mayberg H. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Baig M., Ramsey D., Lisanby S.H., Avery D., McDonald W.M., Li X., Bernhardt E.R., Haynor D.R., Holtzheimer P.E., Sackeim H.A., George M.S., Nahas Z. Prefrontal rTMS for treating depression: Location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimulation. 2013;6:108–117. doi: 10.1016/j.brs.2012.02.003. III. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M. Neuroanatomy: connectome connects fly and mammalian brain networks. Curr. Biol. 2015;25:R416–R418. doi: 10.1016/j.cub.2015.03.039. [DOI] [PubMed] [Google Scholar]
- Kang, G., 2014. Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson's disease: a simulation study1–12. 10.3389/fncom.2014.00032/abstract. [DOI] [PMC free article] [PubMed]
- Karas P.J., Lee S., Jimenez-Shahed J., Goodman W.K., Viswanathan A., Sheth S.A. Deep brain stimulation for obsessive compulsive disorder: evolution of surgical stimulation target parallels changing model of dysfunctional brain circuits. Front Neurosci. 2019:12. doi: 10.3389/fnins.2018.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H., O'Callaghan G., Vidal-Ribas P., Buzzell G.A., Brotman M.A., Leibenluft E., Pan P.M., Meffert L., Kaiser A., Wolke S., Pine D.S., Stringaris A. Reward processing in depression: a conceptual and meta-analytic review across FMRI and EEG studies. Am. J. Psychiatry Appi.AJP. 2018:1–10. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuken M.C., Uylings H.B.M., Geyer S., Schäfer A., Turner R., Forstmann B.U. Are there three subdivisions in the primate subthalamic nucleus? Front Neuroanat. 2012;6:14.. doi: 10.3389/fnana.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisely S., Li A., Warren N., Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anx. 2018;35:468–480. doi: 10.1002/da.22746. [DOI] [PubMed] [Google Scholar]
- Koch K., Reeß T.J., Rus O.G., Gürsel D.A., Wagner G., Berberich G., Zimmer C. Increased default mode network connectivity in obsessive–compulsive disorder during reward processing. Front Psych. 2018;9:625–629. doi: 10.3389/fpsyt.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell B.H., Halverson J., Butson C.R., Dickinson M., Bobholz J., Harsch H., Rainey C., Kondziolka D., Howland R., Eskandar E., Evans K.C., Dougherty D.D. Epidural cortical stimulation of the left dorsolateral prefrontal cortex for refractory major depressive disorder. Neurosurgery. 2011;69 doi: 10.1227/NEU.0b013e318229cfcd. 1015–29– discussion 1029. [DOI] [PubMed] [Google Scholar]
- Kuypers H., Research D.L.B. Elsevier; 1967. Cortical Projections to the Red Nucleus and the Brain Stem in the Rhesus Monkey. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion: clues from the brain. Ann. Rev. Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lehman J.F., Greenberg B.D., McIntyre C.C., Rasmussen S.A., Haber S.N. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J. Neurosci. 2011;31:10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.J., Friston K., Mody M., Wang H.N. A Brain Network Model for Depression: From Symptom Understanding to Disease Intervention. 2018;24:1004–1019. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand L.C., Caan M.W.A., Schuurman P.R., van den Munckhof P., Figee M., Denys D., van Wingen G.A. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimul. 2019;12:353–360. doi: 10.1016/j.brs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Liebrand, L.C., Natarajan, S.J., Caan, M.W.A., Schuurman, P.R., van den Munckhof, P., de Kwaasteniet, B., Luigjes, J., Bergfeld, I.O., Denys, D., van Wingen, G.A., 2019b. Distance to white matter trajectories is associated with treatment response to internal capsule deep brain stimulation in treatment-resistant depression. httpswww.biorxiv.org1–16. 10.1101/19008268. [DOI] [PMC free article] [PubMed]
- Loonen A.J.M., Ivanova S.A. Circuits Regulating Pleasure and Happiness: The Evolution of the Amygdalar-Hippocampal-Habenular Connectivity in Vertebrates. Front Neurosci. 2016;10:1213–1217. doi: 10.3389/fnins.2016.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L., Hendrickx S., Raymaekers S., ls L.G.E., Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psych. 2015;21:1272–1280. doi: 10.1038/mp.2015.124. [DOI] [PubMed] [Google Scholar]
- Makris N., Rathi Y., Mouradian P., Bonmassar G., Papadimitriou G., Ing W.I., Yeterian E.H., Kubicki M., Eskandar E.N., Wald L.L., Fan Q., Nummenmaa A., Widge A.S., Dougherty D.D. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD) Brain Imaging Behav. 2016:1–14. doi: 10.1007/s11682-015-9462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L., Polosan M., Jaafari N., Baup N., Welter M.-L., Fontaine D., Montcel du, S.T. Yelnik, J. Chéreau, I. Arbus, C. Raoul, S. Aouizerate, B. Damier, P. Chabardès, S. Czernecki, V. Ardouin, C. Krebs, M.-O. Bardinet, E. Chaynes, P. Burbaud, P. Cornu, P. Derost, P. Bougerol, T. Bataille, B. Mattei, V. Dormont, D. Devaux, B. Vérin, M. Houeto, J.-L. Pollak, P. Benabid, A.-L. Agid, Y. Krack, P. Millet, B. Pelissolo, A. STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl. J. Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- Malone D.A., Dougherty D.D., Rezai A.R., Carpenter L.L., Friehs G.M., Eskandar E.N., Rauch S.L., Rasmussen S.A., Machado A.G., Kubu C.S., Tyrka A.R., Price L.H., Stypulkowski P.H., Giftakis J.E., Rise M.T., Malloy P.F., Salloway S.P., Greenberg B.D. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psych. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S., Quirk, G. J., 2004, Neuronal signalling of fear memory. nature.com5, 844–852. 10.1038/nrn1535. [DOI] [PubMed]
- Mayberg H.S., Lozano A.M., Voon V., McNeely H.E., Seminowicz D., Hamani C., Schwalb J.M., Kennedy S.H. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McIntyre C.C., Mori S., Sherman D.L., Thakor N.V., Vitek J.L. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin. Neurophys. Offic. J. Int. Federat. Clinical neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Mega M.S., Cummings J.L. Frontal-subcortical circuits and neuropsychiatric disorders. J. Neuropsych. Clin. Neurosci. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- MEYER M. A study of efferent connexions of the frontal lobe in the human brain after leucotomy. Brain. 1949;72:265–296. doi: 10.1093/brain/72.3.265. 3 pl. [DOI] [PubMed] [Google Scholar]
- Milardi D., Cacciola A., Cutroneo G., Marino S., Neuroscience M.I. Vol. 626. Elsevier; 2016. pp. 68–73. (Red Nucleus Connectivity as Revealed by Constrained Spherical Deconvolution Tractography). [DOI] [PubMed] [Google Scholar]
- Miocinovic S., de Hemptinne C., Chen W., Isbaine F., Willie J.T., Ostrem J.L., Starr P.A. Cortical potentials evoked by subthalamic stimulation demonstrate a short latency hyperdirect pathway in humans. J. Neurosci. 2018;38:9129–9141. doi: 10.1523/JNEUROSCI.1327-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monakow, von K.H., Akert K. Springer; 1979. Projections of Precentral and Premotor Cortex to the Red Nucleus and Other Midbrain Areas in Macaca Fascicularis. n.d. [DOI] [PubMed] [Google Scholar]
- Motta, S.C., Carobrez, A.P., Biobehavioral, N.C.N., 2017, 2017.The Periaqueductal Gray and Primal Emotional Processing Critical to Influence Complex Defensive Responses, Fear Learning and Reward Seeking. Elsevier76, 39–47. 10.1016/j.neubiorev.2016.10.012. [DOI] [PubMed]
- Naesström M., psychiatry P.B.N.J.O. 2016. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Taylor Francis. 2016;70:483–491. doi: 10.3109/08039488.2016.1162846. [DOI] [PubMed] [Google Scholar]
- Nambu A., Takada M., Inase M., Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J. Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A., Takada M., Inase M., Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex. Soc Neurosci. 1996 doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A., Tokuno H., Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci. Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Nanda P., Banks G.P., Pathak Y.J., Sheth S.A. Connectivity-based parcellation of the anterior limb of the internal capsule. Hum Brain Mapp. 2017;38:6107–6117. doi: 10.1002/hbm.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Neubert F.-X., Mars R.B., Sallet J., Rushworth M.F.S. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. USA. 2015;112:E2695–E2704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R., Geeraedts L.M.G., Veening J.G. The medial forebrain bundle of the rat. I. General Introduction. J. Comparat. Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Voogd J., van Huijzen C. The Human Central Nervous System. A Synopsis and Atlas. Fourth Edition. Springer; Heidelberg: 2008. [Google Scholar]
- Nuttin B., Gielen F., van Kuyck K., Wu H., Luyten L., Welkenhuysen M., Brionne T.C., Gabriëls L. Targeting bed nucleus of the stria terminalis for severe obsessive-compulsive disorder: more unexpected lead placement in obsessive-compulsive disorder than in surgery for movement disorders. WNEU. 2013;80:S30.e11–S30.e16. doi: 10.1016/j.wneu.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Nioche C., Cabanis E.A., Habas C. Functional connectivity of the human red nucleus in the brain resting state at 3T. Am. Soc. Neuroradiol. 2009;30:396–403. doi: 10.3174/ajnr.A1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttin B.J., Gabriëls L.A., Cosyns P.R., Meyerson B.A., Andréewitch S., Sunaert S.G., Maes A.F., Dupont P.J., Gybels J.M., Gielen F., Demeulemeester H.G. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263–1274. doi: 10.1227/01.neu.0000064565.49299.9a. [DOI] [PubMed] [Google Scholar]
- Oishi K., Faria A., Jiang H., Li X., Akhter K., Zhang J., Hsu J.T., Miller M.I., van Zijl P.C.M., Albert M., Lyketsos C.G., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Mazziotta J., Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Carlsson K., Lundqvist D., Behavior M.I.P. Vol. 92. Elsevier; 2007. pp. 180–185. (On the unconscious Subcortical Origin of Human Fear). [DOI] [PubMed] [Google Scholar]
- Pallanti S., Grassi G. Can we modulate obsessive-compulsive networks with neuromodulation? J. Psychopharmacol. 2015:262–265. Oxford. [Google Scholar]
- Panksepp J. An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the seeking system. Neuropsychoanal. Interdiscipl. 2012 [Google Scholar]
- Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci. Biobehav. Rev. 2011:1–14. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Conscious Cogn. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Nelson E., Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward. evolutionary antecedents and neuropeptide intermediaries. Ann. N Y Acad. Sci. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Peters S.K., Dunlop K., Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst. Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.V., Mlakar J., Haber S.N., Parent M., Smith Y., Strick P.L., Griswold M.A., McIntyre C.C. Holographic reconstruction of axonal pathways in the human brain. Neuron. 2019:1–16. doi: 10.1016/j.neuron.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C., Kelmendi B., Bloch M., Krystal J.H., Coric V. Clinical treatment of obsessive compulsive disorder. Psychiatry (Edgmont) 2005;2:34–43. [PMC free article] [PubMed] [Google Scholar]
- Porrino L.J., Goldman-Rakic P.S. Wiley Online Library; 1982. Brainstem Innervation of Prefrontal and Anterior Cingulate Cortex in the Rhesus Monkey Revealed by Retrograde Transport of HRP. [DOI] [PubMed] [Google Scholar]
- Rasmussen S.A., Noren G., Greenberg B.D., Marsland R., McLaughlin N.C., Malloy P.J., Salloway S.P., Strong D.R., Eisen J.L., Jenike M.A., Rauch S.L., Baer L., Lindquist C. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol. Psych. 2018;84:355–364. doi: 10.1016/j.biopsych.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Rasmussen S.A., Noren G., Greenberg B.D., Marsland R., McLaughlin N.C., Malloy P.J., Salloway S.P., Strong D.R., Eisen J.L., Jenike M.A., Rauch S.L., Baer L., Lindquist C. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol. Psych. 2018;84:355–364. doi: 10.1016/j.biopsych.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Reisert M., Mader I., Anastasopoulos C., Weigel M., Schnell S., Kiselev V. Global fiber reconstruction becomes practical. NeuroImage. 2011;54:955–962. doi: 10.1016/j.neuroimage.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P., Choi K.S., Holtzheimer P.E., Crowell A.L., Garlow S.J., Rajendra J.K., McIntyre C.C., Gross R.E., Mayberg H.S. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psych. 2017;62:10.. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P., Choi K.S., Holtzheimer P.E., McIntyre C.C., Gross R.E., Chaturvedi A., Crowell A.L., Garlow S.J., Rajendra J.K., Mayberg H.S. Defining Critical White Matter Pathways Mediating Successful Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Biol Psych. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Daw N., Jepma M., Wimmer G.E., Wager T.D. Representation of aversive prediction errors in the human periaqueductal gray. Nat. Neurosci. 2014;17:1607–1612. doi: 10.1038/nn.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rück C., Karlsson A., Steele J.D., Edman G., Meyerson B.A., Ericson K., Nyman H., Asberg M., Svanborg P. Capsulotomy for Obsessive-Compulsive Disorder: Long-term Follow-up of 25 Patients. Arch. Gen. Psych. 2008;65:914–921. doi: 10.1001/archpsyc.65.8.914. [DOI] [PubMed] [Google Scholar]
- Safadi Z., Grisot G., Jbabdi S., Behrens T.E., Heilbronner S.R., McLaughlin N.C.R., Mandeville J., Versace A., Phillips M.L., Lehman J.F., Yendiki A., Haber S.N. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J. Neurosci. 2018;38:2106–2117. doi: 10.1523/JNEUROSCI.2335-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi Z., Grisot G., Jbabdi S., Behrens T.E., Heilbronner S.R., McLaughlin N.C.R., Mandeville J., Versace A., Phillips M.L., Lehman J.F., Yendiki A., Haber S.N. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J. Neurosci. 2018;38:2106–2117. doi: 10.1523/JNEUROSCI.2335-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi Z., Grisot G., Jbabdi S., Behrens T.E., Heilbronner S.R., McLaughlin N.C.R., Mandeville J., Versace A., Phillips M.L., Lehman J.F., Yendiki A., Haber S.N. Functional Segmentation of the Anterior Limb of the Internal Capsule: Linking White Matter Abnormalities to Specific Connections. J. Neurosci. 2018;38:2106–2117. doi: 10.1523/JNEUROSCI.2335-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B.F., de O., Gorgulho A., Saraiva C.W.C., Lopes A.C., Gomes J.G.R., Pássaro A.M., Hoexter M.Q., Miguel E.C., De Salles A.A.F. Understanding gamma ventral capsulotomy: Potential implications of diffusion tensor image tractography on target selectivity. Surg. Neurol. Int. 2019;10:136–137. doi: 10.25259/SNI-65-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikcioglu L. Johann Bernhard Aloys von Gudden: an outstanding scientist. J. Neurol. Neurosurg. Psychiatr. 2007;78:195. doi: 10.1136/jnnp.2006.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Bewernick B.H., Kayser S., Hurlemann R., Coenen V.A. Deep brain stimulation of the human reward system for major depression–rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39:1303–1314. doi: 10.1038/npp.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Bewernick B.H., Kayser S., Mädler B., Coenen V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psych. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Schmahmann J., Pandya D. Oxford University Press; New York: 2006. Fiber Pathways of the Brain. 1st ed. [Google Scholar]
- Schoene-Bake J.-C., Parpaley Y., Weber B., Panksepp J., Hurwitz T.A., Coenen V.A. Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology. 2010;35:2553–2563. doi: 10.1038/npp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher L.V., Reisert M., Nitschke K., Egger K., Urbach H., Hennig J., Weiller C., Kaller C.P. Probing the reproducibility of quantitative estimates of structural connectivity derived from global tractography. NeuroImage. 2018;175:215–229. doi: 10.1016/j.neuroimage.2018.01.086. [DOI] [PubMed] [Google Scholar]
- Settell M.L., Testini P., Cho S., Lee J.H., Blaha C.D., Jo H.J., Lee K.H., Min H.-K. Functional circuitry effect of ventral tegmental area deep brain stimulation: imaging and neurochemical evidence of mesocortical and mesolimbic pathway modulation. Front Neurosci. 2017;11 doi: 10.3389/fnins.2017.00104. 1676–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira N.A. Panic and fear induced by deep brain stimulation. J. Neurol. Neurosurg. Psychiatr. 2005;77:410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillery E., Bittar R.G., Robson M.D., Behrens T.E.J., Stein J.F., Aziz T.Z., Johansen-Berg H. Connectivity of the human periventricular-periaqueductal gray region. J. Neurosurg. 2005;103:1030–1034. doi: 10.3171/jns.2005.103.6.1030. [DOI] [PubMed] [Google Scholar]
- Slavich G.M., O donovan A., Epel E.S., Kemeny M.E. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel E., Wycis H., Marks M., Lee A. Stereotaxic apparatus for operations on the human Brain. Science. 1947;106:349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- Spiegel E.A., Wycis H.T., Freed H., Orchinik C. The central mechanism of the emotions: (experiences with circumscribed thalamic lesions) Am. Psych. Assoc. 1951;108:426–432. doi: 10.1176/ajp.108.6.426. [DOI] [PubMed] [Google Scholar]
- Sturm V., Lenartz D., Koulousakis A., Treuer H., Herholz K., Klein J.C., Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J. Chem. Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Taber K.H., Black D.N., Porrino L.J., Hurley R.A. Neuroanatomy of dopamine: reward and addiction. J. Neuropsych. Clin. Neurosci. 2012;24:1–4. doi: 10.1176/appi.neuropsych.24.1.1. [DOI] [PubMed] [Google Scholar]
- Tyagi H., Apergis-Schoute A.M., Akram H., Foltynie T., Limousin P., Drummond L.M., Fineberg N.A., Matthews K., Jahanshahi M., Robbins T.W., Sahakian B.J., Zrinzo L., Hariz M., Joyce E.M. A randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive-compulsive disorder: clinical and imaging evidence for dissociable effects. Biol. Psych. 2019;85:726–734. doi: 10.1016/j.biopsych.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Munckhof P., Bosch D.A., Mantione M.H.M., Figee M., Denys D.A.J.P., Schuurman P.R. Active stimulation site of nucleus accumbens deep brain stimulation in obsessive-compulsive disorder is localized in the ventral internal capsule. Acta Neurochir. Suppl. 2013;117:53–59. doi: 10.1007/978-3-7091-1482-7_9. [DOI] [PubMed] [Google Scholar]
- van Westen M., Rietveld E., Figee M., Denys D. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr. Behav. Neurosci. Rep. 2015;2:41–48. doi: 10.1007/s40473-015-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpini M., Giacobbe P., Cosgrove G.R., Levitt A., Lozano A.M., Lipsman N. The history and future of ablative neurosurgery for major depressive disorder. Stereotact. Funct. Neurosurg. 2017;95:216–228. doi: 10.1159/000478025. [DOI] [PubMed] [Google Scholar]
- Williams N.R., Short E.B., Hopkins T., Bentzley B.S., Sahlem G.L., Pannu J., Schmidt M., Borckardt J.J., Korte J.E., George M.S., Takacs I., Nahas Z. Five-year follow-up of bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Brain Stimulation. 2016;9:897–904. doi: 10.1016/j.brs.2016.06.054. [DOI] [PubMed] [Google Scholar]
- Zacharopoulos G., Lancaster T.M., Bracht T., Ihssen N., Maio G.R., Linden D.E.J. A hedonism hub in the human brain. Cereb. Cortex. 2016;26:3921–3927. doi: 10.1093/cercor/bhw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Ajilore O., Zhan L., GadElkarim J., Korthauer L., Yang S., Leow A., Kumar A. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology. 2013;38:1451–1459. doi: 10.1038/npp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.