Abstract
Dihydroquercetin (DHQ) is a flavonoid in the Chinese traditional herbal medicine Ramulus Euonymi, which has anti-inflammatory, antioxidant and anticancer bioactivity. In the present study, we investigated the protective effects of DHQ on acetaminophen (APAP)-induced liver injury in a mouse model for the first time. The mice received an intraperitoneal dose of APAP for model establishment. After 1 h, they were treated with DHQ at various concentrations. After 48 h of treatment, the mice were sacrificed to determine serum ALT and AST levels and the liver index, examine histopathological changes in the liver through H&E and TUNEL staining, and evaluate TNF-α and IL-6 levels using an ELISA. We also evaluated TNF-α, IL-6, Nrf2 and SOD2 mRNA expression by RT-PCR and Bcl-2, Bax and Pro-caspase-3 expression by Western blot. DHQ treatment significantly attenuated serum ALT and AST levels as well as rescued hepatomegaly. It also down-regulated TNF-α and IL-6, increased Nrf2 and SOD2 mRNA expression, down-regulated Bax, overexpressed Bcl-2 and Pro-caspase-3. Our datas suggest that DHQ treatment can effectively attenuate APAP-induced liver injury by down-regulating inflammatory factors, improving antioxidant capacity and inhibiting hepatocyte apoptosis. DHQ could be a beneficial hepatoprotective agent for the prevention and amelioration of APAP-induced acute liver injury.
Keywords: Dihydroquercetin (DHQ), acetaminophen, liver injury, inflammation, oxidative stress, apoptosis
Introduction
Acute liver injury (ALI) is a common and frequently occurring liver disease, which is the common pathological basis of various additional liver diseases. The causes of acute liver injury include viral infection, drugs, alcoholism, food poisoning and radiation damage [1-3]. APAP is one of the most commonly used antipyretic analgesics, which has a good safety record and offers positive effects at the treatment dose [4]. However, excessive or long-term use of APAP can lead to acute liver injury and even death [5]. APAP is recognized as the leading cause of acute liver failure in the United States and other European countries [6,7]. Generally, APAP is a cytochrome P450 enzyme 2E1 (Cyp2E1) that metabolizes the metabolites of free radical toxicity N-acetyl-p-benzoquinone imine (NAPQI). NAPQI and glutathione (GSH) can be combined to form toxic metabolites of cysteine. However, GSH is depleted, and the remaining DHPQI accumulates with large molecular proteins to form protein complexes that causing oxidative stress, triggering the mitochondrial pathway and leading to liver cell damage [8]. In addition, mitochondrial damage can inhibit respiration and decrease membrane potential and lead to mitochondrial dysfunction, oxidative stress, and inflammation. Previous reports have shown that the direct hepatotoxicity of APAP lies in the increase in oxidative stress and a decrease in the antioxidant defense system [9,10]. The liver inflammation and anti-inflammation cascade of the immune system are activated and further affect the process of liver injury [11,12]. Therefore, medical workers must pay attention to the hepatotoxicity of APAP and identify drugs that can prevent or cure acute liver injury.
The DHQ is the flavonoid bioactive constituent of the herb Ramulus Euonymi, and it has been implicated in a wide range of pharmacological activities. Different studies have shown that DHQ can inhibit oxidative enzymes and reduce the overproduction of ROS to ameliorate cerebral ischemia reperfusion injury [13,14]. It displays hepatoprotective, cardioprotective and neuroprotective properties [15]. DHQ has been shown to inhibite the myocardial apoptosis in mice peritoneal cells [16]. Furthermore, recent studies have reported that DHQ can inhibit autoimmune hepatitis [17]. However, the possible beneficial effects of DHQ on ALI have not been addressed until now. Therefore, the objective of our study was to investigate the potential anti-inflammatory, antioxidation and apoptosis inhibitory effects of DHQ and to find safe and effective drugs for the treatment of acute liver injury at the same time. Our results demonstrated that DHQ effectively ameliorated liver dysfunction and the histopathology of mice, showed protective effects against APAP-induced acute liver injury and supported for DHQ being a potential hepatoprotective agent.
Materials and methods
Chemicals
Acetaminophen (APAP, 99% purity) was purchased from Sigma-Aldrich (St. Louis, USA). APAP was dissolved in normal saline at a concentration of 20 mg/mL for intraperitoneal injection. GSH was provided by the Hospital Affiliated to Hubei Institute for Nationalities. GSH was dissolved in saline at a concentration of 65 mg/ml. DHQ (98% purity) was acquired from Shanghai source leaf Biotechnology Co., Ltd. (source leaf Biotechnology, Shanghai, China). DHQ was dissolved in sodium carboxymethylcellulose at concentrations of 0.2, 1, and 2 mg/mL. Detection ELISA kits for TNF-α and IL-6 were obtained from BD Bioscience (San Jose, USA). ALT and AST were obtained from Nanjing Jiancheng Biology Engineering Institute (Nanjing, Jiangsu, China). A First Strand cDNA Synthesis kit was provided by Thermo Scientific (Thermo Scientific, Waltham, USA). Horseradish peroxidase-conjugated goat anti-rabbit anti-mouse antibodies and the antibodies for Pro-caspase 3, Bcl-2, Bax, and β-actin were purchased from Cell Signaling (Danvers, USA). Other reagents used in the experiment were all of analytical grade.
Animals
Male C57BL/6 mice, 8-10 weeks old weighing approximately 18-20 g, were obtained from the Experimental Animal Center of the China Food and Drug Research Institute (License number: SCXK [Jing] 2012-0001). All animal experiments were performed according to the guidelines of the Animal Welfare Act and the Guide for Care and Use of Laboratory Animals from the National Institutes of Health. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Hubei Institute for Nationalities.
Experimental model and drug treatment
Sixty mice were randomly assigned to 6 groups (n=10/group), including a control group, APAP-induced acute liver injury model group, GSH group at the dose of 1 g/kg•d, DHQ group at low, moderate or high dosages (dose of 4, 20, or 40 mg/kg•d). All mice were subjected to adaptive feeding with regular feeds for one week, and then they were subjected to diet restriction for 12 h prior to the model establishment. Except for the control group, all mice received an intraperitoneal hepatotoxic injection of APAP (300 mg/kg) for the establishment of the acute liver injury model. The mice from the control group were subjected to equivalent saline. An hour later, the DHQ groups were gavage treated, and the GSH group was intraperitoneal treated at the designed dosages twice a day with an interval of 12 h for 48 h. The mice from the control group were subjected to equivalent saline. After 48 h, all groups were sacrificed after being anesthetized with 10% chloraldurate. Blood and liver samples were obtained for further analysis.
Liver index determination
The body weight of each mouse was measured before being sacrificed. Similarly, the wet mouse liver was measured after being sacrificed. The liver index = liver weight (g)/body weight (g) × 100%.
Serum analyses
Blood samples obtained from the mice was placed at room temperature to clot. After centrifugation at the speed of 3000 × g for 5 min at 4°C, the supernatant was collected in new tubes. Serum AST and ALT levels were determined using commercial kits (Jianchen, Nanjing, China) and a scientific a multiskan spectrum (Thermo Scientific, Waltham, USA).
Hematoxylin-Eosin (H&E) staining
The liver tissue from each mouse was fixed in 4% paraformaldehyde, dehydrated in graded ethanol and embedded in paraffin wax. The tissue was cut into sections and stained with hematoxylin, mounted, observed under a light microscope (Nikon Eclipse, E200, Japan) and photographed at 200 × magnification.
Enzyme-linked immunosorbent assay (ELISA)
Tests for TNF-α and IL-6 levels in hepatic tissue were measured with a BD ELISA Reagent kit (BD, USA). Samples were prepared in duplicate according to the manufacturer’s instructions.
Real-time polymerase chain reaction (RT-PCR) detection
Total RNA was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions, and then was reverse transcribed into cDNA with a cDNA-synthesis kit (Thermo Scientific, Waltham, USA). Real-time PCR was performed using 7300 system SDS Software (STRATAGENR, Germany) with SYBR green premix (TOYOBO, Japan) using β-actin as a reference. Sequence of primers shown in the Table 1.
Table 1.
Primer sequence for real time fluorescence quantitative PCR
| Genes (mouse) | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| TNF-α | ACG GCA TGG ATC TCA AAG AC | CGG ACT CCG CAA AGT CTA AG |
| IL-6 | CAT GTT CTC TGG GAA ATC GTG G | GTA CTC CAG GTA GCT ATG GTA C |
| TLR4 | CTT TGG CTA TGG GCT TCC AGT C | GCA AGG ACA GAG TTT ATC GTG |
| SOD2 | TCC CAG ACC TGC CTT ACG ACT AT | GGT GGC GTT GAG ATT GTT CA |
| Nrf-2 | TTG GCA GAG ACA TTC CCA T | GCT GCC ACC GTC ACT GGG |
| β-Actin | TAC CAC CAT GTA CCC AGG CA | CTC AGG AGG AGC AAT GAT CTT GAT |
Western blot assay
Approximately 100 mg of frozen liver tissue, after homogenizing, was lysed with RIPA buffer (Beyotime Institute of Biotechnology, Jiangsu, China) in the presence of PMSF (Beyotime Institute of Biotechnology, Jiangsu, China). After centrifuging, the collected supernatant was added with loading buffer and boiled at 95°C for 5 min. Sequentially, 20 µg of the sample were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membranes. After electrophoresis, the membrane was blocked with 5% skim milk at room temperature for 3 h. The targeted protein on the membrane was probed with primary antibodies such as Pro-caspase-3, Bax or Bcl-2 (Cell Signaling, USA), 1:1000) at 4°C overnight. After being washed with TBS-T buffer three times, the membrane was incubated with secondary antibodies, HRP-labeled with anti-rabbit IgG (Cell Signaling, 1:20000) for 2 h, and then washed with TBS-T buffer three times. Finally, the membrane was imaged with the assistance of an automatic developing machine. The β-actin was probed as an internal reference. The results were analyzed with the software Quantity One 4.6.1 (Bio-Rad Software Inc, California, USA).
Terminal-deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining
Hepatocytes were labeled in situ using a TUNEL apoptotic detection kit (Roche Diagnostics Gmbh, Mannheim, Germany) per the manufacturer’s instructions. The slides of paraffin-embedded liver slices were deparaffinized and rehydrated, and the slides were covered entirely with 3% H2O2 for 15 min at room temperature to inactivate the endogenous peroxidases. The slides were washed in PBS three times (10 min each) and covered with 10% Tirtox-100 for 8 min, followed by incubation with the TUNEL reaction mixture at 37°C in a humidified atmosphere in the dark for 1 h. A total of 50 μl converter-POD was added onto the tissues for a reaction at 37°C for 30 min. Then, the samples were spotted with DAB fluid and hematoxylin. Finally, a light microscope (Nikon Eclipse, Japan) was used for observation. A dark brown DAB signal indicated positive staining (apoptotic cells). At least 1000 cells (TUNEL-positive cells and TUNEL-negative cells) were counted in each of eight separate low-power fields for each sample, and the percentage of TUNEL-positive cells was calculated.
Statistical analyses
All statistical analyses were performed with the GraphPad Prism 6 software (GraphPad Software Inc., San Diego, USA) through Student’s t test (T Test) and Analysis of Variance (ANOVA). Values are expressed as the mean ± standard (M ± SD). The APAP-induced acute liver injury model group were compared with the control group, and the DHQ treatment groups were compared with the APAP-induced acute liver injury model group. The significant difference and very significant difference were considered at P < 0.05 and P < 0.01, respectively.
Results
Effects of DHQ on serum ALT and AST levels
Serum ALT and AST is regarded as a sensitive index of hepatocyte injury. ALT and AST is mainly distributed in the liver and released into the blood when the liver is damaged seriously. Therefore, we detected the levels of ALT and AST in serum as the monitoring index. ALT and AST in the control group were both at a low level. Compared with the control group, the levels of serum ALT and AST in the model group revealed a significant increase (P < 0.05 and P < 0.01, respectively). This indicated that the establishment of the APAP-induced acute liver injury model was successful. In contrast, serum levels of ALT and AST in the DHQ and GSH groups exhibited a significant reduction (P < 0.05 and P < 0.01). Therefore, DHQ treatment can decrease serum ALT and AST levels. The results are shown in Figure 1.
Figure 1.
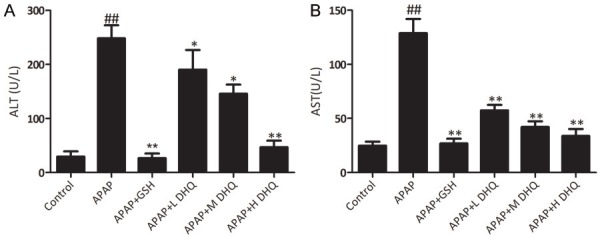
Effects of DHQ on serum ALT and AST levels in APAP-induced acute liver injury. DHQ rescued APAP-induced acute liver damage based on the examination of ALT (A) and AST (B) levels. All data are expressed as the Mean ± SD (n=8). ##P < 0.01 compared with the control group, *P < 0.05 and **P < 0.01 compared with the APAP-induced acute liver injury model group.
Effects of DHQ on histology
We measured the liver index to assess the degree of liver damage. The liver index in the APAP group revealed an obvious increase compared with the control group (P < 0.01), while the treatment with GSH and DHQ at various dosages exhibited a significant decrease (Figure 2A) (P < 0.01). Pathological change is also an important indicator of tissue damage. Therefore we detected the degree of pathological damage of liver tissue. As shown in Figure 2B, H&E staining showed that the liver cells of the normal control group were clear at 200 × magnification under a light microscope, the cell space was even and uniform, and there was no cell edema, degeneration or necrosis. In the APAP group, the arrangement of the liver cells was disordered, the hepatic lobules were not clear, and most of the necrotic hepatocytes were dissolved. While treatment with GSH and DHQ were significantly decreased, inflammatory cell infiltration and the animals showed fewer areas of necrosis. The results are shown in Figure 2.
Figure 2.
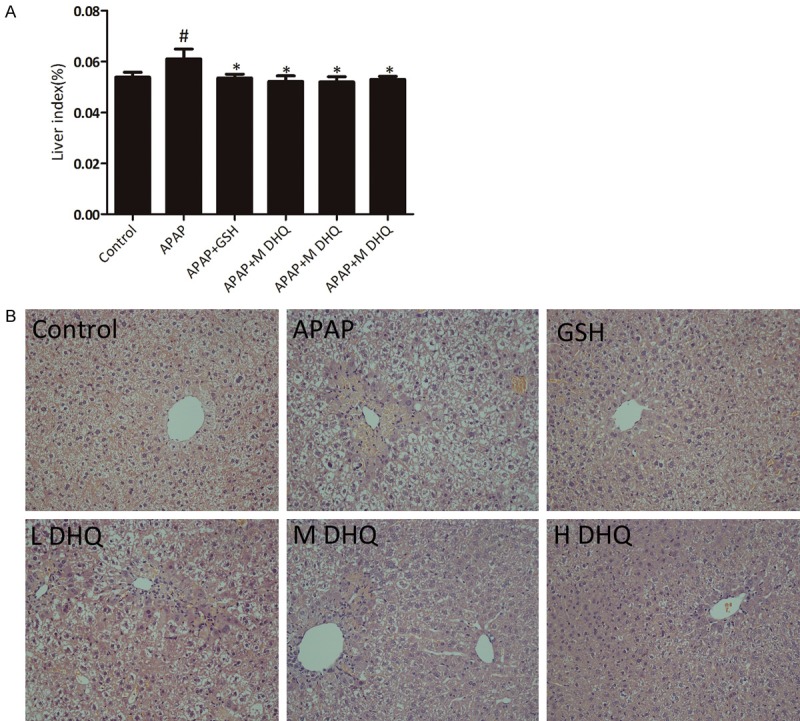
Effects of DHQ on histology. DHQ reduced the liver index (A) as well as alleviated APAP-induced cell necrosis through the evaluation by HE staining (B). All data were expressed as the Mean ± SD (n=8). Original magnification, 200 ×. #P < 0.05 compared with the control group, *P < 0.05 and **P < 0.01 compared with the APAP-induced acute liver injury model group.
Effects of DHQ on inflammatory reaction
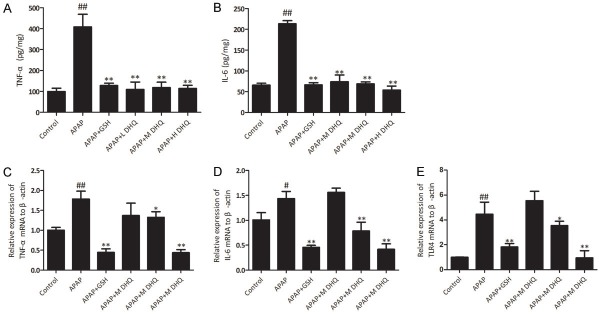
Cytokines such as TNF-α and IL-6 are involved in a variety of inflammatory processes, such as tissue damage and infection. APAP induced a severe inflammatory response [18]. We examined the hepatic TNF-α and IL-6 levels using an ELISA. There was a significant difference in hepatic TNF-α and IL-6 levels between the control and APAP groups (P < 0.01). After treatment with DHQ and GSH, the levels of TNF-α and IL-6 in the GSH and DHQ groups were obviously reduced (P < 0.01). Then, we examined the mRNA expression of TNF-α and IL-6 using RT-PCR. The expression of TNF-α and IL-6 in the GSH and DHQ groups revealed reduction. Toll-like receptors (TLRs) are essential mediators of innate and adaptive immunity in the defense against invading pathogens. TLR4 is a member of the TLR family and it plays a pivotal role in the initiation of the immune response after liver injury [19,20]. We examined TLR4 mRNA expression and the results shown that the expression of TLR4 was obviously increased in the APAP group (P < 0.01). Remarkably, high-dose DHQ treatment significantly reduced the elevated expression of TLR4 mRNA (P < 0.01). The results indicated that DHQ treatment can decrease TLR4 levels. Therefore, DHQ may have the effect of alleviating inflammation. These results are shown in Figure 3.
Figure 3.
Effects of DHQ on inflammatory reactions. DHQ reduced inflammatory responses from APAP-induced acute liver injury in mice through the detection of the levels of TNF-α (A) and IL-6 (B) in liver tissue by an ELISA and the determination of mRNA expression of TNF-α (C), IL-6 (D) and TLR4 (E) by RT-PCR. All data are expressed as the Mean ± SD (n=6) from three independent experiments. #P < 0.05 and ##P < 0.01 compared with the control group, *P < 0.05 and **P < 0.01 compared with the APAP-induced acute liver injury model group.
Effects of DHQ on antioxidant capacity
SOD2 and Nrf2 are important components of the antioxidant system in vivo [21,22]. SOD2 can resist reactive oxygen species produced by APAP toxicity in mitochondria [23]. We examined SOD2 and Nrf2 mRNA expressions to evaluate the antioxidant capacity. The results indicated that APAP-induce liver injury could result in decreases of SOD2 and Nrf2 mRNA expression and DHQ at high dosage treatment could increase SOD2 and Nrf2 mRNA expression (P < 0.05). Therefore, DHQ can improve the antioxidant capacity. The results are shown in Figure 4.
Figure 4.
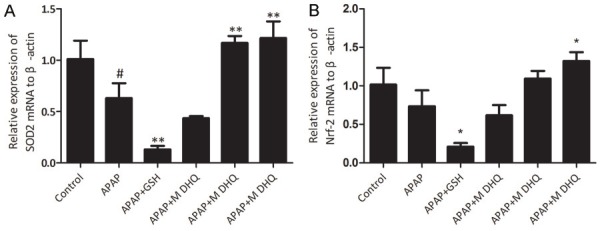
Effects of DHQ on antioxidant capacity. The determination of mRNA expression of SOD2 (A) and Nrf2 (B) by RT-PCR. All data are expressed as the Mean ± SD (n=6) from three independent experiments. #P < 0.05 compared with the control group, *P < 0.05 and **P < 0.01 compared with the APAP-induced acute liver injury model group.
Effects of DHQ on apoptosis of hepatic cells
Massive evidence has demonstrated acetaminophen induces the translocation of Bcl-2 family proteins [24], release of cytochrome C [25] and positive cell staining in a TUNEL assay [26]. APAP can induce caspase-dependent apoptosis in hepatic cells. To verify the effect of DHQ on APAP-induced cell death, we measured apoptosis-related proteins Pro-caspase-3, Bcl-2, and Bax expression. As shown in Figure 5A, compared with the normal control group, APAP-mediated liver injury revealed increased Bax and decreased Bcl-2 and Pro-caspase-3, while DHQ could down-regulate Bax and up-regulate Bcl-2 and Pro-caspase-3 at various doses. Similarly, in the control group, no dark brown granules were observed in the liver by TUNEL staining. The APAP treatment group showed a large number of dark brown particles and TUNEL-positive hepatic cells. In contrast, treatment with DHQ considerably decreased the amount of TUNEL-positive hepatic cells at various doses (Figure 5B). Therefore, DHQ can inhibit hepatic cell apoptosis.
Figure 5.
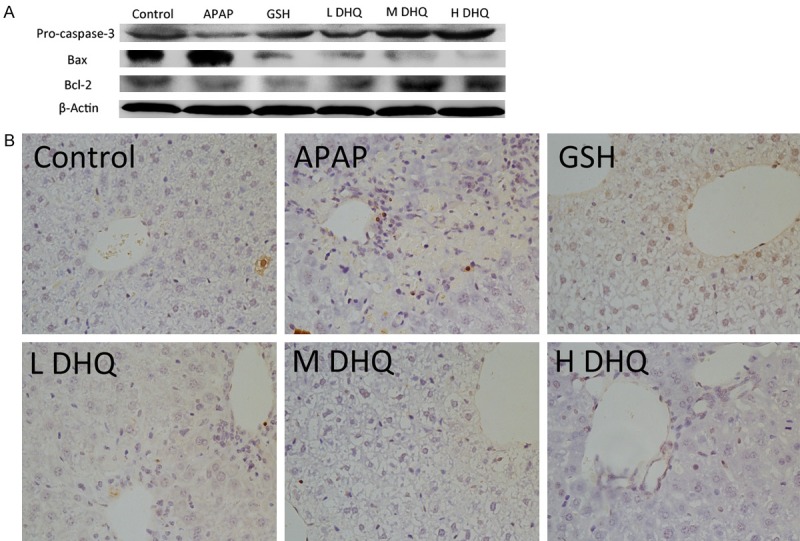
Effects of DHQ on apoptosis of hepatic cells. DHQ down-regulated the expression of apoptosis proteins in acute injury liver cells. DHQ executed hepatoprotection through inhibited apoptosis. DHQ could result in down-regulation of Bax and up-regulation of Pro-caspase-3 and Bcl-2 based on western blot (A) (n=6). Similarly, DHQ could obviously reduce liver cell apoptosis of mice induced by APAP based on TUNEL staining (B) (n=6).
Discussion
In this study, we investigated the protective effects of DHQ on APAP-induced liver injury in mice. Intraperitoneal injection of excessive APAP in mice can result in significant liver damage, which produces an increase in serum biochemical indicators and liver tissue pathological changes. After treatment with DHQ, the parameter indexes were obviously improved. We observed that after treatment with DHQ, TNF-α and IL-6 levels were inhibited, and the expression of SOD2 and Nrf2 mRNA was improved. There was also a decrease in the protein expression of Bax and an increase in the expression of Pro-caspase-3 and Bcl-2. These results show that DHQ had a protective effect on APAP-induced liver injury including inflammation, antioxidation, and cell apoptosis.
APAP metabolic activation and oxidative stress are the key pathological events of APAP hepatotoxicity. The mechanism for hepatic toxicity of APAP is related to mitochondrial damage and the generation of oxygen free radicals. SOD2 can rapidly eliminate the active oxygen produced by mitochondria and alleviate the oxidative stress as an antioxidant enzyme in mitochondria. The transcription factor nuclear factor erythroid 2 p45-related factor 2 (Nrf2) regulates the expression of a wide array of genes involved in GSH synthesis, the antioxidative system, and drug metabolism via binding to the antioxidant response element (ARE) for hepatoprotection [27]. There are several pieces of evidence to corroborate a protective role for DHQ against oxidative injury via the Nrf2/ARE pathway and Nrf2-mediated gene regulation [28]. After activation of Nrf2 in mice deficient for kelch-like ECH associated protein 1 (keap 1), an Nrf2 inhibitor protein, they were more resistant to toxic doses of APAP [29]. Conversely, in Nrf2-deficient mice, the higher susceptibility to APAP led to a greater severity of hepatic damage and increased lethality [30]. ROS can be eliminated by endogenous antioxidants, antioxidant enzymes and antioxidant defense systems, and this process is mainly dependent on Nrf2 signals [31,32]. The antioxidant defense transcription factor Nrf2/antioxidant responsive element (ARE) pathway determines cellular ROS levels [33]. There is evidence that Nrf2 is involved in the process of liver injury induced by APAP and that it controls the expression of a series of antioxidant genes [34]. In this study, DHQ can enhance the expression of Nrf-2 and SOD2, suggesting that the protective effects of DHQ on APAP-induced liver injury may be correlated with the ascending antioxidant capacity.
APAP mediates the release of a large number of DAMP molecules after hepatocyte necrosis, triggers the inflammatory response by activating the DMAPS receptor, which further amplifies APAP-induced liver injury [35,36]. Therefore, inhibiting the secretion of inflammatory cytokines can help improve the inflammatory reaction in the liver and block the vicious cycle of liver injury caused by APAP. In this study, DHQ can significantly reduce the abnormal increase of TNF-α and IL-6 in hepatic tissues, and it alleviated the secondary damage of the liver in sterile inflammation. TLR4 has been reported to be involved in APAP-induced liver injury and TLR4 signaling deficiency protection against APAP-induced hepatotoxicity in TLR4 mutant mice [37]. In this study, the expression of TLR4 mRNA in the APAP group was significantly increased. This indicated that TLR4 was involved in APAP-induced acute liver injury. This result was consistent with previous reports. Dihydroquercetin decreased NF-κB activation in CI/R-injured rats and inhibited NF-κB transcriptional activation in the transient transfected macrophage cell line with a plasmid containing a triple NF-κB binding site. At high doses, DHQ could inhibit TLR4 mRNA expression and reduced the expression of TNF-α and IL-6 mRNA. Therefore, we suggest that the TLR4 mediated TLR4/NF-κB pathway is involved in APAP-induced acute liver injury and may affect the inflammatory response in liver tissue. On the one hand, maybe DHQ acts by reducing the inflammatory factors and avoiding stimulation of overexpression, and inhibiting TLR4/NF-κB signaling pathway activation; on the other hand, it may directly inhibit the TLR4/NF-κB signaling pathway and downstream cytokines TNF-α and IL-6 and reduce liver damage through inhibiting inflammatory reaction. Therefore, inhibition of the inflammatory response may be one of the mechanisms by which DHQ protects against APAP-induced liver injury.
The mitochondrial apoptosis pathway is one of the major pathways of apoptosis. ROS changes the mitochondrial membrane permeability, leading to activation of caspases [38], resulting in intracellular antioxidant depletion (e.g., glutathione), and intracellular GSH depletion triggers apoptosis pathways [39]. A large body of evidence has suggested that APAP is excessive in apoptosis of hepatocytes. Hepatocyte apoptosis is a cascade reaction. Caspase-3 is involved in the apoptotic pathway, which is an important cell death pathway. Another important component of the death program is the Bcl-2 family [40]. Bcl-2 and Bax are two important members of the Bcl-2 family. Bcl-2 can prevent the occurrence of all early signs of apoptosis and Bax has a role in promoting apoptosis [41]. In this study, the expression of Bcl-2 and pro-caspase-3 increased, but the expression of Bax was inhibited, and the results of TUNEL detection showed that DHQ could significantly reduce the apoptosis of hepatocytes. Therefore, we suggest that DHQ can inhibit the apoptosis of hepatocytes, which may be one of the mechanisms of DHQ protection against APAP-induced liver injury.
In conclusion, DHQ showed protective effect on acute liver injury induced by APAP in the aspects of antioxidation, anti-inflammatory activity and inhibition of hepatocyte apoptosis. However, due to the mechanism of APAP-induced liver injury being complex, whether there are other regulatory mechanisms of DHQ require further study. These results suggest that DHQ may be a candidate for a hepatoprotective drug. However, further studies are needed to determine the exact protective mechanisms.
Acknowledgements
This investigation was supported by a grant from the National Natural Science Foundation of China (No. 81560675).
Disclosure of conflict of interest
None.
References
- 1.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Davis ML, Hashemi N. Acute liver failure as a rare initial manifestation of peripheral T-cell lymphoma. World J Hepatol. 2010;2:384–386. doi: 10.4254/wjh.v2.i10.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svobodova AR, Galandakova A, Sianska J, Dolezal D, Ulrichova J, Vostalova J. Acute exposure to solar simulated ultraviolet radiation affects oxidative stress-related biomarkers in skin, liver and blood of hairless mice. Biol Pharm Bull. 2011;34:471–479. doi: 10.1248/bpb.34.471. [DOI] [PubMed] [Google Scholar]
- 4.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 5.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blieden M, Paramore LC, Shah D, Ben-Joseph R. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev Clin Pharmacol. 2014;7:341–348. doi: 10.1586/17512433.2014.904744. [DOI] [PubMed] [Google Scholar]
- 7.Twisk F MSJ. FDA asks physicians to stop prescribing high-dose acetaminophen products. J Rehabil Res Dev. 2013;50:vii–viii. [Google Scholar]
- 8.Jaeschke H, Xie Y, McGill MR. Acetaminophen-induced liver injury: from animal models to humans. J Clin Transl Hepatol. 2014;2:153–161. doi: 10.14218/JCTH.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, Sultana S. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208:149–161. doi: 10.1016/j.toxlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Tai M, Zhang J, Song S, Miao R, Liu S, Pang Q, Wu Q, Liu C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int Immunopharmacol. 2015;27:164–170. doi: 10.1016/j.intimp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr. 2014;3:331–343. doi: 10.3978/j.issn.2304-3881.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 13.Vladimirov YA, Proskurnina EV, Demin EM, Matveeva NS, Lubitskiy OB, Novikov AA, Izmailov DY, Osipov AN, Tikhonov VP, Kagan VE. Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis. Biochemistry (Moscow) 2009;74:301–307. doi: 10.1134/s0006297909030092. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Wang WY, Chang CC, Liou KT, Sung YJ, Liao JF, Chen CF, Chang S, Hou YC, Chou YC, Shen YC. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of NFkappa B activation. J Biomed Sci. 2006;13:127–141. doi: 10.1007/s11373-005-9031-0. [DOI] [PubMed] [Google Scholar]
- 15.Schauss AG, Tselyico SS, Kuznetsova VA, Yegorova I. Toxicological and genotoxicity assessment of a dihydroquercetin-rich dahurian larch tree (Larix gmelinii Rupr) extract (Lavitol) Int J Toxicol. 2015;34:162–181. doi: 10.1177/1091581815576975. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, Yang LJ, Sun XB. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Chen J, Zhu P, Fujino M, Takahara T, Toyama S, Tomita A, Zhao L, Yang Z, Hei M, Zhong L, Zhuang J, Kimura S, Li XK. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int Immunopharmacol. 2015;28:938–944. doi: 10.1016/j.intimp.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 19.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–244. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res. 2011;35:782–786. doi: 10.1111/j.1530-0277.2010.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palliyaguru DL, Chartoumpekis DV, Wakabayashi N, Skoko JJ, Yagishita Y, Singh SV, Kensler TW. Withaferin A induces Nrf2-dependent protection against liver injury: role of keap1-independent mechanisms. Free Radic Biol Med. 2016;101:116–128. doi: 10.1016/j.freeradbiomed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Zhang S, Cheng H, Lv H, Cheng G, Ci X. Nrf2-mediated liver protection by esculentoside a against acetaminophen toxicity through the AMPK/Akt/GSK3beta pathway. Free Radic Biol Med. 2016;101:401–412. doi: 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60:907–915. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- 25.El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GEN. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury. Toxicol Appl Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- 26.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 28.Liang L, Gao C, Luo M, Wang W, Zhao C, Zu Y, Efferth T, Fu Y. Dihydroquercetin (DHQ) induced HO-1 and NQO1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J Agric Food Chem. 2013;61:2755–2761. doi: 10.1021/jf304768p. [DOI] [PubMed] [Google Scholar]
- 29.Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD. Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci. 2009;109:31–40. doi: 10.1093/toxsci/kfp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, Kitteringham NR, Park BK. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 32.Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR. Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Jin F, Wan C, Li W, Yao L, Zhao H, Zou Y, Peng D, Huang W. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PLoS One. 2017;12:e0170900. doi: 10.1371/journal.pone.0170900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald B, Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology. 2016;151:1087–1095. doi: 10.1053/j.gastro.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 36.Mehendale HM. Once initiated, how does toxic tissue injury expand? Trends Pharmacol Sci. 2012;33:200–206. doi: 10.1016/j.tips.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Yohe HC, O’Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, Bement WJ, Szakacs JG, Wrighton SA, Jacobs JM, Kostrubsky V, Sinclair PR, Sinclair JF. Involvement of toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 38.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 39.Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology. 1995;21:1725–1741. doi: 10.1002/hep.1840210635. [DOI] [PubMed] [Google Scholar]
- 41.Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]