Abstract
Patients with hepatocellular carcinoma (HCC) have a poor survival rate because of its high invasion ability. Therefore, it is necessary to elucidate the mechanisms of HCC migration and invasion. Our previous study showed that follistatin-like 5 (FSTL5), which was associated with the prognosis of HCC patients, acts as an inhibitor of HCC cell proliferation. It also promotes the transition of cell morphology from mesenchymal to epithelial, which is associated with the process of mesenchymal-to-epithelial transition. In this study, we used two HCC cell lines (SK-Hep1 and SMMC-7721) to explore the effect of FSTL5 on HCC invasion and migration. We found that up-regulated FSTL5 restrained HCC invasion and migration by transwell, wound healing, detachment, and attachment assays. Decreased expression of YAP was found upon over-expression of FSTL5, as well as inhibition of the Wnt/β-catenin signaling pathway. YAP is a downstream gene of the Wnt/β-catenin signaling pathway and plays an important role in HCC metastasis. Thus, we speculate that FSTL5 inhibits the invasion of HCC through the Wnt/β-catenin/YAP pathway. In conclusion, FSTL5 exerts an inhibitory effect on HCC metastasis and proliferation through the Wnt/β-catenin/YAP pathway and may be a target gene for anti-tumor therapy.
Keywords: Hepatocellular carcinoma, follistatin-like 5, metastasis, wnt/β-catenin, epithelial to mesenchymal transition
Introduction
In general, hepatocellular carcinoma (HCC) is considered as one of the most common malignancies and accounts for 90% of primary liver cancers [1]. In clinical practice, a large number of patients cannot undergo curative surgery because of metastasis, resulting in survival times of only 3-6 months [2]. Therefore, it is necessary to explore the underlying mechanisms of metastasis in HCC to achieve a better treatment outcome for HCC patients.
As a member of the follistatin family, follistatin-like 5 (FSTL5) is a protein that binds directly to activins. FSTL5 is an activin antagonist that inhibits the secretion of follicle-stimulating hormone. It regulates cell differentiation and plays an important role in embryogenesis. Follistatin is a single glycosylated polypeptide chain of approximately 37 kDa and is not a member of the inhibin family. Very few studies have shown that FSTL5 is a tumor suppressor gene in tumor cells, except our own [3]. With a low expression level in HCC, FSTL5 has a suppressive role in the proliferation of HCC cells. To further explore the function of FSTL5 in the invasion of HCC cells and its mechanism, we undertook this study.
Materials and methods
Cell culture
HCC cell lines SK-Hep1 and HepG2 were purchased from the American Type Culture Collection (Portland, Oregon). SMMC-7721 cells were purchased from the Cell Bank of the Chinese Academy of Sciences. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C with 5% CO2 in a humidified incubator.
Quantitative real-time PCR (qRT-PCR)
Trizol reagent (Takara, Dalian, China) was used for total RNA extraction. The Prime Script RT-PCR kit (Takara) was used for reverse transcription, according to the manu facturer’s instructions. qRT-PCR analyses were performed with SYBR Premix ExTaq (Takara) on a VIIA7 (Applied Biosystems Inc, USA). The primers in this study were as follows: GAPDH, forward, 5’-GGAGCGAGATCCCTCCAAAAT-3’, reverse, 5’-GGCTGTTGTCATACTTCTCATGG-3’; FSTL5, forward, 5’-TGAAGTGCACAGAGCTGCTT-3’, reverse, 5’-AGCATATTTTTCATCTTGCTGTATTC-3’; YAP, forward, 5’-ACCCACAGCTCAGCATCTTCG-3’, reverse, 5’-TGGCTTGTTCCCATCCATCAG-3’. The relative expression of FSTL5 was analyzed by the comparative cycle threshold method, which was normalized to GAPDH mRNA levels.
Western blotting
Total protein was extracted using RIPA lysis buffer (P0013B, Beyotime, China) following the manufacturer’s protocol, and 30-50 µg of proteins were separated by reduced SDS polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Then, the membrane was blocked in TBS buffer containing 5% bovine serum albumin (Sangon, China) for 1 h. The membrane was then incubated overnight with primary antibodies against FSTL5 (1:1000, Proteintech, USA), YAP (1:1000, Signaling Technology, USA), or GAPDH (1:5000, Cell Signaling Technology), followed by a horseradish peroxidase (HRP)-linked secondary antibody (Cell Signaling). An ImmobilonTM Western Chemiluminescent HRP Substrate kit (Millipore, Germany) was used for detection.
Over-expression of FSTL5 in HCC cell lines
The expression vector containing the open reading frame of FSTL5 was purchased from Genecopoeia (Guangzhou, China). SK-Hep1 cells (5×105/well) were seeded in a 6-well plate and transfected with 2 µg of the over-expression vector using Lipofectamine Reagent (Invitrogen, USA). After 48 h of incubation, stably transfected cells were selected by treatment with 2 µg/ml puromycin in DMEM for 2 weeks. Puromycin-resistant colonies were isolated by the limited dilution approach. They were expanded and then maintained in regular growth medium containing 2 µg/ml puromycin [3].
Recombinant human FSTL5 treatment
SMMC-7721 cells (4×103) were seeded in a 96-well plate well and cultured in 90 µl and 1 ml conditioned DMEM medium with 5% FBS (v/v). Cells were treated without or with 50 nM recombinant human FSTL5 (rFSTL5) (Proteintech) to analyze cell migration and invasion abilities.
Wound healing assay
HCC cells were seeded in a 12-well plate. At 85%-90% confluence, a scratch wound was made on the surface of the plate with a pipette tip. Cells were cultured further in serum-free medium. Images were obtained at 0 and 24 h by microscopy.
Cell attachment and detachment assays
For the attachment assay, HCC cells were seeded in 12-well plates at 1×105 cells per well. Unattached HCC cells were washed out after 1 h, and attached cells were collected by trypsinization and counted under a microscope. The results are shown as a percentage of the attached cells compared with total cells. For the detachment assay, the cells were cultured for 24 h and then treated with 0.05% trypsin for 3 min. Then, trypsinization was stopped by adding FBS, and detached cells were collected and counted under a microscope. The attached cells were treated with 0.25% trypsin and counted. These data are presented as a percentage of the detached cells to the total cells [4].
Transwell invasion assays
Twenty-four-well Transwell chambers (Corning) with a gelatin-coated polycarbonate membrane filter were used for invasion assays. Matrigel (BD Biosciences) was applied to the upper chamber, and then HCC cells were seeded in serum-free medium while growth medium was added to the bottom chamber. After incubation for 24 h, medium in the upper chamber was discarded and the cells were scrapped off with a cotton swab. The invaded HCC cells were fixed with 4% paraformaldehyde for 20 min and then stained with a Giemsa solution for 15 min. The invaded cells were photographed and counted under a microscope in 10 randomly selected fields.
Statistical analysis
Data are shown as means ± standard deviation and comparisons between groups were made by Student’s t-test. A value of P<0.05 was considered as statistically significant.
Result
Migration activities of HCC cell lines and expression of FSTL5
To compare migration activities, we conducted transwell assays using three HCC cell lines (HepG2, SMMC-7721, and SK-Hep1). SK-Hep1 and SMMC-7721 cell lines had much higher migration compared with HepG2 cells in transwell assays (Figure 1A and 1B). However, the expression of FSTL5 in SK-Hep1 and SMMC-7721 cells was much lower than that in HepG2 cells, although they all have low expression of FSTL5 as reported previously [3]. Therefore, we used SK-Hep1 and SMMC-7721 cells for the following assays.
Figure 1.
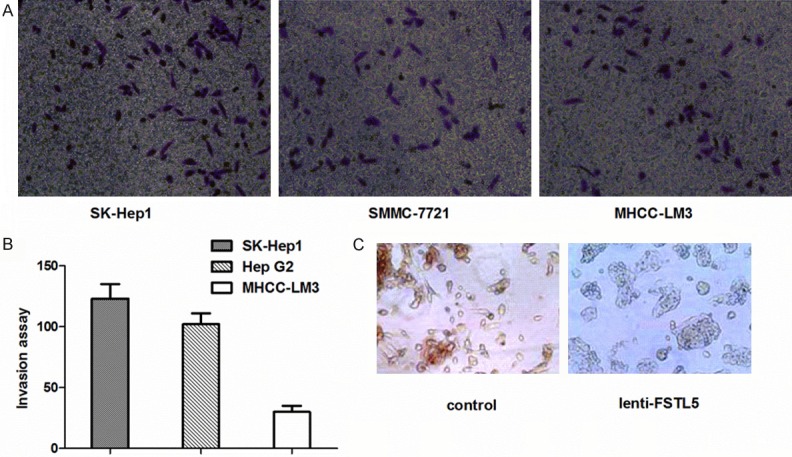
Invasion ability and morphological changes of HCC cells. A. Transwell assays showed the invasion ability of various HCC cell lines. B. Migration of HCC cells. Cells were counted at ×100 magnification in more than 10 microscopic fields. C. Cell morphology changes were observed by microscopy in SK-Hep1 cells of lenti-FSTL5 and control groups (×200).
We used an expression vector containing the open reading frame of FSTL5 to establish HCC cell lines over-expressing FSTL5 [3]. After over-expression of FSTL5, the morphology of SK-Hep1 had changed compared with the group control. As depicted in Figure 1C, SK-Hep1 cells in the lenti-FSTL5 group gained cell polarity and showed decreased formation of pseudopodia, leading to an epithelioid appearance. In contrast, the control group displayed an elongated, irregular fibroblastoid morphology.
Up-regulated FSTL5 enhances the invasion and migration activities of HCC cells
The morphology of cancer cells changes from epithelioid to fibroblastoid, indicating increased activities of invasion and migration. This change prompted us to explore the function of FSTL5 in HCC cell metastasis by assays to examine the invasion and migration abilities of HCC cells.
As depicted in Figure 2A, the transwell invasion assay showed that the lenti-FSTL5 group had much more cell migration compared with the control group. The same result was found in the rFSTL5 group (Figure 2B). The wound healing assay showed that cells of the control group covered the scratched area after 24 h of culture, but the lenti-FSTL5 group did not (Figure 2C). In addition, the rFSTL5 group showed that FSTL5 had the same function in HCC cells as shown in Figure 2D. These results indicate that up-regulated FSTL5 expression in HCC cells inhibits cell invasion and migration abilities.
Figure 2.
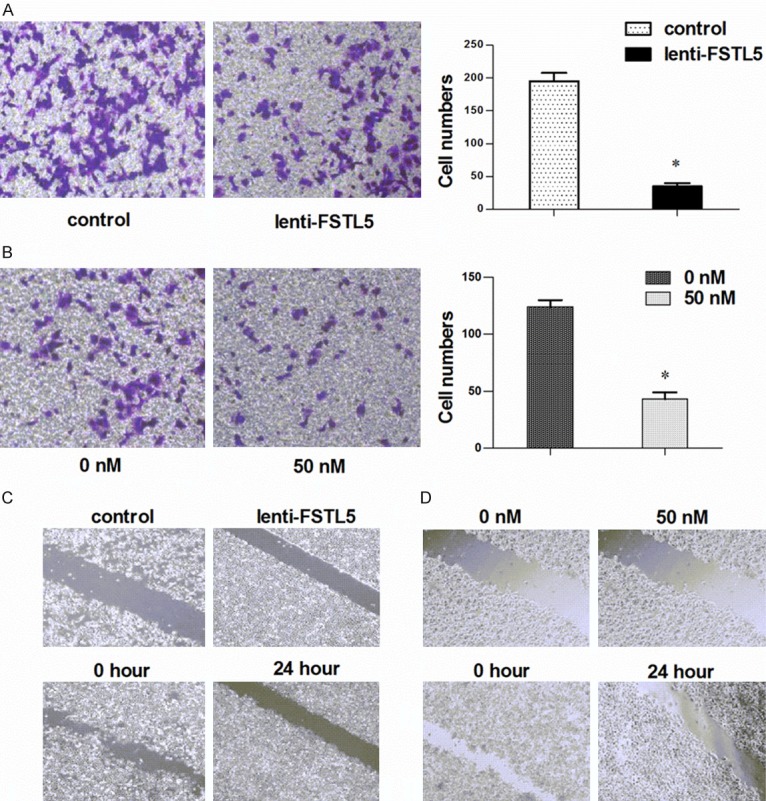
Effect of FSTL5 on the invasion and migration activities of HCC cells. A. Transwell assays showed that up-regulated FSTL5 inhibited HCC invasion in the lenti-FSTL5 group. B. Transwell assays showed the same results in the rFSTL5 group. C. Wound healing assays showed inhibition of FSTL5 in the lenti-FSTL5 group. D. The same results were obtained in the rFSTL5 group by transwell assays. *P<0.05 (with the statistical analysis method of Student’s t-test).
Stable FSTL5 over-expression in HCC cell lines lowers their detachment and attachment abilities
Tumor cell detachment from the matrix is a pivotal event in cancer metastasis and often results in tumor recurrence. It is a hallmark of the tumor metastatic process when tumor cells attach to other organs. To confirm the function of FSTL5 in HCC migration, we carried out detachment and attachment assays. We found that a low number of cells in the rFSTL5 group had detached from the plate compared with the control group in the SMMC-7721 cell line. In addition, the number of cells attached to the plate in the rFSTL5 group was less than that in the control group (Figure 3A). The same result was obtained in the lenti-FSTL5 group as shown in Figure 3B.
Figure 3.
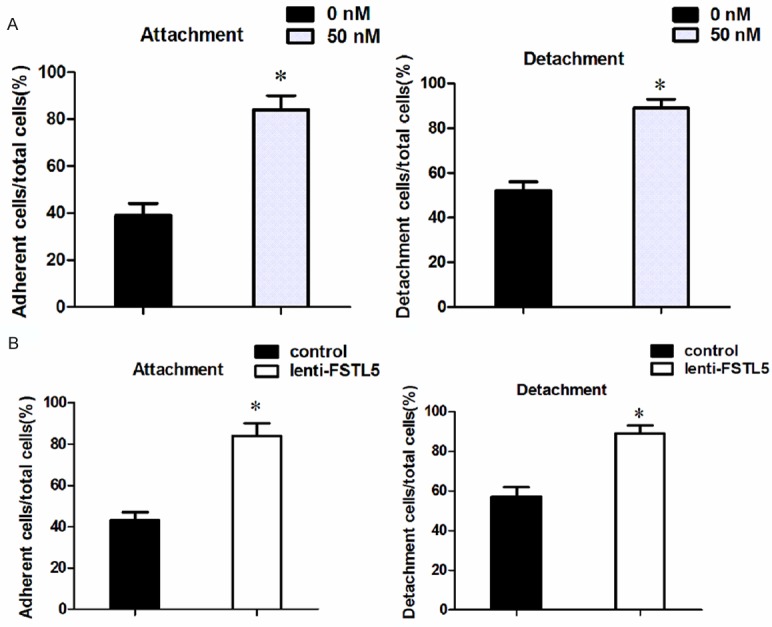
Detachment and attachment abilities of HCC cells. A. Both attachment and detachment assays showed that SMMC-7721 cells of the rFSTL5 group had a low adhesive capacity. B. The same result was obtained in the lenti-FSTL5 group. *P<0.05 (with the statistical analysis method of Student’s t-test).
FSTL5 inhibits the expression of YAP in HCC cell lines
To further explore whether the molecular changes induced by over-expression of FSTL5 in HCC cells were consistent with their metastasis, we detected YAP expression by qRT-PCR and western blot analyses. We found that the metastasis-related gene YAP was significantly reduced in lenti-FSTL5 and rFSTL5 groups at both gene and protein levels (Figure 4A-D). Expression of other genes, such as matrix metalloproteinase (MMP) 1, MMP2, MMP3, and MMP9, showed no significant change in PCR assays (Figure 4E).
Figure 4.
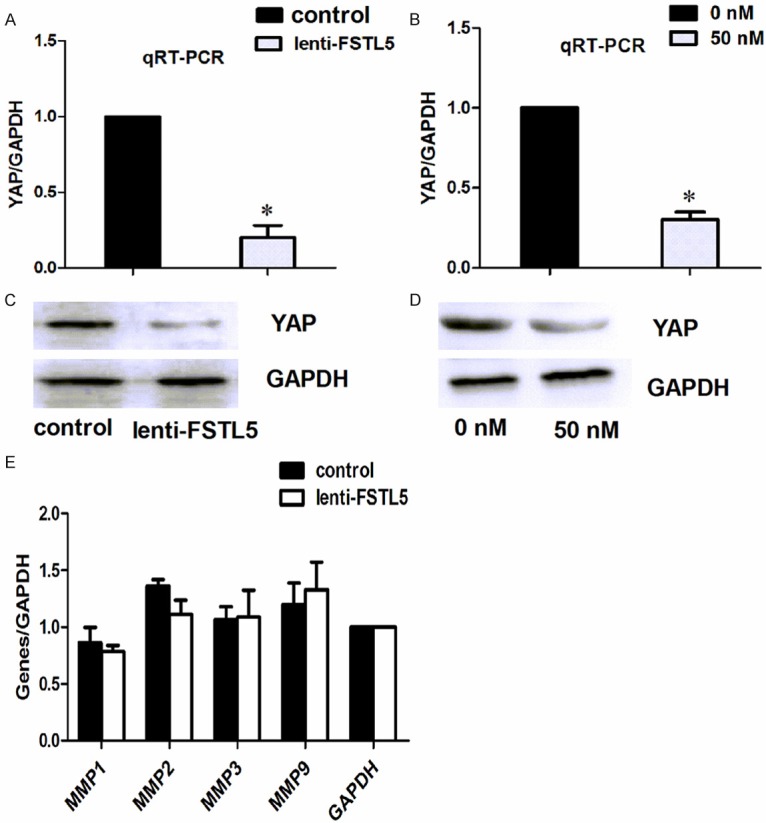
Influence of FSTL5 on the expression of YAP and MMPs. (A) qRT-PCR and western blot assays (C) showed inhibition of YAP in the lenti-FSTL5 group. The same results were obtained in the rFSTL5 group (B, D). (E) qRT-PCR assays showed no changes in the expression of MMPs in the lenti-FSTL5 group. *P<0.05 (with the statistical analysis method of Student’s t-test).
Discussion
HCC is one of the most malignant tumors with characteristics of high invasion and chemoresistance, resulting in a poor prognosis for patients [2,5]. In the clinic, a large number of patients have a metastatic neoplasm in the liver, lungs, or other tissues when first referred to hospital, leading to a high proportion of unresectable tumors in patients with 3-5 months of survival. Therefore, it is necessary and urgent to explore the mechanism of HCC metastasis.
The FSTL family includes FSTL1-5 that all have similar functions in humans. A previous study showed that FSTL1 plays an inhibitory role in nasopharyngeal carcinoma cells in terms of proliferation and invasion activities [6,7]. FSTL1 has been detected in ovarian cancer, endometrial carcinoma, as well as clear cell and renal cell carcinomas, and functions as a potential tumor metastasis-associated gene [8-10] with important roles in inflammatory diseases [11]. The high expression of FSTL1 in HCC is associated with its epithelial-to-mesenchymal transition (EMT) [12]. FSTL3 shows low expression in HCC and functions as a suppressive gene through binding to transforming growth factor-β family genes [13]. Whether FSTL5 has the same characteristic in tumor cells is unclear. Studies have revealed high expression of FSTL5 in adult rat brain, ventricles, neurons, and spinal cord, which is attributed to development of olfactory and nervous systems [14,15]. In addition, FSTL5 may function as a marker of poor prognosis in non-WNT/Non-SHH medulloblastoma [16,17] and plays an inhibitory role through extracellular matrix metabolism in bone marrow suppression [18].
By exploring the function of FSTL5 in HCC, we detected low expression of FSTL5 in HCC tissues compared with adjacent tissues. Moreover, through up-regulated FSTL5 expression, we found suppression of HCC cell proliferation [3].
In terms of metastasis of tumors, especially malignant tumors, studies of FSTL5 are limited. We found that over-expression of FSTL5 inhibits the invasion and migration activities of HCC. FSTL5 is structurally related to genes related to the extracellular matrix, such as MMP1 and MMP2, and the MMP inhibitor TIMP1 [19]. Therefore, we detected the expression of MMPs by qRT-PCR in HCC upon over-expression of FSTL5, but no significant changes were found. Down-regulated FSTL5 activates expression of YAP and contributes to reduced sensitivity to XPO1 inhibitors in KRAS-mutant lung cancer cells [20]. Expression of YAP is high in HCC and low in paracancerous tissue [21], which is inversely proportional compared with FSTL5 in HCC. YAP also has a critical role in HCC proliferation and chemosensitivity, and decreased expression of YAP correlates with inhibition of EMT in both HCC and mammary epithelial cells [22-24].
We evaluated the expression of YAP upon up-regulation of FSTL5 in HCC. Reduced expression of YAP was found at both gene and protein levels, indicating that FSTL5 attenuates the expression of YAP in HCC (Figure 3B). In general, Wnt plays a pivotal role in destroying the structure of the complex comprising Axin, APC, and GSK3, resulting in accumulation of β-catenin in the nucleus, which regulates the expression of other downstream genes. YAP and TAZ work as partners in tumor cells, which have the same mechanism as β-catenin regulated by Wnt [25-27].
Together with the results in our previous study, up-regulated FSTL5 inhibits the expression of β-catenin and GSK3β, indicating suppression of the Wnt/β-catenin signaling pathway. We speculate that FSTL5 plays a suppressive role in HCC migration and invasion by inhibiting YAP through the Wnt/β-catenin Signaling pathway.
The morphological changes in HCC upon over-expression of FSTL5 led us to speculate that FSTL5 may also function as an inhibitor of EMT. As shown Figure 1C, the lenti-FSTL5 group gained cell polarity and decreased formation of pseudopodia, leading to an epithelioid appearance. In contrast, the control group displayed an elongated, irregular fibroblastoid morphology. It is accepted that HCC acquires much more invasion and proliferation abilities upon EMT and vice versa [28]. Our finding is associated with this conclusion, which leads us to consider a relationship between FSTL5 and EMT in HCC. The detailed mechanism should be explored.
In conclusion, as a secretory glycoprotein, FSTL5 has low expression in HCC cells and plays a tumor-suppressive role during HCC proliferation and invasion, as well as EMT. Thus, it may become a target gene for HCC treatment. Because of its suppression role, FSTL5 may be used in therapies such as endovascular embolotherapy therapy for liver cancer patients. Further study of FSTL5 as well as the detailed mechanism of EMT should be performed in animals.
Acknowledgements
This study was supported by the Key Research and Development Plan of Anhui Province, China (1604a0802088). We thank M. Arico from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Ma X, Sun W, Cui P, Lu Z. Down-regulated FSTL5 promotes cell proliferation and survival by affecting Wnt/β-catenin signaling in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:3386–3394. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Li Y, Wang R, Qin S, Liu J, Su F, Yang Y, Zhao F, Wang Z, Wu Q. MiR-130a-3p regulates cell migration and invasion via inhibition of Smad4 in gemcitabine resistant hepatoma cells. J Exp Clin Cancer Res. 2016;35:1–11. doi: 10.1186/s13046-016-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Wu S, Huang S, Yin S, Zou G, Huang K, Zhang Z, Tang A, Wen W. Follistatin-like protein 1 contributes to dendritic cell and T-lymphocyte activation in nasopharyngeal carcinoma patients by altering nuclear factor κb and Jun N-terminal kinase expression. Cell Biochem Funct. 2016;34:34. doi: 10.1002/cbf.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Xiao X, Huang T, Du C, Wang S, Mo Y, Ma N, Murata M, Li B, Wen W, Huang G, Zeng X, Zhang Z. Epigenetic inactivation of follistatinlike 1 mediates tumor immune evasion in nasopharyngeal carcinoma. Oncotarget. 2016;7:16433–44. doi: 10.18632/oncotarget.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo-saito C, Fuwa T, Murakami K, Kawakami Y. Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res. 2013;73:6185–6193. doi: 10.1158/0008-5472.CAN-13-1364. [DOI] [PubMed] [Google Scholar]
- 9.Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suooressor effect of follistatin-like1 in ovarian and endometrial carcinogenesis, a differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 10.Tan X, Zhai Y, Chang W, Hou J, He S, Lin L, Yu Y, Xu D, Xiao J, Ma L, Wang G, Cao T, Cao G. Global analysis of metastasis-associated gene expression in primary cultures from clinical specimens of clear-cell renal-cell carcinoma. Int J Cancer. 2008;123:1080–1088. doi: 10.1002/ijc.23637. [DOI] [PubMed] [Google Scholar]
- 11.Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. doi: 10.1007/s12026-014-8526-z. [DOI] [PubMed] [Google Scholar]
- 12.Slany A, Haudek VJ, Zwickl H, Gundacker NC, Grusch M, Weiss TS, Seir K, Rodgarkia-Dara C, Hellerbrand C, Gerner C. Cell characterization by proteome profiling applied to primary hepatocytes and hepatocyte cell lines Hep-G2 and Hep-3B. J Proteome Res. 2010;9:6–21. doi: 10.1021/pr900057t. [DOI] [PubMed] [Google Scholar]
- 13.Eli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, Schulte-Hermann R, Rodgarkia-Dara C, Grusch M. Activins and activin antagonists in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1699–1709. doi: 10.3748/wjg.14.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuda T, Sakuma C, Nagaoka A, Yamagishi T, Ueda S, Nagase T, Yaginuma H. Follistatinlike5 is expressed in restricted areas of the adult mouse brain: implications for its function in the olfactory system. Congenit Anom (Kyoto) 2014;54:63–66. doi: 10.1111/cga.12022. [DOI] [PubMed] [Google Scholar]
- 15.Masuda T, Kai N, Sakuma C, Kobayashi K, Koga H, Yaginuma H. Laser capture microdissection and cDNA array analysis for identification of mouse KIAA/FLJ genes differentially expressed in the embryonic dorsal spinal cord. Brain Res. 2009;1249:61–67. doi: 10.1016/j.brainres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M, Sturm D, Witt H, Haag D, Toedt G, Wittmann A, Schöttler A, von Bueren AO, von Deimling A, Rutkowski S, Scheurlen W, Kulozik AE, Taylor MD, Lichter P, Pfister SM. FSTL5 is a marker of poor prognosis in non-WNT/Non-SHH medulloblastoma. J. Clin. Oncol. 2011;29:3852–3861. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 17.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Benner A, Ryzhova M, Sturm D, Witt H. FSTL5 improves prognostic subclassification of medulloblastoma. Cancer Res. 2011;71:3447–3447. [Google Scholar]
- 18.Zabala W, Cruz R, Barreiro-de Acosta M, Chaparro M, Panes J, Echarri A, Esteve M, Carpio D, Andreu M, García-Planella E, Domenech E, Carracedo A, Gisbert JP, Barros F EIGA & ENEIDA investigators. New genetic associations in thiopurine-related bone marrow toxicity among inflammatory bowel disease patients. Pharmacogenomics. 2013;14:631–640. doi: 10.2217/pgs.13.38. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrini M, Marcotte EM, Thompson MJ, Eisenberg D, Yeates TO. Assigning protein functions by comparative genome analysis: protein phylogenetic profiles. Proc Natl Acad Sci U S A. 1999;96:4285–4288. doi: 10.1073/pnas.96.8.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, McMillan E, Kim HS, Venkateswaran N, Makkar G, Rodriguez-Canales J, Villalobos P, Neggers JE, Mendiratta S, Wei S, Landesman Y, Senapedis W, Baloglu E, Chow CB, Frink RE, Gao B, Roth M, Minna JD, Daelemans D, Wistuba II, Posner BA, Scaglioni PP, White MA. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538:114–117. doi: 10.1038/nature19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Fan Q, Li Y, Yang Z, Yang L, Zong Z, Wang B, Meng X, Li Q, Liu J, Li H. Transforming growth factor-beta1 suppresses hepatocellular carcinoma proliferation via activation of Hippo signaling. Oncotarget. 2017;8:29785–29794. doi: 10.18632/oncotarget.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Li H, Wang G, Zhang T, Fu B, Ma M, Quan Z, Chen G. Yes-associated protein (YAP) expression is involved in epithelial-mesenchymal transition in hepatocellular carcinoma. Clin Transl Oncol. 2016;18:172–177. doi: 10.1007/s12094-015-1353-4. [DOI] [PubMed] [Google Scholar]
- 23.Bai N, Zhang C, Liang N, Zhang Z, Chang A, Yin J, Li Z, Luo N, Tan X, Luo N, Luo Y, Xiang R, Li X, Reisfeld RA, Stupack D, Lv D, Liu C. Yes-associated protein (YAP) increases chemosensitivity of hepatocellular carcinoma cells by modulation of p53. Cancer Biol Ther. 2013;14:511–520. doi: 10.4161/cbt.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Kim T, Johnson RL, Lim DS. Transcriptional Co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11:270–282. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. doi: 10.1016/j.jhep.2016.05.007. [DOI] [PubMed] [Google Scholar]


