Abstract
Laryngeal squamous cell carcinoma (LSCC) is the main type of human laryngeal cancer which is one of the most common malignant head and neck tumors and the outcomes of LSCC patients are always poor. The intrinsic molecular mechanisms in initiation, development, growth and metastasis of LSCC remain unclear. Further researches are necessary and urgent. In this article, we examined artemin (ARTN) promoted both cell proliferation and metastasis of human LSCC cells Hep-2 by siRNA mediated ARTN knocking down using MTT assay, cell migration assay and cell invasion assay. Moreover, we examined the expression level of ARTN in LSCC tissues was much higher than that in benign laryngeal polyp tissues. In addition, ARTN was identified as a direct target of miR-223 and miR-223 suppressed the expression of ARTN in LSCC cells. Supplement to our former study, we demonstrated ARTN was oncogenic both in vitro and in clinical tissues. As a result, ARTN could be used as a potential therapeutic target for human LSCC.
Keywords: Artemin, glioma, proliferation, metastasis, miR-223
Introduction
Laryngeal cancer is one of the most common head and neck malignancies worldwide, and is considered as the most common throat malignancy in China [1,2]. Laryngeal squamous cell carcinoma (LSCC) accounts for more than 90% of laryngeal cancers diagnosed [2,3]. Traditional treatment for LSCC is surgery, chemotherapy and radiotherapy, but the rate of recurrence and metastasis remains high for patients with advanced LSCC [2,4]. Lack of efficient molecular markers suppresses development of targeted therapy for LSCC. Further study for molecular mechanisms in initiation, development, growth and metastasis of LSCC will be helpful to develop new methods for diagnosis and therapy of LSCC.
Artemin (ARTN) belongs to glial cell line-derived neurotrophic factor (GDNF) family ligands (GFL) and is a potent neurotrophic factors [5,6].Molecular pathways involved in the downstream of ARTN is GFRα3 and receptor tyrosine kinase RET signaling [5,7]. As reported previously, ARTN promoted cell proliferation and metastasis in human breast cancer, endometrial carcinoma, non-small cell lung carcinoma, esophageal carcinoma, pancreatic cancer, and hepatocellular carcinoma [5-10]. Moreover, high expression levels of ARTN in these kinds of human cancers were correlated with poor survival of patients [5-10]. In our previous study, we reported ARTN and its receptor GFRα1 were positively associated with worse clinicopathological parameters in 76 clinical LSCC tissues [11]. But the exact role of ARTN in human LSCC cells was still unclear.
In this article, we performed systematical experiments to examine the role of ARTN in human LSCC cells. With depressed expression of ARTN using siRNA method, LSCC cells Hep-2 showed decreased cell viability, cell migration and cell invasion as determined by MTT assay, cell migration assay and cell invasion assay. Moreover, the expression level of ARTN in LSCC tissues was much higher than that in benign laryngeal polyp tissues, which was concordant with our pervious study [11]. Furthermore, we examined miR-223 directly targeted ARTN and negatively regulated the expression of ARTN. As a result, ARTN promoted cell proliferation and metastasis of human LSCC cells. ARTN could be used as a new potential biomarker for diagnosis and therapy of human LSCC.
Materials and methods
Cell lines and cell culture
Human LSCC cells Hep-2 was used in this study, which was obtained from the American Type Culture Collection (ATCC) (Rockville, MD). As recommended, Hep-2 cells was cultured in a humidified incubator at 37°C and 5% CO2.
RNA oligonucleotides transfection
ARTN siRNA/negative control siRNA, miR-223 mimics/negative control miRNA mimics and miR-223 ASO/negative control miRNA ASO were synthesized by GenePharma (Shanghai, China). As recommended and described previously, RNA oligonucleotides transfection were carried out using lip2000 (QIAGEN) [12].
Plasmid constructs and transfection
Luciferase reporter plasmid PsiCHECK2 was used in this study. We cloned human ARTN 3’UTR sequence into PsiCHECK2 and designated it as PsiCHECK2-ARTN 3’-UTR. Plasmid transfection and co-transfection with plasmid and miRNA mimics were performed using lip2000 (QIAGEN) as described previously [12].
Cell oncogenicity assays
Celloncogenicity assays containing 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, cell migration assay and cell invasion assay were performed in this study to evaluate the role of ARTN in human LSCC cells Hep-2 and were all carried out essentially as described previously [12]. In MTT assay, cells (2000 per well) were plated in 96-well plates and were tested after 24 hours, 48 hours, 72 hours, 96 hours and 120 hours; cell growth curves were analyzed. Cell migration assay and cell invasion assay were carried out using transwellchambers. In cell migration assay, 100000 cells per well were plated and were tested after 18 hours; in cell invasion assay, 500000 cells per well were plated and were tested after 30 hours.
RT-Quantitative PCR (RT-qPCR)
RT-Quantitative PCR (RT-qPCR) was essentially performed as described in previous studies [12,13]. We determined mRNA levels of ARTN in human LSCC cells Hep-2 transfected with ARTN siRNA/negative control siRNA, miR-223 mimics/negative control miRNA mimics, miR-223 ASO/negative control miRNA ASO and in human LSCC tissues/benign laryngeal polyp tissues. GAPDH was used as a control.
Clinical tissue samples
We collected 16 fresh LSCC tissues and 14 fresh benign polyp tissues from patients who underwent surgery between 2012 and 2015 at the First Affiliated Hospital of Anhui Medical University (Hefei, Anhui, China). All of the patients approved the use of their tissues for scientific research and we have got the approval of the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University for this study.
Luciferase reporter assay
For luciferase reporter assay, we used Dual Luciferase Reporter Assay System (Promega Corp.) to test the Renilla luciferase activity as described earlier [12]. Firefly luciferase activity was also tested as a reference control.
Statistics
At least three independent replicated experiments were carried out for each assay. The variances were analyzed in this study using unpaired two-tailed t test. P<0.05 was considered as statistically significant.
Results
ARTN promoted proliferation and metastasis of human LSCC cells
To evaluate the role of ARTN in human LSCC cells, Hep-2 cells were transfected with ARTN siRNA or negative control siRNA. Compared with negative control siRNA, ARTN-siRNA significantly decreased the mRNA level of ARTN in Hep-2 cells (Figure 1A). As shown in Figure 1B, cell viability decreased dramatically over a period of 120 hours after transfected with ARTN-siRNA compared with negative control siRNA. Moreover, both cell migration and invasion decreased significantly in Hep-2 cells with depressed expression of ARTN compared with negative control respectively (Figure 1C). Therefore, ARTN promoted both proliferation and metastasis of human LSCC cells.
Figure 1.
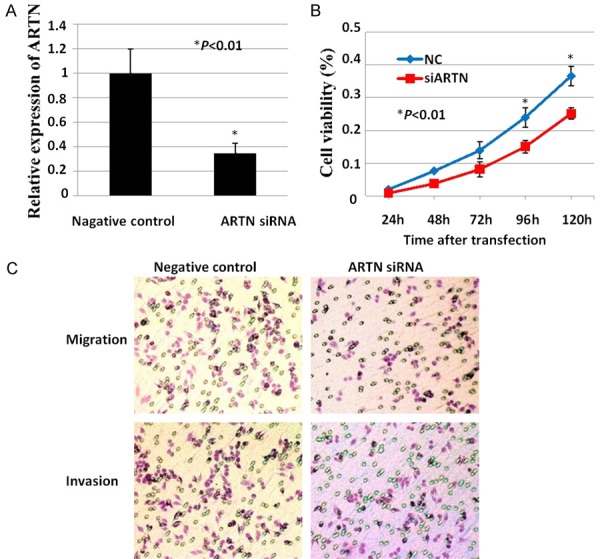
ARTN promoted proliferation and metastasis of human LSCC cells. A: mRNA level of ARTN was examined after transfected with ARTN siRNA or negative control siRNA in Hep-2 cells using RT-qPCR. B: MTT assay. C: Cell migration assay and invasion assay were carried out in Hep-2 cells after transfected with ARTN siRNA or negative control siRNA respectively. *, P<0.01.
Expression levels of ARTN in LSCC tissues and benign laryngeal polyp tissues
To evaluate the expression levels of ARTN in tissues from patients with laryngeal neoplasm, we collected 16 LSCC tissues and 14 benign polyp tissues and examined the mRNA level of ARTN using RT-qPCR. As shown in Figure 2, the expression level of ARTN was dramatically higher in LSCC tissues compared with benign laryngeal polyp tissues (P<0.01).
Figure 2.
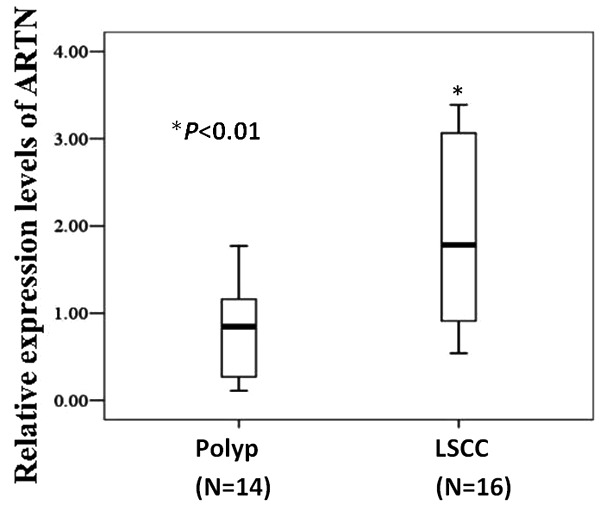
Expression levels of ARTN in LSCC tissues and benign laryngeal polyp tissues. mRNA levels of ARTN in 16 LSCC tissues and 14 benign laryngeal polyp tissues from patients were examined using RTqPCR. *, P<0.01.
ARTN was a direct target of miR-223
For further study, we used online software Target Scanto search for potential miRNAs that directly targeted and regulated ARTN in human LSCC cells. MiR-223 was found to be a candidate miRNA that could directly target ARTN, and the miR-223-binding site with the 3’-UTR of ARTN mRNA was 5’-GUCAGUU-3’ (Figure 3A). QRT-PCR analysis showed that forced expression of miR-223 significantly suppressed the mRNA level of ARTN; depressed expression of miR-223 with ASO significantly increased the mRNA level of ARTN (Figure 3B). Furthermore, luciferase reporter assay was performed to evaluate the interaction of miR-223 and ARTN.When co-transfected with luciferase reporter plasmid PsiCHECK2-ARTN 3’-UTR and miR-223 mimics in Hep-2 cells, the luciferase activity was dramatically decreased compared with co-transfected with negative control miRNA mimics/PsiCHECK2 vector, negative control miRNA mimics/PsiCHECK2-ARTN 3’-UTR or miR-223 mimics/PsiCHECK2 vector respectively (Figure 3C). Therefore, miR-223 directly targeted and regulated ARTN.
Figure 3.
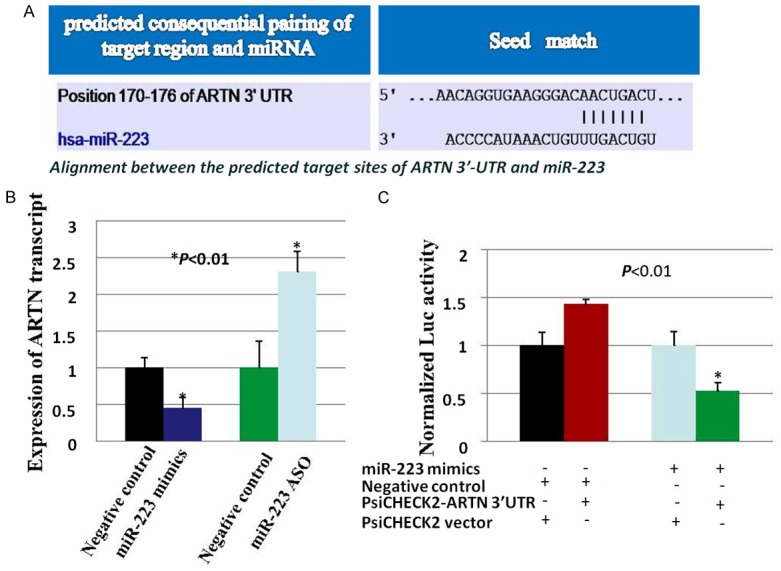
ARTN was a direct target of miR-223. A. Predicted consequential pairing of miR-223 and the 3’UTR of ARTN. B. mRNA levels of ARTN were examined after transfected with miR-223 mimics/negative control miRNA mimics and miR-223 ASO/negative control miRNA ASO in Hep-2 cells using RT-qPCR. C. Luciferase assay of Hep-2 cells cotransfected with miR-223 mimics/negative control miRNA mimics, and luciferase reporter plasmid PsiCHECK2 containing ARTN 3’UTR (PsiCHECK2-ARTN 3’UTR)/PsiCHECK2 vector. *, P<0.01.
Discussion
LSCC was the main type of human laryngeal cancer, which was one of the most common fatal diseases in human head and neck. Despite of progresses in diagnosis and therapy methods for human LSCC, the survival rate of patients with advanced LSCC remained low [2,4]. As reported previously, many genes contributed to the development and progress of LSCC, such as ETS-1, SOX 1, SOX 2, IGF1R, HOXA9 etc. [14-17]. Herein, we reported ARTN promoted both proliferation and metastasis of human LSCC cells. The expression level of ARTN in LSCC tissues was much higher than that in benign laryngeal polyp tissues. As reported in our former article, 53.9% of LSCC tissues were positive for ARTN whilst only 26.9% of benign laryngeal polyp tissues were positive for ARTN [11]. Moreover, high expression level of ARTN was correlated with advanced pTNM stage, lower five-year RFS (relapse-free survival) or OS (overall survival) in patients with LSCC [11]. These data was concordant with our present data. As a result, ARTN was oncogenic for human LSCC.
As reported previously, ARTN increased cell survival, cell migration, cell invasion, cell anchorage-independent growth and tumor xenograft growth in human breast cancer; the expression level of ARTN was much higher in breast cancer tissues compared with normal breast tissues [5]. Moreover, ARTN was estrogen regulated and promoted antiestrogen resistance in ER positive breast cancer by regulating the downstream gene BCL-2 [18]. In ER negative breast cancer, ARTN also contributed to tumor metastasis and poor survival outcome in patients by regulating the downstream gene TWIST1 [19]. Furthermore, ARTN also contributed to radio-resistance, chemo-resistance and trastuzumab-resistance in human breast cancer by promoting TWIST1-BCL-2-dependent cancer stem cell like behavior [20,21]. In other types of human cancers including endometrial carcinoma, non-small cell lung carcinoma, esophageal carcinoma, pancreatic cancer, and hepatocellular carcinoma, ARTN also acted as a tumor promoter [6-10]. Herein, we examined that ARTN was oncogenic in human LSCC. Therefore, ARTN was a widely important oncogene in nearly all kinds of human cancers.
We examined miR-223 directly targeted ARTN and suppressed the expression of ARTN in LSCC cells. Li S et al demonstrated that miR-223 suppressed cell migration and invasion by directly targeted ARTN in human esophageal carcinoma [9]. MiR-223 was also reported to suppress cell proliferation and enhance cell apoptosis in human acute myeloid leukemia [22]. However, miR-223 promoted cell growth and invasion in human pancreatic cancer [23]; miR-223 promoted tumor progression of human lung cancer [24]. Therefore, miR-223 could function as either an oncogene or a tumor suppressor gene. MiR-223 showed tissue specificity in different human cancers [25]. The exact role of miR-223 in human LSCC remained unclear, which will be well studied in our future work.
In a word, this study systematically examined the role of ARTN in human LSCC cells. Supplement to our former tissue results, we demonstrated ARTN promoted cell proliferation and metastasis of LSCC cells in vitro, and ARTN was dramatically associated with clinicopathological parameters and survival rate of patients with LSCC. As an oncogene, ARTN could be used as a potential therapeutic target for human LSCC.
Acknowledgements
This work was supported by Natural Science Research Project for Universities of Anhui Province (KJ2015A036), Natural Science Foundation of Anhui Province (1608085MH189).
Disclosure of conflict of interest
None.
References
- 1.Li Y, Wang K, Yin SK, Zheng HL, Min DL. Xanthohumol inhibits proliferation of laryngeal squamous cell carcinoma. Oncol Lett. 2016;12:5289–5294. doi: 10.3892/ol.2016.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussein S, Mosaad H, Rashed HE, El-Anwar MW. Up-regulated miR-221 expression as a molecular diagnostic marker in laryngeal squamous cell carcinoma and its correlation with Apaf-1 expression. Cancer Biomark. 2017 doi: 10.3233/CBM-160444. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Li P, Yang Y, Liu H, Yang AK, Di JM, Tan GM, Wang HF, Qiu JG, Zhang WJ, Jiang QW, Zheng DW, Chen Y, Wei MN, Huang JR, Wang K, Shi Z, Ye J. MiR-194 functions as a tumor suppressor in laryngeal squamous cell carcinoma by targeting Wee1. J Hematol Oncol. 2017;10:32. doi: 10.1186/s13045-017-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenckel F, Knecht R. State of the art in the treatment of laryngeal cancer. Anticancer Res. 2013;33:4701–4710. [PubMed] [Google Scholar]
- 5.Kang J, Perry JK, Pandey V, Fielder GC, Mei B, Qian PX, Wu ZS, Zhu T, Liu DX, Lobie PE. Artemin is oncogenic for human mammary carcinoma cells. Oncogene. 2009;28:2034–2045. doi: 10.1038/onc.2009.66. [DOI] [PubMed] [Google Scholar]
- 6.Pandey V, Qian PX, Kang J, Perry JK, Mitchell MD, Yin ZN, Wu ZS, Liu DX, Zhu T, Lobie PE. Artemin stimulates oncogenicity and invasiveness of human endometrial carcinoma cells (vol 151, pg 909, 2010) Endocrinology. 2012;153:540–540. doi: 10.1210/en.2009-0979. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Zhang W, Wu Z, Liu S, Sun L, Zhong Y, Zhang X, Kong X, Qian P, Zhang H, Lobie PE, Zhu T. Artemin is hypoxia responsive and promotes oncogenicity and increased tumor initiating capacity in hepatocellular carcinoma. Oncotarget. 2016;7:3267–3282. doi: 10.18632/oncotarget.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang JZ, Kong XJ, Kang J, Fielder GC, Steiner M, Perry JK, Wu ZS, Yin ZA, Zhu T, Liu DX, Lobie PE. Artemin-stimulated progression of human non-small cell lung carcinoma is mediated by BCL2. Mol Cancer Ther. 2010;9:1697–1708. doi: 10.1158/1535-7163.MCT-09-1077. [DOI] [PubMed] [Google Scholar]
- 9.Li SJ, Li ZG, Guo FJ, Qin XB, Liu B, Lei Z, Song ZQ, Sun LY, Zhang HT, You JC, Zhou QH. miR-223 regulates migration and invasion by targeting Artemin in human esophageal carcinoma. J Biomed Sci. 2011;18:24. doi: 10.1186/1423-0127-18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng LX, Chi YH, Wang XX, Ding ZJ, Fei LC, Zhang H, Mou L, Cui W, Xue YJ. Neurotrophic artemin promotes motility and invasiveness of MIA PaCa-2 pancreatic cancer cells. Asian Pac J Cancer Pre. 2012;13:1793–1797. doi: 10.7314/apjcp.2012.13.5.1793. [DOI] [PubMed] [Google Scholar]
- 11.Gao C, Cheng X, Li X, Tong B, Wu K, Liu Y. Prognostic significance of artemin and GFRalpha1 expression in laryngeal squamous cell carcinoma. Exp Ther Med. 2014;8:818–822. doi: 10.3892/etm.2014.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding K, Wu Z, Wang N, Wang X, Wang Y, Qian P, Meng G, Tan S. MiR-26a performs converse roles in proliferation and metastasis of different gastric cancer cells via regulating of PTEN expression. Pathol Res Pract. 2017;213:467–475. doi: 10.1016/j.prp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, Zhao B, Li XC, Wan JH. ARPP-19 promotes proliferation and metastasis of human glioma. Neuroreport. 2016;27:960–966. doi: 10.1097/WNR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SY, Lu ZM, Lin YF, Chen LS, Luo XN, Song XH, Chen SH, Wu YL. miR-144-3p, a tumor suppressive microRNA targeting ETS-1 in laryngeal squamous cell carcinoma. Oncotarget. 2016;7:11637–11650. doi: 10.18632/oncotarget.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang N, Wang Y, Hui L, Li XT, Jiang XJ. SOX 1, contrary to SOX 2, suppresses proliferation, migration, and invasion in human laryngeal squamous cell carcinoma by inhibiting the Wnt/beta-catenin pathway. Tumor Biol. 2015;36:8625–8635. doi: 10.1007/s13277-015-3389-z. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Wu J, Li Z, Qin H, Wang B, Wong TS, Yang W, Fu QL, Lei W. miR-375 suppresses IGF1R expression and contributes to inhibition of cell progression in laryngeal squamous cell carcinoma. Biomed Res Int. 2014;2014:374598. doi: 10.1155/2014/374598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Liu B, Ji WY, Ma XL, Wang XQ, Gu H. The role of HOXA9 in human laryngeal squamous cell carcinoma. Oncol Res. 2012;20:467–472. doi: 10.3727/096504013x13685487925257. [DOI] [PubMed] [Google Scholar]
- 18.Kang J, Qian PX, Pandey V, Perry JK, Miller LD, Liu ET, Zhu T, Liu DX, Lobie PE. Artemin is estrogen regulated and mediates antiestrogen resistance in mammary carcinoma (vol 29, pg 3228, 2010) Oncogene. 2012;31:402–402. doi: 10.1038/onc.2010.71. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A, Wu ZS, Qian PX, Kang J, Pandey V, Liu DX, Zhu T, Lobie PE. ARTEMIN synergizes with TWIST1 to promote metastasis and poor survival outcome in patients with ER negative mammary carcinoma. Breast Cancer Res. 2011;13:R112. doi: 10.1186/bcr3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee A, Qian PX, Wu ZS, Ren XG, Steiner M, Bougen NM, Liu SL, Liu DX, Zhu T, Lobie PE. Artemin stimulates radio- and chemo-resistance by promoting TWIST1-BCL-2-dependent cancer stem cell-like behavior in mammary carcinoma cells. J Biol Chem. 2012;287:42502–42515. doi: 10.1074/jbc.M112.365163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding KS, Banerjee A, Tan S, Zhao JS, Zhuang Q, Li R, Qian PX, Liu SL, Wu ZS, Lobie PE, Zhu T. Artemin, a member of the glial cell line-derived neurotrophic factor family of ligands, Is HER2-regulated and mediates acquired trastuzumab resistance by promoting cancer stem cell-like behavior in mammary carcinoma cells. J Biol Chem. 2014;289:16057–16071. doi: 10.1074/jbc.M113.529552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Y, Su CL, Deng TR. miR-223 decreases cell proliferation and enhances cell apoptosis in acute myeloid leukemia via targeting FBXW7. Oncol Lett. 2016;12:3531–3536. doi: 10.3892/ol.2016.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He D, Huang C, Zhou QX, Liu DW, Xiong LH, Xiang HX, Ma GN, Zhang ZY. HnRNPK/miR-223/FBXW7 feedback cascade promotes pancreatic cancer cell growth and invasion. Oncotarget. 2017;8:20165–20178. doi: 10.18632/oncotarget.15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Li F, Deng PB, Hu CP. MicroRNA-223 promotes tumor progression in lung cancer A549 cells via activation of the NF-kappa B signaling pathway. Oncol Res. 2016;24:405–413. doi: 10.3727/096504016X14685034103437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Lin L, Li T, Yang J, Wei Y. The role of miRNA-223 in cancer: function, diagnosis and therapy. Gene. 2017;616:1–7. doi: 10.1016/j.gene.2017.03.021. [DOI] [PubMed] [Google Scholar]


