Abstract
Desmoid tumors, also known as aggressive fibromatoses, are clinically rare soft-tissue tumors that arise from such connective tissues as the musculoaponeurotic structures. The majority of the reported intra-abdominal desmoid tumors occur in the small intestinal mesentery and peritoneum, whereas stomach desmoid tumors are extremely rare. The present study reports a case of stomach desmoid tumor and reviews the related available literature. The diagnosis of the stomach desmoid tumors can be made with routine pathological and immunohistochemical examinations. Compared with desmoid tumors at other sites, the currently preferred treatment of the stomach desmoid tumors with negative margins is radical resection of tumors. All the follow-ups of patients with the stomach desmoid tumors show no obvious recurrence, suggesting that the prognosis of the stomach desmoid tumors is good.
Keywords: Stomach desmoid tumor, aggressive fibromatosis, desmoid tumor, fibrous tissue tumor-like hyperplasia, gastrointestinal stromal tumor
Introduction
Desmoid tumor, also known as aggressive fibromatosis or tumor-like fibrous tissue hyperplasia, is a fibroblastic/myofibroblastic tumor originated from mesenchymal cells. In 2002, the WHO defined the desmoid tumor as an infiltrating tumor originated from deep soft tissue [1]. Clinically, desmoid tumor is a rare disease, with significant clinical diversity and variability [2]. Although histopathologies show that desmoid tumor is benign, but it has a high rate of local recurrence. However, it has no clinical features of distant metastasis. Desmoid tumor can occur in a wide variety of locations, including the extremities, trunk, head, neck, abdominal wall, abdomen, perineum, and foreskin. Based on the anatomical location of the occurrence, desmoid tumors can be divided into superficial and deep types. The deep desmoid tumors can be further subdivided into intra-abdominal and extra-abdominal tumors[3]. Currently, the clinically reported intra-abdominal desmoid tumors are rare, and the stomach desmoid tumors are extremely rare.
The present study reports a case of a patient with the gastric desmoid tumor, including the diagnosis and treatment, and the literature regarding the stomach desmoid tumor is systematically reviewed and summarized in Table 1 in order to provide a comprehensive understanding of this rare disease.
Table 1.
Summary of 8 cases of the stomachdesmoid tumors
| NO. | Sex | Age (year) | Symptoms | Tumor location | Tumor size (mm) | Ultrasonography and endoscopy findings | Surgical resection | Adjuvant therapy | Immunohistochemistry | Recurrence | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 47 | Upper abdominal pain | The posterior wall of the antrum | 45 × 35 × 40 | Ulcer lesions, ultrasonography hypoechoic mass with less clear boundaries | R1 | No | Positive: SMA, β-catenin | No | ANED |
| Negative: CD117, CD34, S100 | |||||||||||
| 2 | M | 45 | Abdominal pain, hematemesis | The posterior wall of the gastric body | 68 × 52 | Irregular mucosal uplift, surface deep ulcers, no ultrasonography description | R0/R1 | NA | Positive: SMA, S100, β-catenin | NA | NA |
| Negative: CD117, Des, CD34 | |||||||||||
| 3 | F | 42 | Abdominal discomfort | Greater curvature of the stomach | 70 × 70 × 60 | Submucosal tumor, ulcer lesions, ultrasonography hypoechoic mass with clear boundaries | R0/R1 | NA | Positive: - | NA | NA |
| Negative: SMA, CD34, CD117 | |||||||||||
| 4 | M | 28 | Dysphagia, vomiting | Gastroesophageal junction | 50 × 18 | Mucosal erosion, ultrasonography hypoechoic mass with less clear boundaries | R0/R1 | Radiotherapy | Positive: SMA, CD117 (dot +) | No | ANED |
| Negative: S100, CD34 | |||||||||||
| 5 [20] | M | 9 | Abdominal pain, vomiting | Gastroesophageal junction | NA | diffuse gastric wall thickening in ultrasonography, endoscopy did not pass the cardia | R0 | No | Positive: - | No | ANED |
| Negative: S100, CD34, Des | |||||||||||
| 6 [13] | M | 67 | NA | The posterior wall of the stomach | NA | NA | NA | Chemotherapy | NA | NA | AWD |
| 7 [21] | F | 15 | NA | Gastroesophageal junction | NA | NA | NA | NA | NA | NA | NA |
| 8 [22] | M | 56 | NA | The remaining greater curvature of the stomach | 40 × 40 | submucosal tumor, ultrasonography hypoechoic mass with clear boundaries | R0/R1 | Positive: β-catenin | No | ANED | |
| Negative: - |
Notes: NA: not available; ANED: alive with no evidence of disease; AWD: alive with disease; No. 8 underwent early gastric distal gastrectomy 18 months ago; No. 1 follow-up 63 months; No. 4 follow-up 22 months; No. 5 follow-up 12 months; No. 8 follow-up 12 months.
Case report
Patient No. 1 in Table 1 was the reported patient in this paper. The patient was a female, 47 years old. She visited our hospital because of “upper abdominal pain for more than a year” in September, 2010. The patient had no history of abdominal surgery. After admission to the hospital, the patient underwent a whole abdominal CT scan, including plain and contrast enhanced scans as shown in Figure 1A and 1B. As shown in Figure 1, there was a shadow of soft tissue mass measuring 20 × 25 mm in size in the antrum, lesser curvature side. The shadow of the mass was significantly heterogeneously enhanced and protruded locally into the stomach cavity. A diagnosis of gastrointestinal stromal tumor (GIST) was considered initially. Endoscopic ultrasonography (Figure 1C and 1D) showed that there was an ulcer with a diameter of about 15 mm in the posterior wall of the antrum. The bottom of the ulcer was significantly below the level of the surrounding mucosa. Ultrasonography findings showed that there was a hypoechoic mass in the lesion, with a size of about 22 × 25 mm protruding to the gastric cavity and a less clear boundary. It seemed that the mass originated from muscularis propria. No preoperative biopsy was performed. The preoperative diagnosis of the patient was GIST. The patient underwent distal gastrectomy (Billroth-I) following a perfect preoperative preparation. Intraoperative findings showed that there was a mass near pyloric antrum, with a size of about 45 × 45 mm. Significantly enlarged lymph nodes (No. 5 and 8) were found, No other obvious abnormalities were found in pelvic and abdominal cavities. The operation was performed successfully and the postoperative recovery was good. Postoperative microscopic pathological findings (Figure 2A) showed that diffuse infiltrative growth of tumor cells in the muscle layer of stomach. The tumor cells were spindle-shaped or short spindle-shaped, with a mitotic index (MI) of about 1-2 cells/50 HPF. No tumor involvement was found in the upper margin and gastroepiploic tissue. However, there was tumor involvement in the lower margin. No lymph node metastasis was found. Main immunohistochemistry findings included SMA (++), DES (foci +), CD117 (-), S100 (-), Ki-67 (about 1%), CD34 (-) (Figure 2B), β-catenin (+) (Figure 2C). The pathological diagnosis was the stomach desmoid tumor. The patient did not receive any postoperative adjuvant therapy. No tumor recurrence occurred after follow-up for 63 months. The patient was currently in good condition with no obvious discomfort.
Figure 1.
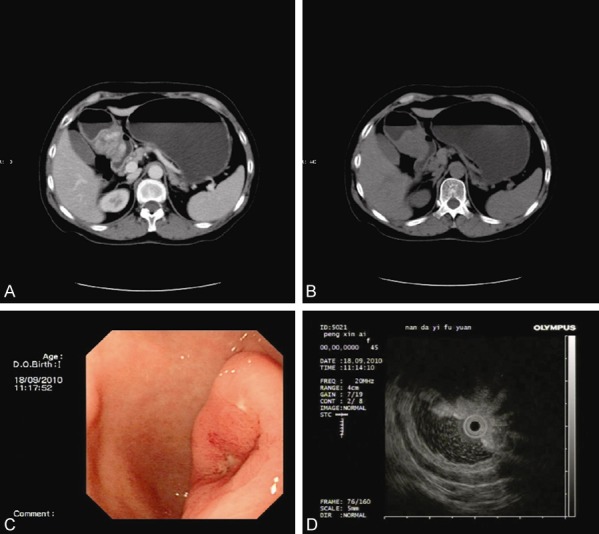
Whole abdominal CT scan (A and B) shows that there is a shadow of soft tissue mass measuring 20 × 25 mm in size in the antrum. Endoscopy (C) shows that there is an ulcer with a diameter of about 15 mm in the posterior wall of the antrum. Endoscopic ultrasonography (D) findings show that there is a hypoechoic mass in the lesion, with a size of about 22 × 25 mm protruding to the gastric cavity and a less clear boundary. (A) Plain CT scan; (B) Enhanced CT scan; (C) Endoscopy; (D) Endoscopic ultrasonography.
Figure 2.
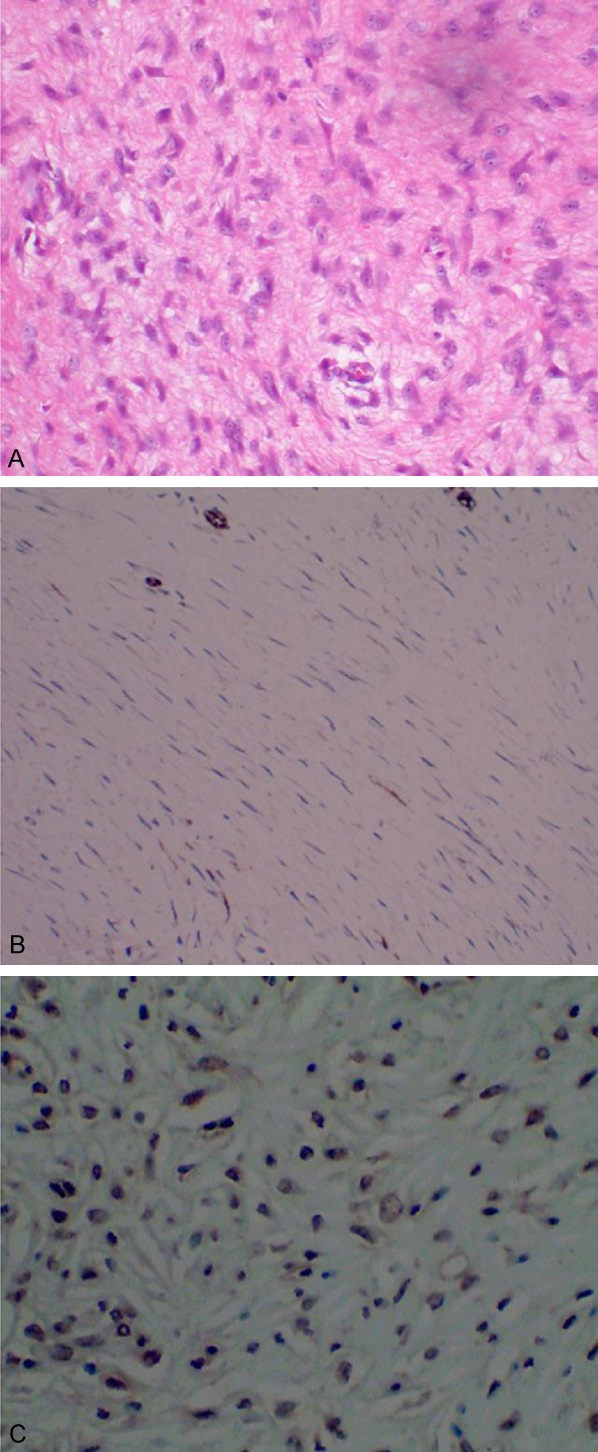
Microscopic pathological findings (A) indicate the diffuse infiltrative growth of tumor cells in the muscle layer of stomach. Main immunohistochemistry findings include CD34 (-) (B) and β-catenin (+) (C). (A) H&E stain, × 400; (B) CD34, × 400; (C) β-catenin, × 400.
Discussion
The biological behavior and clinical manifestations of desmoid tumors at different anatomical locations are significantly different. Therefore, individualized treatment for desmoid tumor is required for the treatment of the tumors at different anatomical locations. Thus, it is necessary and of great value to summarize the clinical features, diagnosis and treatment of the stomach desmoid tumors.
As shown in Table 1, the stomach desmoid tumors occur mainly in the middle-aged patients, which is different from desmoid tumors in general that usually occur in younger patients. This difference may be related to the fact that the stomach desmoid tumors grow slowly without obvious symptoms. The Symptoms of the stomach desmoid tumors are not directly correlated with the size of the tumors. For example, among the eight patients shown in Table 1, the patient with the largest tumor with a diameter of 70 mm only showed some nonspecific abdominal discomfort. In contrast, the clinical manifestations of the stomach desmoid tumors are more directly related to the location of the tumors. The tumors located at the gastroesophageal junction or near pyloric antrum usually produce more obvious symptoms. In addition, the symptoms of the stomach desmoid tumors are also related to whether the blood vessels supplying the stomach are infiltrated with tumor. Of the eight patients shown in Table 1, one patient had hematemesis, which might be related to the infiltration of tumor cells into the superficial blood vessels of the stomach.
Even though imaging data can help the diagnosis of the stomach desmoid tumor, overall, their values are quite limited. Plain CT scan shows a mass with a more uniform density, sometimes shows striped low-density shadows, which may be related to mucinous matrix. The contrast enhanced scan demonstrates a lesion with homogeneous or heterogeneous enhancement, the degree of which is from mild to significant. However, these image findings lack specificity. The MRI imaging findings have certain specificity and therefore can provide a basis for the initial diagnosis of the stomach desmoid tumor. In general, moderate signal intensity on T1W images and inhomogeneous signal intensity on T2W images of the tumors are presented. These MRI image features may be related to the different components of the tumors, including fibroblasts, extracellular collagen, and mucinous matrix. It is these image features that help to diagnose the stomach desmoid tumor[4]. In nearly 90% of patients with the stomach desmoid tumor, the presence of non-enhanced stripes with low signal intensity on T2W images formed by fibrosis within the mass is the key basis for the diagnosis [5].
With regard to the differential diagnosis of the stomach desmoid tumor, other tumors occurred in the stomach should be excluded, such as low grade fibrosarcoma, neuroendocrine tumors, ectopic pancreas, and GIST. Among these tumors, GIST is the most difficult one from which to differentially diagnose. Moreover, the differential diagnosis of the stomach desmoid tumor from GIST is crucial since the treatment and prognosis, and the corresponding influencing factors are quite different. The preoperative diagnosis of all the eight cases of the stomach desmoid tumors shown in Table 1 were misdiagnosed as GIST.
Endoscopic ultrasonography and CT can provide certain values for the differential diagnosis of the stomach desmoid tumors. An ultrasonography study consisting of 42 patients with 44 lesions of fibromatosis indicated that the number of lesions with well-defined and regular margins are similar to that with poorly defined and irregular margins [6]. The ultrasonography features of desmoid tumors are generally shown as different solid masses, mainly with hypoechoic appearance. In the eight cases of the stomach desmoid tumor patients shown in Table 1, four patients had the endoscopic ultrasonography data. 50% of the lesions in these four patients had clear margins whereas the other 50% had an unclear margin. In addition, whether the margins are clear or not are not closely correlated with the tumor size, i.e., a large lesion can have a clear margin while a small lesion may have a poorly defined margin. On contrary, in patients with GIST, a lesion with a diameter of less than 50 mm usually has a clear margin, whereas a lesion with a diameter of greater than 50 mm generally has a poorly defined margin. Therefore, a small mass in the submucosa, muscularis, or serosa of the stomach with an unclear margin in endoscopic ultrasonography images, in combination with other findings similar to GIST may prompt the diagnosis of the stomach desmoid tumor.
With regard to the application of CT to the differential diagnosis of desmoid tumors, several key points have been proposed as follows in one study on the differential diagnosis of intra-abdominal desmoid tumors and GIST [7]. 1. Since intra-abdominal desmoid tumors originate from connective tissue in muscle, fascia or aponeuroses, they are more likely to occur in the extra-gastrointestinal tract, and rarely found in the gastrointestinal tract. In contrast, GIST occurs less likely in the peritoneum, mesentery, retroperitoneum [8], and is more commonly found in the gastrointestinal tract. 2. Intra-abdominal desmoid tumors are mostly ovoid or irregular contour, while GIST is predominantly lobulated or round. 3. GIST usually shows heterogeneous enhancement. In contrast, intra-abdominal desmoid tumors generally show homogeneous enhancement since there are large amounts of collagen matrix in the tumors. 4. Faria et al [9] believed that the necrotic foci found in intra-abdominal desmoid tumors are not typical, and the low-density mucus matrix in the cross-section could be confusing and mistakenly considered as necrosis in CT images. On contrary, necrosis is commonly found in GIST lesions. In order to avoid the confusions, an intralesional CT attenuation value of < 20 Hounsfield unit (HU) is defined as necrosis. 5. In intra-abdominal desmoid tumors, the cut-off values are set to the degree of enhancement of less than 40.5 HU in the arterial phase, and less than 46.5 HU in the portal venous phase, which is primarily because the GIST is much richer in blood vessels. 6. In intra-abdominal desmoid tumors, a lesion/aorta (L/A) CT attenuation ratio of < 0.315 in the arterial phase versus 0.525 in the portal phase are defined. With these differential diagnostic criteria, a specificity of 89.5% for the diagnosis of intra-abdominal desmoid tumors can be achieved. Similarly, the comprehensive utilization of these CT features may provide great values for the differential diagnosis of the stomach desmoid tumor and GIST.
In addition to endoscopic ultrasonography and CT, immunohistochemistry has the highest value for the differential diagnosis of the stomach desmoid tumor from GIST. Microscopically, both the stomach desmoid tumor and GIST are characterized by spindle-cell hyperplasia. Therefore, the differential diagnosis of these two types of tumors cannot rely on the use of conventional hematoxylin and eosin staining, but mainly on the immunohistochemistry. However, even with the immunohistochemistry the differential diagnosis of these two types of tumors is still difficult. For example, immunostaining of CD117 is widely used for differential diagnosis of the stomach desmoid tumors and GIST, while positive expression of CD117 is found in most of the GIST patients (10), it is also found in 0-60% of the stomach desmoid tumors (Patient NO. 4 in Table 1 is positive) [11]. In other word, negative expression of CD117 can be found not only in the stomach desmoid tumors but also in some GIST, which might be attributed to the fact that some GIST patients have PDGFRA mutation with wildtype C-KIT gene. When patients are difficult to diagnose, immune staining of β-catenin and CD34 can be used [12]. Since patients with desmoid tumors usually have activation mutations in β-catenin, therefore, abnormal nuclear accumulation of β-catenin is seen in most patients. In contrast, no nuclear staining of β-catenin could be seen in patients with GIST since the β-catenin signaling pathway in GIST patients is mostly normal [11]. In contrast to β-catenin, CD34 is expressed in about 70-90% of GIST patients whereas CD34 staining is negative in patients with desmoid tumors [12]. Therefore, immune staining of CD34 and nuclear staining of β-catenin are powerful tools for differential diagnosis of the stomach desmoid tumors from GIST. Interestingly, among the eight patients in Table 1, patient No. 6 was initially diagnosed as GIST and treated with imatinib (400 mg/d). Thirty five months later, a new tumor was found and diagnosed as the stomach desmoid tumor, raising a question of whether there is a certain relationship between the occurrence of GIST and the stomach desmoid tumors. A study on the potential relationship between GIST and desmoid tumors indicated a nonrandom association between these two types of tumors, i.e., GIST patients are predisposed to developing desmoid tumor and vice versa. However, the specific underlying molecular and biological mechanisms responsible for the nonrandom relationship are still unknown [13]. Therefore, more attention should be paid to the differences and connections between these two types of tumors.
With regard to the treatment of the stomach desmoid tumor, R0 resection with a negative margin is currently the major surgical treatment option. Recent studies have found that some patients with desmoid tumors will resolve spontaneously for unknown reasons. In addition, some patients with desmoid tumors, even those with local recurrence, the tumors stop growing and are stable for many years. Therefore, a conservative initial watch-and-wait policy is advocated for the treatment of desmoid tumors. However, given the special nature of the anatomical location of the stomach, and the fact that desmoid tumors are usually located at gastroesophageal junction, or near pylorus antrum and the quality of life of patients is affected progressively, therefore surgery is still the first choice for patients with the stomach desmoid tumors. Complete resection of the stomach desmoid tumor is not difficult. Currently, the main surgical approach is laparoscopic-assisted stomach tumor resection. In some hospitals, a less invasive robot-assisted laparoscopic resection for the stomach desmoid tumors has been carried out. Since the stomach has good compensatory function, partial gastrectomy has little impact on the quality of life, which is different from the tumors occurred in the extremities and trunk, where resection of tumors may be associated with the risk of disability. Moreover, resection of tumors is helpful for the final diagnosis of the disease. For asymptomatic patients found during physical examination and verified with endoscopic ultrasound-guided biopsy, a conservative watch and wait policy can be implemented to closely monitor the disease. When local recurrence occurs, or complete surgical removal of the tumor is not feasible, radiotherapy, chemotherapy and other comprehensive therapies should be performed. The recommended radiotherapy protocol is a single dose of 2 Gy, once a day for 28 days, with a total dose of 56 Gy. This protocol can ensure a high local control rate for most of the patients [2]. Since there is not any one specific drug that is effective for all desmoid tumors, thus individualized treatment plan is required for the chemotherapeutic treatment of the stomach desmoid tumors. The pharmacological treatment of the stomach desmoid tumors includes estrogen receptor antagonists such as tamoxifen, non-steroidal anti-inflammatory drugs (NSAIDs) such as methotrexate, and low dose of chemotherapeutic drugs, such as vinblastine and vinorelbine. In addition, any one of the tyrosine kinase inhibitors (TKI), including imatinib, sunitinib, and sorafenib can be used. Liposomal doxorubicin is also recommended for the treatment of the stomach desmoid tumors [14]. Anti-estrogen therapy or anti-estrogen therapy in combination with NSAIDs therapy can be applied clinically. These hormonal agents have low toxicity, low incidence of local adverse events, and cheap, they can be used as the first treatment protocol [15]. Compared with conventional doxorubicin, liposomal doxorubicin has more tolerable toxicity, including reduced cardiotoxicity. Therefore, many physicians and young patients prefer to use liposomal doxorubicin. Currently the recommended dose of liposomal doxorubicin is 50 mg/m2 intravenously every four weeks (one cycle). Lower dose with extended treatment cycle can also be used, which, however, does not make much sense since it can lead to an increased toxicity [2]. When patients suffer from both GIST and desmoid tumors at the same time, sorafenib may be the best drug of choice for the treatment [16].
Since the rarity of the stomach desmoid tumor, information about the prognosis of the disease is very limited. Regarding the desmoid tumors occurred at any parts of the body, a study on 426 patients with desmoid tumors indicates that the prognosis is closely correlated with the ages, size and location of tumors, and the margin of resection (R2 vs R1/R0). Patient with an age of greater than 37 years, tumor size of less than 70 mm, an R1 margin of resection, the tumor in the abdominal wall or abdomen has a better prognosis. R0 or R1 margin of resection does not make any statistical difference in the prognosis of patients [17]. However, another study indicates that the R0 margin of resection is very important for the prognosis of the patients [18]. Interestingly, recurrence in patients with R0 margin of resection and no recurrence in patients with R1 margin of resection have been frequently reported. In the case of our reported patient (No. 1 in Table 1), an R1 resection was performed and no recurrence was found after 63 months of follow-ups. Therefore, in patients with the gastric desmoid tumors, we recommend that an intraoperative frozen pathological examination should be performed in order to ensure an R0 margin of resection. However, if an extended resection will seriously affect the function of the stomach or result in greater damages, an R1 margin of resection should be performed. In addition, it has also been found that the location of tumor is the most important factor affecting the local recurrence [18]. All the eight patients with the gastric desmoid tumors summarized in Table 1 are sporadic, with no recurrence after follow-ups, indicating that the desmoid tumors occurred at the stomach may have a very good prognosis. Nevertheless, given the local recurrence rate of 13% reported for intra-abdominal sporadic desmoid tumors [19], a regular postoperative follow-up is still required.
In summary, the stomach desmoid tumor is a clinically rare soft tissue tumor, which has the clinical features of easy local recurrence without distant metastasis. The stomach desmoid tumor usually needs to be differentiated from GIST. The diagnosis can be made with routine pathological and immunohistochemical examinations. Complete tumor resection with a negative margin is the first-line treatment. The prognosis of the stomach desmoid tumor is good.
Disclosure of conflict of interest
None.
References
- 1.Fletcher C UK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. 69009, Lyon, France: IARC Press; 2002. World Health Organization Classification of Tumours. [Google Scholar]
- 2.Kasper B, Baumgarten C, Bonvalot S, Haas R, Haller F, Hohenberger P, Moreau G, van der Graaf WT, Gronchi A Desmoid Working Group. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise-a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer. 2015;51:127–136. doi: 10.1016/j.ejca.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C BJ, Hogendoorn PCW. WHO Classification of Tumours of Soft Tissue and Bone. 69009, Lyon, France: IARC Press; 2013. [Google Scholar]
- 4.Lee JC, Thomas JM, Phillips S, Fisher C, Moskovic E. Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol. 2006;186:247–254. doi: 10.2214/AJR.04.1674. [DOI] [PubMed] [Google Scholar]
- 5.Hartman TE, Berquist TH, Fetsch JF. MR imaging of extraabdominal desmoids: differentiation from other neoplasms. AJR Am J Roentgenol. 1992;158:581–585. doi: 10.2214/ajr.158.3.1738999. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Tang J, Luo Y. Sonographic diagnosis of fibromatosis. J Clin Ultrasound. 2008;36:330–334. doi: 10.1002/jcu.20483. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Chen H, Zhang S, Peng W. Intra-abdominal fibromatosis: differentiation from gastrointestinal stromal tumour based on biphasic contrast-enhanced CT findings. Clin Radiol. 2013;68:1133–1139. doi: 10.1016/j.crad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Long KB, Butrynski JE, Blank SD, Ebrahim KS, Dressel DM, Heinrich MC, Corless CL, Hornick JL. Primary extragastrointestinal stromal tumor of the pleura: report of a unique case with genetic confirmation. Am J Surg Pathol. 2010;34:907–912. doi: 10.1097/PAS.0b013e3181d9f18f. [DOI] [PubMed] [Google Scholar]
- 9.Faria SC, Iyer RB, Rashid A, Ellis L, Whitman GJ. Desmoid tumor of the small bowel and the mesentery. AJR Am J Roentgenol. 2004;183:118. doi: 10.2214/ajr.183.1.1830118. [DOI] [PubMed] [Google Scholar]
- 10.Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat. 2010;42:135–143. doi: 10.4143/crt.2010.42.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26:1296–1301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa N, Iseki H, Tsunozaki H, Hayashi M, Baba H, Matsuyama T, Uetake H, Sugihara K. Intra-abdominal desmoid tumor difficult to distinguish from a gastrointestinal stromal tumor: report of two cases. Surg Today. 2014;44:2174–2179. doi: 10.1007/s00595-013-0681-7. [DOI] [PubMed] [Google Scholar]
- 13.Dumont AG, Rink L, Godwin AK, Miettinen M, Joensuu H, Strosberg JR, Gronchi A, Corless CL, Goldstein D, Rubin BP, Maki RG, Lazar AJ, Lev D, Trent JC, von Mehren M. A nonrandom association of gastrointestinal stromal tumor (GIST) and desmoid tumor (deep fibromatosis): case series of 28 patients. Ann Oncol. 2012;23:1335–1340. doi: 10.1093/annonc/mdr442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–190. doi: 10.1093/annonc/mdg064. [DOI] [PubMed] [Google Scholar]
- 15.Skapek SX, Anderson JR, Hill DA, Henry D, Spunt SL, Meyer W, Kao S, Hoffer FA, Grier HE, Hawkins DS, Raney RB. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer. 2013;60:1108–1112. doi: 10.1002/pbc.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gounder MM, Lefkowitz RA, Keohan ML, D’Adamo DR, Hameed M, Antonescu CR, Singer S, Stout K, Ahn L, Maki RG. Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res. 2011;17:4082–4090. doi: 10.1158/1078-0432.CCR-10-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salas S, Dufresne A, Bui B, Blay JY, Terrier P, Ranchere-Vince D, Bonvalot S, Stoeckle E, Guillou L, Le Cesne A, Oberlin O, Brouste V, Coindre JM. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-andsee policy according to tumor presentation. J. Clin. Oncol. 2011;29:3553–3558. doi: 10.1200/JCO.2010.33.5489. [DOI] [PubMed] [Google Scholar]
- 18.Bonvalot S, Eldweny H, Haddad V, Rimareix F, Missenard G, Oberlin O, Vanel D, Terrier P, Blay JY, Le Cesne A, Le Péchoux C. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34:462–468. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson MJ, Fitzgerald JE, Thomas JM, Hayes AJ, Strauss DC. Surgical resection for nonfamilial adenomatous polyposis-related intraabdominal fibromatosis. Br J Surg. 2012;99:706–713. doi: 10.1002/bjs.8703. [DOI] [PubMed] [Google Scholar]
- 20.Koyluoglu G, Yildiz E, Koyuncu A, Atalar M. Management of an esophagogastric fibromatosis in a child: a case report. J Pediatr Surg. 2004;39:640–642. doi: 10.1016/j.jpedsurg.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Ure BM, Holschneider AM, Gharib M, Halsband H, Hinselmann D. Clinical aspects, classification and prognosis of 7 cases of pediatric fibromatosis. Z Kinderchir. 1988;43:27–30. doi: 10.1055/s-2008-1043407. [DOI] [PubMed] [Google Scholar]
- 22.Date K, Shima Y, Okabayashi T, Iwata J, Sumiyoshi T, Kozuki A. Desmoid tumor of the stomach. Endoscopy. 2015;47(Suppl 1 UCTN):E242–243. doi: 10.1055/s-0034-1391870. [DOI] [PubMed] [Google Scholar]


