Abstract
Tumor-associated macrophages (TAMs), the most important immune cells in tumor microenvironment, were reported to play a key role in cancer progression, but the correlation of TAMs and Kazakh esophageal squamous cell carcinoma (ESCC) was still not clear, so we sought to identify the function of TAMs in Kazakh ESCC clinicopathological and prognostic evaluation. CD68 as the TAMs marker, and immunohistochemistry (IHC) was used to quantify the TAMs infiltrated in tumor nest and stroma, the IHC staining was also used to evaluate the expression of MMP-9 in Kazakh ESCCs. The density of CD68-TAMs in ESCCs tumor nest and stromal, were significantly higher than those of CANs (P<0.05). The increasing number of CD68-positive TAMs in tumor nest and stromal were positively associated with tumors lymph node metastasis and clinical stage (P<0.05). The expression of MMP-9 in Kazakh ESCCs was higher than that of CAN tissues (P<0.05). Increased MMP-9 expression in ESCCs was significantly associated with lymph node metastasis and tumor clinical stage (P<0.05). Importantly, the number of CD68-positive TAMs in ESCCs was significantly correlated with the expression of MMP-9 (P<0.05). Furthermore, the survival analyses demonstrated that high-density of CD68-TAMs in tumor nest was positively related to the shorter overall survival time of patients (P<0.05). Increasing numbers of CD68-TAMs promote higher expression of MMP-9 and may play an important role in the occurrence and progression of Kazakh ESCCs, and which could be used as important prognostic markers for Kazakh ESCCs.
Keywords: Tumor associated macrophages, CD68, MMP-9, esophageal squamous cell carcinoma, Kazakh
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors in the world that threaten human life and health [1]. World Health Organization (WHO) released the data showed that the number of new EC cases in 2008 was 482,000, with an incidence of 7.0/1,000,000, ranking eighth in the global incidence of malignant tumors. The Kazak nationality, lived in Xinjiang, the northwest of China, has a high incidence rate of EC. Due to special geographical, ethnic, economic backwardness and other factors, the mortality rate of EC is up to 68.95/1,000,000, which is significantly higher than the average level of China (14.95/1,000,000) [2]. Although the diagnosis and treatment have improved, the 5-year survival rate of esophageal cancer is still very low due to tumor invasion, metastasis and recurrence.
Tumor microenvironment plays an important role in the tumorigenesis and tumor metastasis [3]. In addition to tumor cells, the tumor microenvironment also contains many mesenchymal cells, such as macrophages, lymphocytes, fibroblasts and so on [4], in which macrophages account for a large proportion. Macrophages infiltrated in tumor microenvironment (TME) are define as tumor-associated macrophages (TAMs), which play a critical role in regulating tumor growth and progression [5]. Studies have confirmed that increased density of TAMs has a closely correlated with the invasion, metastasis and poor prognosis of some cancers [6]. CD68 as a marker of macrophage in tumor microenvironment has been identified by many scholars. Some studies have confirmed the mechanism of TAMs promoting tumor cell invasion and metastasis is closely related to their secretion of vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP). Matrix metalloproteinase-9 (MMP-9) is a member of the MMP family, which is the enzyme for degradation of the extracellular matrix of the tumor, destroys the tissue barrier, plays a key role in tumor cell invasion and metastasis [7]. Some studies have showed that TAMs infiltration in tumor tissue produce many pro-angiogenic factors and express high levels of MMP-9, which promotes cancer cell invasion and metastasis. However, the precise role of TAMs in Kazakh EC has yet to be elucidated, we aimed to investigate whether TAMs correlate with MMP-9 expression, play a role in Kazakh EC clinicopathological and prognostic evaluation.
Material and methods
Ethics statement
All participants were recruited from the Yili Friendship Hospital in Xinjiang, China. Each participant provided written, informed consent before enrolling in this study. Protocols were approved by the institutional ethics committee of Yili Friendship Hospital in accordance with Helsinki Declaration ethical guidelines.
Patients and tissue specimens
A total of 200 paraffin-embedded human tissues were collected, including 100 Kazakh ESCC tissues and 100 Kazakh cancer adjacent normal tissues (CANs) (collected from 2008 to 2014) in this study. Mean age of the Patients (70 males and 30 females) was 58.47±7.8 years. According to the World Health Organization histological tumor classification criteria [8], there were 29 cases of well-differentiated, 47 cases of moderately differentiated and 24 cases of poorly differentiated ESCCs. From these, 35 and 65 cases exhibited invasive depths of T1-T2 and T3-T4, respectively. There were 51 cases with lymph node metastasis, 62 cases in clinical stages I-II, and 38 cases in clinical stages III-IV. All cases were confirmed and diagnosed as squamous cell carcinoma by postoperative pathological examination. None of the patients had received any preoperative radiation-chemotherapy. A total of 100 patients had at least a 2-year follow-up after treatment, and the time for loss of visitor were identified by telephone follow-up and from outpatient care records.
Immunohistochemical staining
Paraffin-embedded, formalin fixed tissues were cut into 4-µm thick sections, deparaffinized in xylene and rehydrated in ethanol and water. The CD68 (Zhongshan Goldenbridge Biotechnology Co. LTD. Beijing, China) and MMP-9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were chosen in this study, the Immunohistochemistry method as described previously [9].
Immunoreactivity evaluation
The positive expression of CD68-TAMs in the cytoplasm of macrophages appeared as yellowish-brown to brown granules. The five most representative hot spots were selected from low-power fields (LPFs 100×) per slide using an Olympus BX51TF microscope (Olympus, Japan). Tumor nest and stroma areas were defined and numbers of CD68-TAMs were counted in high-power fields (HPFs, 400×) by two pathologists who were blinded to the clinical patient data. When cell counts differed by more than 10 cells per HPF, samples would be counted again a week later until recording differences were below 10 counts. The mean number of macrophages per HPF across five hot spots for every sample (tumor nest and tumor stroma) was defined as the CD68-TAMs density.
MMP-9 positive expression was located in the cytoplasm of the cell, which was brown yellow particles. Each slice was then selected 5 high power microscope (400×) image acquisition. The results were determined according to the percentage of positive cells and the depth of positive staining. Scoring criteria: IHC staining slides were scored as positive or negative by percentage and intensity of positive cells, where the scoring percentage of positively stained cells was as follows: 0≤5%, 1 = 6%-25%, 2 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%; Staining intensity scoring was: 0 = absent, 1 = weak, 2 = moderate, and 3 = strong. A final score was based on multiplying both scores from individual slides [10,11], where: 0-1 was negative (-), 2-3 was weak positive (1+), 4-6 was moderate positive (2+), and 8-12 was strong positive (3+).
Statistical analysis
SPSS version 17.0 was used for all statistical analyses. For comparisons of independent samples, t-test and one-way analysis of variance (ANOVA) were used to evaluate the density of CD68-positive TAM correlations between ESCCs and CANs, and between the density of CD68-positive TAM and different clinicopathological features. The X2 test was adopted for analysis of correlations between MMP-9 expression and ESCC clinical pathological features. Spearman’s rank correlation method was used to evaluate the correlations between the CD68 and MMP-9. Prognosis were estimated by the Kaplan Meier, univariate and Cox proportional hazard regression analysis method. P-values were calculated using the Epi-Info program, and p-values<0.05 were considered significant.
Results
The distribution of CD68-positive TAMs in Kazakh ESCC and CAN tissues
In this study, we used CD68 as marker of TAMs to assess the distribution of TAMs in Kazakh ESCCs and CANs. We found TAMs mainly distributed in the tumor stroma, little TAMs distributed in tumor nest (Figure 1). The density of TAMs in Kazakh ESCC tumor nests (approximately 9/HPF, 2-34) and stroma (approximately 43/HPF, 6-136) were significantly higher compared to CAN epithelial (approximately 2/HPF, 0-7) and stroma (approximately 9/HPF, 3-35, Figure 2).
Figure 1.
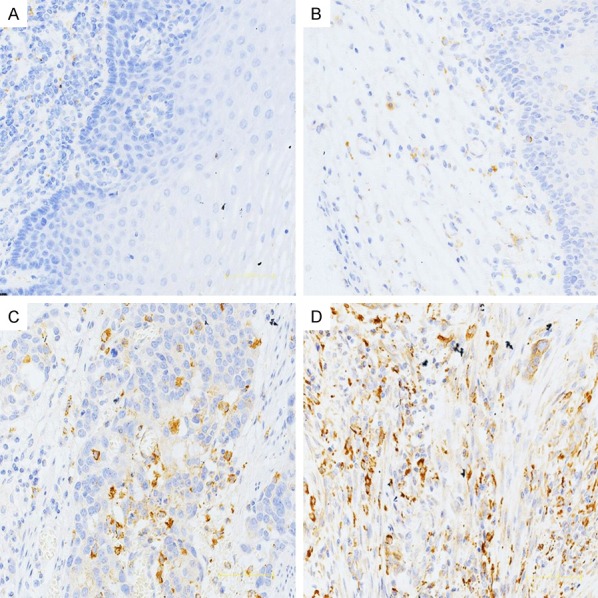
The distribution of CD68-positive TAMs in Kazakh ESCC tumor nest tissue, CAN epithelial, and CAN stroma. Immunohistochemical staining of CD68, which was used to mark TAMs and to evaluate the density of TAMs in ESCC and CAN tissues. A and B. Show the distribution of TAMs in CAN epithelial and stroma. A Small number of CD68-positive TAMs appear in CAN tissues. C and D. Show the distribution of TAMs in ESCC tumor nest and stromal tissues, demonstrating that CD68 reveals diffuse staining of membranes and cytoplasm of TAMs, and showing the high density of TAMs located in ESCC tissues (especially in tumor stroma).
Figure 2.
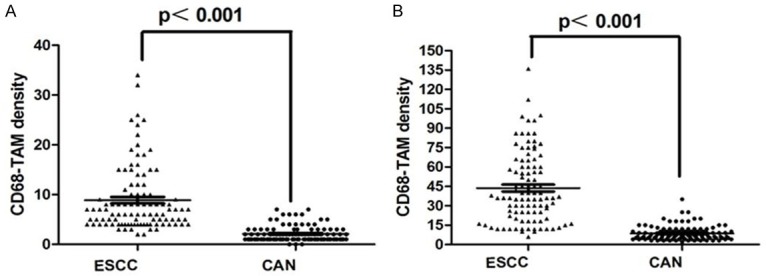
Comparison with the density of CD68 positive TAM in ESCC and CAN. A. The density of CD68 positive TAM was significantly higher in ESCC nest than that in CAN epithelium (P<0.001). B. The density of CD68 positive TAM was significantly higher in ESCC stroma than that in CAN stroma (P<0.001).
The density of CD68-positive TAMs in tumor nest and stroma were close related with clinicopathological factors of Kazakh ESCCs
We investigated whether CD68-positive TAMs have association with clinicopathological factors of Kazakh ESCCs, found that the density of CD68-positive TAMs in tumor nests were significantly associated with nodal metastasis and later clinical stages (all P<0.05). Similar trends were more obvious appeared in tumor stroma (all P<0.001). Also we found the density of CD68-positive TAMs in tumor stroma were significantly associated with tumor location (P<0.05). No significant correlations were found between TAMs distribution and other parameters (P>0.05 Table 1).
Table 1.
Correlation between density of CD68-positive TAMs and clinicopathological parameters in Kazakh ESCCs tumor nest and stroma
| Variable | Cases (N) | Tumor Nest Mean ± s.d | P | Tumor stroma Mean ± s.d | P |
|---|---|---|---|---|---|
| Age (y) | |||||
| ≤58 | 53 | 8.91±7.19 | 0.955 | 44.32±30.87 | 0.823 |
| >58 | 47 | 8.83±5.99 | 43.09±23.00 | ||
| Gender | |||||
| Male | 70 | 9.14±7.33 | 0.532 | 43.51±28.41 | 0.900 |
| Female | 30 | 8.23±4.58 | 44.27±25.07 | ||
| Tumor location | |||||
| Upper | 2 | 12±9.89 | 0.062 | 42.50±10.61 | 0.018* |
| Middle | 74 | 14.20±13.30 | 48.16±28.35 | ||
| Lower | 24 | 15.82±10.45 | 30.21±20.09 | ||
| Histologic grade | |||||
| Well | 29 | 9.00±6.01 | 0.794 | 45.14±29.54 | 0.237 |
| Moderate | 48 | 9.50±7.58 | 46.92±25.66 | ||
| Poor | 23 | 7.39±5.04 | 35.35±27.30 | ||
| Depth of invasion | |||||
| T1-T2 | 36 | 7.19±5.25 | 0.057 | 40.11±27.22 | 0.322 |
| T3-T4 | 64 | 9.81±7.14 | 45.78±27.39 | ||
| Nodal status | |||||
| pN- | 50 | 7.18±5.09 | 0.010* | 33.32±21.05 | <0.001* |
| pN+ | 50 | 10.56±7.53 | 54.16±29.05 | ||
| Clinical stage | |||||
| I-II | 62 | 7.19±5.20 | 0.001* | 34.97±22.29 | <0.001* |
| III-IV | 38 | 11.61±7.77 | 58.05±28.98 |
Note: pN-: no lymph node metastasis; pN+: node metastasis.
P<0.05.
Expression of MMP-9 in Kazakh ESCCs and CANs, and its relationship with ESCC clinicopathological parameters
Some studies have showed that TAM could produce MMP-9, promoting cancer cell invasion and metastasis. So we investigated a correlation between MMP-9 and TAMs in occurrence and progression of Kazakh ESCC. As shown in Figure 3, the expression of MMP-9 mainly focused on tumor stromal cells (located in cell membranes and cytoplasm), some also focused on tumor cells. Only 7.0% of ESCC cases were negative for MMP-9 antibody staining, and most cases showed strong staining (3+). MMP-9 antibody negative staining was 55.0% in CAN cases and only a few examples of CAN tissues showed strong staining (3+). We compared three categories of MMP-9 positive staining combinations (1+, 2+/3+, and 1+/2+/3+) to MMP-9 negative (0) staining (Table 2). The MMP-9 positive rate (1+/2+/3+) in ESCCs was higher than in CAN tissues (97.0% vs 45.0%, P<0.001). Differences in MMP-9 positive rates from ESCC to CAN tissues were more prominent in strong staining cases (2+/3+, 59.0% vs 19.0%, P<0.001).
Figure 3.
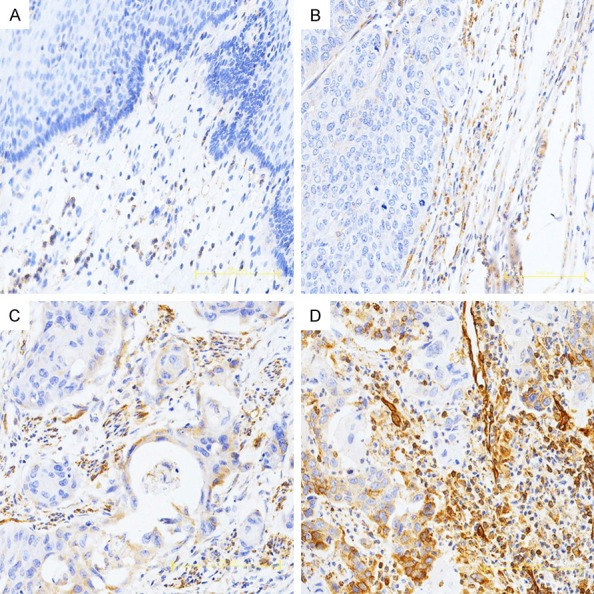
Immunohistochemical staining of MMP-9 in Kazakh ESCC and CAN tissues. MMP-9 staining is primarily observed in tumor stroma (cell membranes and cytoplasm); some ESCC cells also show staining. A. Negative MMP-9 staining is shown in CAN tissues (scored as 0). B. Weak MMP-9 staining is shown in Kazakh ESCC tissues (scored as 1). C and D. Show moderate and strong MMP-9 staining in Kazakh ESCC tissues, respectively (scored as 2 and 3, respectively).
Table 2.
The expression of MMP-9 in Kazakh ESCC and CAN tissues
| Characteristics | Cases (N) | Negative 0 (%) | Positive combination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1+ (%) | X2 | P | 2+3+ (%) | X2 | P | 1+2+3+ | X2 | P | |||
| ESCCs | 100 | 7 (7.0%) | 34 (34.0%) | 26.141 | <0.001* | 59 (59.0%) | 54.851 | <0.001* | 93 (93.0%) | 51.636 | <0.001* |
| CANs | 100 | 55 (55.0%) | 26 (26.0%) | 19 (19.0%) | 45 (45.0%) | ||||||
Note: ESCCs: Esophageal squamous cell carcinoma tussies. CANs: Cancer adjacent normal tissues.
P<0.05.
For comparison of MMP-9 expression to Kazakh ESCC clinical parameters, we divided ESCC cases into two categories according to MMP-9 expression level: low expression (0/1+) and high expression (2+/3+). We found the expression level of MMP-9 was significantly higher in nodal status (pN+ vs pN- = 78.0% vs 40.0%, P<0.001), and were clearly present in advanced ESCC stages (III-IV vs I-II = 81.6% vs 45.2%, P<0.001, Table 3).
Table 3.
Correlation between expression of MMP-9 and clinicopathological parameters in Kazakh ESCCs
| Variable | Cases (N) | MMP9 low expression | MMP9 high expression | X2 | P |
|---|---|---|---|---|---|
|
|
|
||||
| 0/1+ (%) | 2+/3+ (%) | ||||
| Age (y) | |||||
| ≤58 | 53 | 25 (47.2%) | 28 (52.8%) | 1.273 | 0.259 |
| >58 | 47 | 19 (40.4%) | 28 (59.6%) | ||
| Gender | |||||
| Male | 70 | 29 (41.4%) | 41 (58.6%) | 0.333 | 0.564 |
| Femle | 30 | 12 (40.0%) | 18 (60.0%) | ||
| Tumor location | |||||
| Upper | 2 | 1 (50.0%) | 1 (50.0%) | / | 0.116 |
| Middle | 74 | 28 (37.8%) | 46 (62.2%) | ||
| Lower | 24 | 13 (54.2%) | 11 (45.8%) | ||
| Histologic grade | |||||
| Well | 29 | 5 (17.2%) | 24 (82.8%) | 1.009 | 0.605 |
| Moderate | 47 | 18 (38.3%) | 29 (61.7%) | ||
| Poor | 24 | 8 (33.3%) | 16 (66.7%) | ||
| Depth of invasion | |||||
| T1-T2 | 36 | 17 (47.2%) | 19 (52.8%) | 0.543 | 0.461 |
| T3-T4 | 64 | 24 (37.5%) | 40 (62.5%) | ||
| Nodal status | |||||
| pN- | 50 | 30 (60.0%) | 20 (40.0%) | 13.394 | <0.001* |
| pN+ | 50 | 11 (22.0%) | 39 (78.0%) | ||
| Clinical stage | |||||
| I-II | 62 | 34 (54.8%) | 28 (45.2%) | 11.455 | <0.001* |
| III-IV | 38 | 7 (18.4%) | 31 (81.6%) |
Note: pN-: no lymph node metastasis; pN+: node metastasis.
P<0.05.
Correlation between the density of CD68-positive TAMs and MMP-9 expression in Kazakh ESCCs and CANs
To found the role of TAMs in tumor invasion and metastasis, we investigate the relationship between the CD68 and MMP-9. The density of CD68-positive TAMs, either in the tumor nest or tumor stroma, was positively associated with MMP-9 expression, As shown in Table 4, the levels of CD68 expression both in the tumor nest or stroma cells were positively associated with the expression of MMP-9 (r = 0.359, P<0.001 and r = 0.373, P<0.001, respectively). However in CAN tissues, CD68-positive TAMs in stroma or epithelium were not positively associated with the expression of MMP-9. We can also found strong positive correlations between the density of TAMs in stroma and in the tumor nest or epithelium (in ESCC, r = 0.801, P<0.001 and in CAN, r = 0.666, P<0.001, respectively).
Table 4.
Cross correlation analyses reveal strong relationships among density of CD68 in tumor nest, tumor stroma and expression of MMP-9 in Kazakh ESCCs and CANs
| CD68 density in tumor nest | CD68 density in tumor stroma | MMP-9 | |
|---|---|---|---|
| ESCCs | |||
| CD68 density in tumor nest | 1 | ||
| CD68 density in tumor stroma | 0.801* | 1 | |
| MMP-9 | 0.359* | 0.373* | 1 |
| CANs | |||
| CD68 density in epithelium | 1 | ||
| CD68 density in stroma | 0.666* | 1 | |
| MMP-9 | -0.008 | -0.049 | 1 |
Note: ESCCs: Esophageal squamous cell carcinoma tussies. CANs: Cancer adjacent normal tissues. The numbers shown in the table are correlation coefficient r values. Spearman rank correlation analysis was used.
P<0.05.
Correlation of CD68-positive TAMs with patient survival after surgery
Kaplan-Meier method was used to predict the prognostic value of TAMs for Kazakh ESCCs, All ESCC patients who received curative surgery from 2008 to 2014 and were followed up for 1-7 years. We found the density of CD68-positive TAMs (P = 0.017, Figure 4) in tumor nest was significantly associated with poor prognosis of Kazakh ESCCs. Similarly, the tendency was also observed in tumor stroma, but the relation has no statistical significant (P = 0.078, Figure 4). Multivariate Cox proportional hazard egression analysis was performed for Kazakh ESCCs prognosis predicts. As shown in Table 5, Univariate analysis showed that the increased density of CD68-TAMs infiltrated in tumor nest, nodal metastasis, later clinical stage were poor prognosis factors for patients of Kazakh ESCCs. But in multivariate analysis, there was no independent prognosis factor for patients of Kazakh ESCCs.
Figure 4.
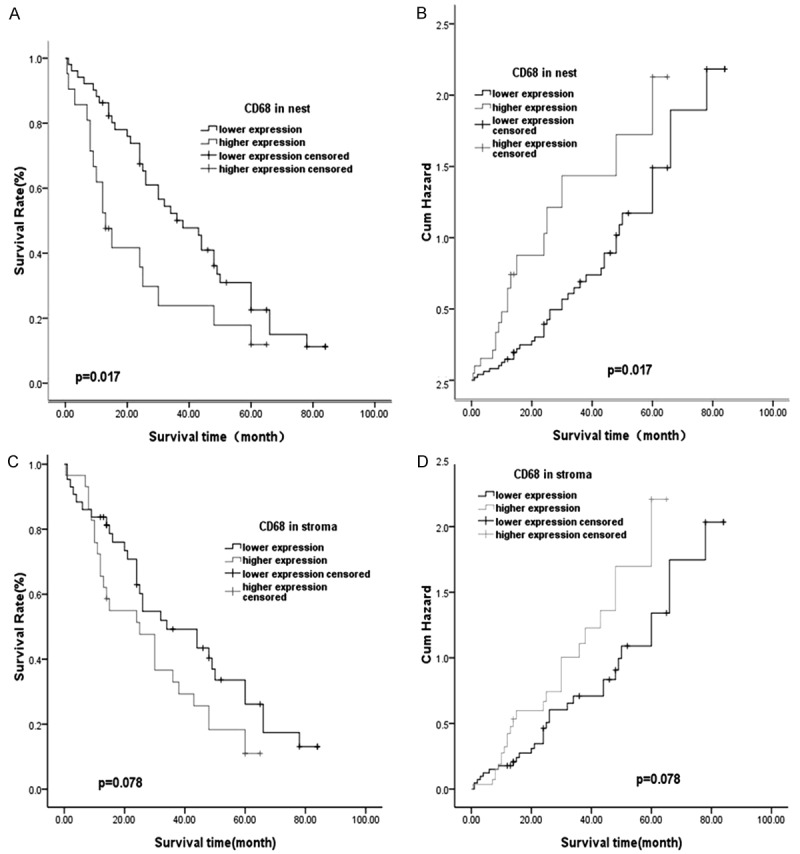
Kaplan-Meier survival curves showing 100 ESCC patients with expression of CD68. A. Patients with high expression of CD68 in nest experienced a significantly shorter postoperative survival time than those with low CD68 levels (P = 0.017). B. Patients with CD68 over expression had a higher risk of death than those with lower CD68 levels (P = 0.017). C. Patients with high expression of CD68 in stroma has negative relationship than those with low CD68 levels (P = 0.078). D. Patients with CD68 over expression had no risk of death than those with lower CD68 levels (P = 0.078).
Table 5.
Univariate and multivariate survival analyses of clinicopathological characteristics and CD68 macrophage with overall survival for Kazakh esophageal carcinoma (ESCC)
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (≥58 y) | 0.611 | 0.359 | 1.041 | 0.070 | 0.467 | 0.259 | 0.841 | 0.074 |
| Sex (female) | 1.202 | 0.674 | 2.144 | 0.533 | 1.313 | 0.686 | 2.512 | 0.411 |
| Differentiation (moderate/poor) | 1.536/1.022 | 0.758/0.526 | 3.112/1.985 | 0.234/0.948 | 2.513/1.432 | 1.115/0.658 | 5.661/3.117 | 0.026/0.365 |
| L/N metastasis (positive) | 1.956 | 1.139 | 3.360 | 0.015* | 1.108 | 0.423 | 2.902 | 0.834 |
| Clinical stage (III + IV) | 2.093 | 1.226 | 3.575 | 0.007* | 1.894 | 0.746 | 4.812 | 0.179 |
| Depth of invasion (T1-T2/T3-T4) | 1.001 | 0.579 | 1.733 | 0.996 | 0.216 | 0.343 | 1.273 | 0.661 |
| Tumor location (Middle/Lower) | 2.283/1.292 | 0.282/0.606 | 18.51/2.753 | 0.439/0.507 | 6.661/1.271 | 0.619/0.560 | 71.632/2.888 | 0.118/0.566 |
| CD68+ macrophages counts in tumor nest (High) | 1.898 | 1.060 | 3.399 | 0.031* | 1.849 | 0.870 | 3.929 | 0.110 |
| CD68+ macrophages counts in tumor stroma (High) | 1.544 | 0.899 | 2.652 | 0.115 | 0.900 | 0.448 | 1.808 | 0.767 |
Note: L/N, lymph node; CI, confidence interval; HR, hazard radio.
Significant difference that 95% CI was not including 1.
Discussion
Macrophage is a highly heterogeneous cell population, and it exhibit unique phenotype and function in vivo complex microenvironment [12]. TAMs are the major tumor infiltrating leukocytes in human tumors that play an important role in the development of tumor. Clinical studies have also demonstrated a strong correlation between TAMs infiltration and poor prognosis in some human tumors [13-16]. However, the interaction between TAMs and the occurrence and development of esophageal cancer has not been elucidated clearly. In this study we found CD68 positive macrophages mainly located in tumor stroma of Kazakh ESCCs, and the density of CD68 positive TAMs in esophageal cancer nests and stroma was significantly higher than in normal tissues. Such result was similar to the report in esophageal carcinoma by Xie et al, and they showed that the mean density of TAMs infiltrated in esophageal carcinoma tissues was significantly higher than that in normal adjacent tissue [17]. Moreover, increasing number of CD68 positive TAMs in cancer nests and stroma was a significant correlation with lymph node metastasis and later clinical stages of Kazakh ESCCs. The result was similar with those reported in papillary thyroid carcinoma and melanoma [18,19]. At the same time, we found that the density of CD68 positive TAMs was significantly higher in ESCC nest and stroma than that in CAN stroma and epithelium. These results were consistent with previous findings that CD68 molecules in malignant tumors can be used as a marker to identify tumor-associated macrophages [20,21].
TAMs can promote the progression of tumor through some mechanisms. It was reported that TAMs could produce some important molecules which can promote angiogenesis, tissue remodeling and immunosuppression in some tumors [22-24]. According to previous studies we know that TAMs can release MMP that promote cancer invasion and metastasis [5]. Because of the change of cell matrix, it is beneficial to the migration of tumor cells, which is the key role to the infiltration and distant metastasis of tumor cells [25-28]. MMP-9 is one of the important members of MMP family, which overexpression was related to the occurrence and progression of tumor [29]. In order to further explore the interaction between MMP-9 and TAMs, we first evaluated specific expression of MMP-9 in Kazakh esophageal squamous cell carcinoma, then found that MMP-9 was mainly expressed in tumor stroma cells, such as macrophages. In addition, we found that the expression of MMP-9 in cancer tissues was significantly higher than that in normal tissues. Similar results were also reported in lung cancer, in which MMP9 was mainly expressed in tumor stroma cells or tumor cells, whereas less expressed in lung normal tissues stroma cells or epithelium [30]. According to the clinical parameters analysis, we found the high expression of MMP-9 in Kazakh esophageal carcinoma was significantly positive correlated with lymph node metastasis and later clinical stage, these results were consistent with previous studies in breast cancer and laryngeal squamous cell carcinoma (LSCC) [31,32].
In order to explore the possible mechanism of TAMs promoting tumor development, we analyzed the correlation between the number of CD68 positive TAMs and the expression of MMP-9, found that increasing number of CD68 positive TAMs was close relationship with high expression of MMP-9 (P<0.05), and both of which closely linked to tumor lymphatic metastasis and later clinical stage, suggested that the infiltration of TAMs in esophageal squamous cell carcinoma may promote the invasion and metastasis of esophageal squamous cell carcinoma by secreting MMP-9.
Some studies found increasing number of TAMs infiltrated in tumor nest and stroma was associated with tumor distant metastasis and poor prognosis [33]. In our study, the results revealed that the high density of CD68 positive TAMs in cancer nests was associated with shorter overall survival, but not CD68 positive TAMs in cancer stroma, which suggest the increasing number of CD68 positive TAMs, especially in cancer nests, has a close correlation with the occurrence, progression and poor prognosis of Kazakh ESCCs.
There are still some deficiencies in this study. Firstly, the CD68 molecule as a marker of macrophages has been widely accepted by scholars, and which could not only mark M1 type macrophages, but also mark M2 type macrophages, however, as this widespread marker and lack of specificity, some studies thought it may be not completely suitable to as a labeled of TAMs. Secondly, the criteria for counting macrophages in cancer tissue specimens is subject to some level of error. Related research finding that counted CD68-positive TAMs stratified as those localized to cancer stroma and those in contact with cancer cells or penetrating into a cancer nest was an independent prognostic factor for survival in ESCC patients [34]. So the counts of CD68-positive TAMs are important, the use of image analysis software might be one way to reduce subjective error. In order to better evaluate the density of TAM, The mean number of macrophages across five hot spots for every sample (tumor nest and tumor stroma) was defined as the CD68-TAMs density by two pathologists likely kept error to a minimum, and may prove to be the preferred method for TAM density evaluation.
Conclusion
The density of TAMs in Kazakh ESCCs tumor stroma and nests were significantly higher than that of CANs, and the increased number of CD68-positive TAMs in tumor nest and tumor stroma correlation with high expression of MMP-9, which predicts the high aggressive of Kazakh ESCCs (lymph node metastasis and later clinical stage progression), and which could be used as important prognostic markers for Kazakh ESCCs.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.81460363, 81760428 and 81560399).
Disclosure of conflict of interest
None.
References
- 1.Wang Y, Wu J, Guo W, Sun Q, Chen X, Zhang W, Dong Z, Zhao G. α-Solanine modulates the radiosensitivity of esophageal cancer cells by inducing MicroRNA 138 expression. Cell Physiol Biochem. 2016;39:996–1010. doi: 10.1159/000447807. [DOI] [PubMed] [Google Scholar]
- 2.Ayxiam H, Ma H, Ilyar S, Zhang LW, Ablizi A, Batur M, Lu XM. [Metabonomic variation of esophageal cancer within different ethnic groups in Xinjiang, China] . Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43:591–6. [PubMed] [Google Scholar]
- 3.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumorassociated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kankuri E, Cholujova D, Comajova M, Vaheri A, Bizik J. Induction of hepatocyte growth factor/scatter factor by fibroblast clustering directly promotes tumor cell invasiveness. Cancer Res. 2005;65:9914–22. doi: 10.1158/0008-5472.CAN-05-1559. [DOI] [PubMed] [Google Scholar]
- 5.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 6.Jakovic LR, Mihaljevic BS, Jovanovic MD, Bogdanovic AD, Andjelic BM, Bumbasirevic VZ. Prognostic significance of Bcl-2, tumor-associated macrophages, and total neoplastic and inflammatory lymph node involvement in advanced stage classical hodgkin’s lymphoma. Onkologie. 2012;35:733–9. doi: 10.1159/000343664. [DOI] [PubMed] [Google Scholar]
- 7.Lamar JM, Pumiglia KM, Dipersio CM. An immortalization-dependent switch in integrin function upregulates MMP-9 to enhance tumor cell invasion. Cancer Res. 2008;68:7371–9. doi: 10.1158/0008-5472.CAN-08-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li ZS, Li Q. The latest 2010 WHO classification of tumors of digestive system. Zhonghua Bing Li Xue Za Zhi. 2011;40:351–4. [PubMed] [Google Scholar]
- 9.Hu JM, Chang AM, Chen YZ, Yuan XL, Li F. Regulatory role of miR-203 in occurrence and progression of Kazakh esophageal squamous cell carcinoma. Sci Rep. 2016;6:23780. doi: 10.1038/srep23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Yang L, Chen YZ, Liu CX, Li SG, Cui XB, Zou H, Pang LJ, Zhao J, Qi Y, Liang WH, Yuan XL, Li F. The increased number of tumor-associated macrophage is associated with overexpression of VEGF-C, plays an important role in Kazakh ESCC invasion and metastasis. Exp Mol Pathol. 2017;102:15–21. doi: 10.1016/j.yexmp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, Cui XB, Yang L, Zhao J, Shen XH, Jiang JF, Liang WH, Yuan XL, Li F. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squmous cell carcinoma. Oncotarget. 2017;8:21526–21538. doi: 10.18632/oncotarget.15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 13.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, Ostrowski MC. An Ets2-specific transcriptional program in tumor associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–33. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Wu D, Zhao L, Huang L, Chen G, Shen G, Huang J, Chai Y. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 2016;7:40451–40460. doi: 10.18632/oncotarget.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu HY, Wang JS, Duan XY. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2016;9:3975–83. doi: 10.2147/OTT.S103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigeoka M, Urakawa N, Nakamura T, Nishio M, Watajima T, Kuroda D, Komori T, Kakeji Y, Semba S, Yokozaki H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1112–9. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie R, Dong B, Pang H, et al. Tumor-associated macrophages in esophageal carcinoma and its impact on prognosis. Chinese Journal of Clinical Oncology. 2011;38:83–86. [Google Scholar]
- 18.Wei Q, Fang W, Ye L, Shen LY, Zhang XF, Fei XC, Chen X, Wang WQ, Li XY, Xiao JC, Ning G. Density of tumor associated macrophage correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012;22:905–10. doi: 10.1089/thy.2011.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee onCancer stage I/II melanoma. J. Clin. Oncol. 2009;27:3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 20.Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Suefuji N, Sugita Y, Takeya M, Ohshima K. Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T-cell lymphoma (AITL) Pathol Int. 2010;60:278–283. doi: 10.1111/j.1440-1827.2010.02514.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, Li Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One. 2013;8:e76147. doi: 10.1371/journal.pone.0076147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–64. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, Steidl C, Gordon LI, Horning SJ, Gascoyne RD. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280–7. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, Ostrowski MC. An Ets2-specific transcriptional program in tumor associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–33. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roeb E, Matern S. Metalloproteinases: promoters of tumor invasion and metastasis - A review with focus on gastrointestinal tumors. Z Gastroenterol. 2001;39:807–13. doi: 10.1055/s-2001-17197. [DOI] [PubMed] [Google Scholar]
- 26.Szabo KA, Singh G. Modulation of monocyte matrix metalloproteinase-2 by breast adenocarcinoma cells. Breast Cancer Res. 2005;7:R661–8. doi: 10.1186/bcr1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry. 2003;68:711–7. doi: 10.1023/a:1025051214001. [DOI] [PubMed] [Google Scholar]
- 28.Bargostavan MH, Eslami G, Esfandiari N, Shams Shahemabadi A. MMP9 promoter polymorphism (-1562 C/T) does not affect the serum levels of soluble MICB and MICA in breast cancer. Iran J Immunol. 2016;13:45–53. [PubMed] [Google Scholar]
- 29.Liu Y, Liu H, Luo X, Deng J, Pan Y, Liang H. Overexpression of SMYD3 and matrix metalloproteinase-9 are associated with poor prognosis of patients with gastric cancer. Tumor Biol. 2015;36:4377–86. doi: 10.1007/s13277-015-3077-z. [DOI] [PubMed] [Google Scholar]
- 30.Schütz A, Schneidenbach D, Aust G, Tannapfel A, Steinert M, Wittekind C. Differential expression and activity status of MMP-1, MMP-2 and MMP-9 in tumor and stromal cells of squamous cell carcinomas of the lung. Tumour Biol. 2002;23:179–84. doi: 10.1159/000064034. [DOI] [PubMed] [Google Scholar]
- 31.Wu QW, Yang QM, Huang YF, She HQ, Liang J, Yang QL, Zhang ZM. Expression and clinical significance of matrix metalloproteinase-9 in lymphatic invasiveness and metastasis of breast cancer. PLoS One. 2014;9:e97804. doi: 10.1371/journal.pone.0097804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin G, Li W, Sun X, Qin G, Li W, Sun X. Expression and significance of CD44v6 and MMP-9 in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19:688–91. [PubMed] [Google Scholar]
- 33.Lu X, Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc Natl Acad Sci U S A. 2009;106:9385–90. doi: 10.1073/pnas.0900108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo SJ, Lin DM, Li J, Liu RZ, Zhou CX, Wang DM, Ma WB, Zhang YH, Zhang SR. Tumor-associated macrophages and CD3-zeta expression of tumor-infiltrating lymphocytes in human esophageal squamous-cell carcinoma. Dis Esophagus. 2007;20:107–16. doi: 10.1111/j.1442-2050.2007.00655.x. [DOI] [PubMed] [Google Scholar]


