Abstract
Circulating microRNAs (miRNAs) have been demonstrated to be potential biomarkers in various cancers including head and neck squamous cell carcinoma (HNSCC). The aim of this study was to assess the diagnostic and prognostic significance of serum miR-138 in HNSCC. Quantitative reverse-transcription PCR (qRT-PCR) was used to measure serum miR-138 levels in 113 HNSCC cases and 60 controls. The results showed that serum miR-138 expression was remarkably down-regulated in HNSCC patients compared to healthy volunteers. Moreover, serum miR-138 levels in 32 patients after receiving surgical treatment were significantly increased. Also, the receiver operating characteristic (ROC) curve analysis revealed that serum miR-138 could discriminate HNSCC patients from controls with high accuracy. Furthermore, a positive correlation was observed between decreased serum miR-138 and worse clinical outcome, as well as shorter survival. Then serum miR-138 was confirmed to be an independent prognostic indicator for HNSCC. Our results demonstrated that serum miR-138 might be a potential biomarker for detection and prognosis prediction of HNSCC.
Keywords: Head and neck squamous cell carcinoma, serum mir-138, prognosis, biomarker
Introduction
Head and neck squamous cell carcinoma (HNSCC), which mainly occurs in the oral cavity, the pharynx and the larynx, is the sixth most common cause of cancer mortality around the world [1,2]. Annually, an estimated 600,000 emerging cases and 350,000 deaths with HNSCC are reported worldwide. Despite of considerable advances in the surgical and medical treatments for HNSCC, the survival rate in patients with HNSCC has not improved throughout the last three decades [3,4]. Therefore, identifying new effective indicators for early detection of this malignancy will be of great value.
MicroRNAs (miRNAs) are a group of non-coding small (19-25 nucleotides in length) RNAs that post-transcriptionally regulate the expression of target messenger RNAs (mRNAs), resulting in mRNA degradation or translational repression [5]. miRNAs have been demonstrated to regulate multiple cellular processes such as differentiation, proliferation, migration and apoptosis. MiRNAs may function as either oncogenes or tumor suppressors under certain conditions [6]. MiRNAs are very stable in blood, and circulating miRNAs are used as potential biomarkers for HNSCC. For instance, increased serum miR-186-5p levels were significantly correlated with shorter overall survival and progression free survival, indicating that miR-186-5p played an oncogenic role in HNSCC [7]. Similarly, serum miR-99a levels were greatly elevated in HNSCC patients after receiving surgery, suggesting that serum miR-99a could serve as biomarker for monitoring the therapeutic responses of HNSCC [8].
MiR-138, a family of microRNA precursors, has been extensively studied and found to act as a tumor suppressor in various tumor types, such as HNSCC [9], non-small cell lung cancer [11], colorectal cancer [12], ovarian cancer [13], osteosarcoma [14], hepatocellular carcinoma [15], gallbladder carcinoma [16], cervical cancer [17] and bladder cancer [18]. However, the expression level and clinical value of serum miR-138, especially with respect to the diagnosis and prognosis of HNSCC remains poorly known. Therefore, the aim of this study was to investigate the potential clinical significance of serum miR-138 in HNSCC.
Materials and methods
Ethics statement
This study was conducted with the approval of, and all participants provided written informed consents.
HNSCC patients and blood samples
In this study, a total of 113 subjects diagnosed with HNSCC were enrolled. Patients were excluded if they had received chemotherapy, radiotherapy or other treatments before surgery. Tumors were staged according to the criteria of the Union for International Cancer Control (UICC) Staging System. Detailed clinical variables, including age, gender, tobacco consumption, alcohol consumption, primary tumor sites, pT stage, pN stage, differentiation and clinical stage, were listed in Table 1. Overall survival (OAS) was defined as the time from diagnosis to the date of death or last follow-up. Disease-free survival (DFS) was defined as the time from diagnosis to the date of recurrence or death or last follow-up. Sixty healthy individuals were recruited as controls.
Table 1.
Association between serum miR-138 levels and clinical features in 113 HNSCC patients
| Clinical parameters | Cases (N=113) | MiR-138 levels | P value | |
|---|---|---|---|---|
|
| ||||
| High (n=57) | Low (n=56) | |||
| Age | 0.7838 | |||
| ≤61 | 62 | 32 | 30 | |
| >61 | 51 | 25 | 26 | |
| Gender | 0.5201 | |||
| Men | 101 | 52 | 49 | |
| Women | 12 | 5 | 7 | |
| Tobacco consumption | 0.2667 | |||
| Yes | 40 | 23 | 17 | |
| No | 73 | 34 | 39 | |
| Alcohol consumption | 0.1420 | |||
| Yes | 44 | 26 | 18 | |
| No | 69 | 31 | 38 | |
| Primary tumor sites | 0.2983 | |||
| Oral cavity/oropharynx | 59 | 27 | 32 | |
| Others | 54 | 30 | 24 | |
| pT stage | 0.1091 | |||
| T0-2 | 55 | 32 | 23 | |
| T3-4 | 58 | 25 | 33 | |
| Differentiation | 0.0467* | |||
| Well | 34 | 22 | 12 | |
| Moderate/poor | 79 | 35 | 44 | |
| pN stage | 0.0994 | |||
| N0-1 | 30 | 19 | 11 | |
| N2-3 | 83 | 38 | 45 | |
| Clinical stage | 0.0046* | |||
| I/II | 43 | 29 | 14 | |
| III/IV | 70 | 28 | 42 | |
P<0.05 was considered to be statistically significant.
Venous blood (~5 mL) was taken from each participant prior to any treatment. Thirty-two cases among all 113 patients underwent tumor resection, and 32 plasma samples from these patients six months after surgical resection were collected. All blood specimens were centrifuged and the serum was separated, and the separated supernatant was stored at -80°C until further analysis.
Isolation of total RNA and quantitative reverse-transcription PCR (qRT-PCR)
Total RNA was isolated from serum samples using a miRNeasy RNA isolation Kits (Qiagen, Venlo, Netherlands) following the manufacturer’s protocol. Reverse transcription was performed using the TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). PCR reactions for quantification of miR-138 were carried out in triplicate using TaqMan 2×Universal PCR Master Mix, and run on a 7500 Real-Time PCR System (Applied Biosystems, CA, USA). The relative expression of miR-138 in serum was normalized to endogenous reference gene RNU6, and calculated using the 2-ΔΔCt method.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, California, USA) and MedCalc 16.4.3 (MedCalc, Ostend, Belgium). Difference of serum miR-138 expression levels between groups was analyzed using Mann-Whitney U test. The correlation between serum miR-138 expression and clinical parameters was assessed by the Pearson Chi-square test. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were constructed to evaluate the predictive power of serum miR-138 for HNSCC. OAS and DFS curves were plotted using Kaplan-Meier method, and the log-rank test was used to determine P values. Multivariate Cox proportional hazards model was used to estimate the effect of serum miR-138 expression and clinical characteristics on OAS/DFS. A p value less than 0.05 was defined statistically significant.
Results
Serum miR-138 was markedly decreased in HNSCC patients and it diagnostic value
The expression levels of serum miR-138 in 113 HNSCC cases and 60 healthy volunteers were detected by qRT-PCR. As shown in Figure 1, serum miR-138 expression levels were greatly down-regulated in HNSCC patients compared to those in controls (P<0.05). Moreover, a significant increase in serum miR-138 levels was found in subjects (n=34) with well differentiation in comparison with those (n=79) with moderate/poor differentiation (P<0.05, Figure 2A). In addition, serum miR-138 expression in stage III/IV subjects (n=70) was much lower than that in stage I/II patients (n=43, P<0.05, Figure 2B).
Figure 1.

Serum miR-138 levels was significantly down-regulated in HNSCC patients.
Figure 2.
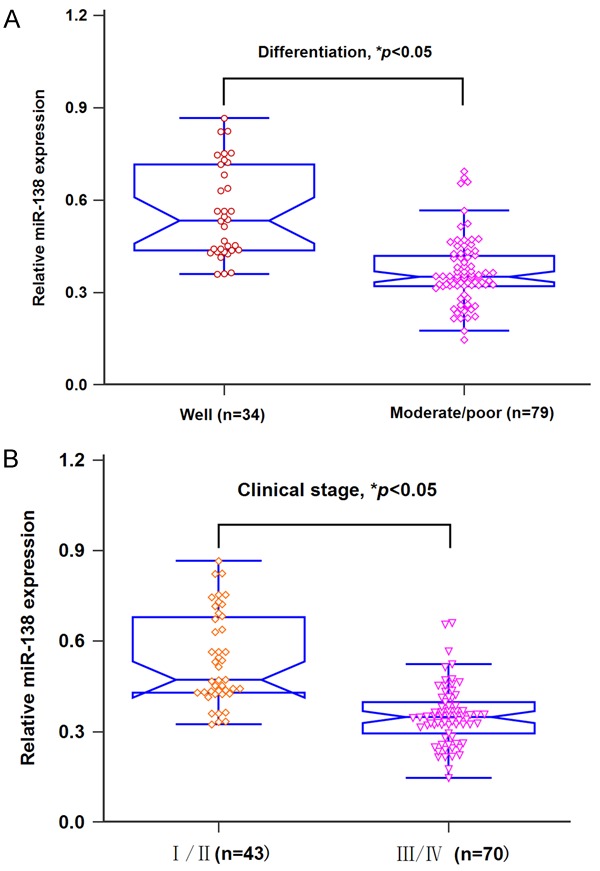
A. Patients with well differentiation had higher miR-138 expression than those with moderate/poor differentiation. B. Patients in I/II stage had higher miR-138 expression than those in III/IV stage.
A ROC curve analysis was used to evaluate the diagnostic accuracy of miR-138 in serum between HNSCC patients and normal controls. As shown in Figure 3, serum miR-138 levels could discriminate patients with HNSCC from healthy subjects with an AUC value of 0.836, and the specificity and sensitivity were 76.67% and 81.42%, respectively. These findings implied that serum miR-138 could be a potential indicator for HNSCC monitoring.
Figure 3.

Serum miR-138 yielded an AUC value of 0.836 in differentiating HNSCC patients from controls.
Pre-operative and post-operative serum miR-138 in HNSCC patients
Circulating miR-138 levels in 32 paired blood specimens of HNSCC patients with tumor resection were measured, and serum miR-138 levels in the post-operative samples were dramatically elevated compared to those in the pre-operative samples (P<0.05, Figure 4).
Figure 4.

Serum miR-138 expression in post-operative blood samples was greatly elevated compared with paired pre-operative samples.
Down-regulation of serum miR-138 correlated with poor clinical variables of HNSCC patients
To analyze the correlation of serum miR-138 with clinicopathological factors, we divided all the HNSCC patients into a low (n=56) and a high (n=57) expression group according to the median serum miR-138 level. As presented in Table 1, serum miR-138 expression was strongly associated with differentiation (P=0.0467) and clinical stage (P=0.0046), but not associated with other characteristics such as age, gender, tobacco consumption, alcohol consumption, primary tumor sites, pT stage and pN stage (all P>0.05).
Down-regulation of miR-138 correlated with worse survival in HNSCC patients
The Kaplan-Meier analysis showed that HNSCC patients with lower serum miR-138 levels had significantly worse OAS (P=0.018, Figure 5A) and DFS (P=0.007, Figure 5B) than those with higher expression of this miRNA.
Figure 5.

A. Patients in low miR-138 expression group had shorter overall survival. B. Patients in low miR-138 expression group had worse disease free survival.
Serum miR-138 was an independent prognostic indicator for HNSCC patients
Multivariate Cox proportional hazard regression model was employed and showed that serum miR-138 expression (OAS: HR=3.55, 95% CI=1.39-5.68, P=0.023; DFS: HR=4.13, 95% CI=1.46-6.97, P=0.019), clinical stage (OAS: HR=4.81, 95% CI=1.68-8.06, P=0.012; DFS: HR=5.31, 95% CI=1.87-8.92, P=0.005) and differentiation (OAS: HR=2.56, 95% CI=1.21-3.95, P=0.032; DFS: HR=2.82, 95% CI=1.34-4.45, P=0.028) were independent prognostic biomarkers for predicting poorer OAS/DFS in HNSCC patients (Table 2).
Table 2.
Multivariate Cox regression analysis for OAS and DFS
| Factors | Multivariate analysis | |
|---|---|---|
|
| ||
| HR (95% CI) | P value | |
| Overall survival (OAS) | ||
| Differentiation (Moderate/poor vs. well) | 2.56 (1.21-3.95) | 0.032* |
| Clinical stage (III/IV vs. I/II) | 4.81 (1.68-8.06) | 0.012* |
| MiR-138 expression (low vs. high) | 3.55 (1.39-5.68) | 0.023* |
| Disease free survival (DFS) | ||
| Differentiation (Moderate/poor vs. well) | 2.82 (1.34-4.45) | 0.028* |
| Clinical stage (III/IV vs. I/II) | 5.31 (1.87-8.92) | 0.005* |
| MiR-138 expression (low vs. high) | 4.13 (1.46-6.97) | 0.019* |
Footnote: CI: confidence interval; HR: hazard ratio.
P <0.05 was considered to be statistically significant.
Discussion
In this study, we found that serum miR-138 expression was remarkably reduced in HNSCC patients compared to healthy subjects. Also, serum miR-138 levels were significantly elevated in post-operative blood samples taken from patients with tumor resection. Furthermore, ROC curve analysis showed that miR-138 in serum could distinguish HNSCC patients from controls with relatively high accuracy. Subsequently, a positive correlation was observed between down-regulated serum miR-138 expression and aggressive clinical factors, as well as shorter OAS/DFS. Finally, serum miR-138 level was verified to be an independent prognostic indicator for HNSCC. Taken together, our results suggested that miR-138 might act as an anti-oncogenic miRNA in HNSCC.
In accordance with our findings, some previous studies demonstrated that miR-138 exerted a tumor suppressive function in HNSCC. For example, Liu and colleagues showed that miR-138 overexpression repressed cell invasion and induced cell cycle arrest, apoptosis in HNSCC cell lines. Moreover, knockdown of miR-138 stimulated cell invasion and restrained apoptosis [9]. MiR-138 was reduced in both primary HNSCC tumor and cell lines, and up-regulated miR-138 expression suppressed cell motility, invasion colony and fiber formation. RhoC was proved to be a downstream target of miR-138 [10].
Besides HNSCC, miR-138 was reported to act as a tumor suppressor in some other types of cancers. In non-small cell lung cancer, miR-138 expression was markedly reduced in cancerous tissues and miR-138 underexpression was associated with poor clinical outcome. Additionally, ectopic miR-138 expression significantly suppressed cancer cell growth, migration and invasion by targeting yes-associated protein 1 [11]. In colorectal cancer, Long et al provided in vitro and in vivo evidence to reveal that decreased miR-138 expression greatly stimulated cancer cell migration and invasion through inversely regulating twist basic helix-loop-helix transcription factor 2 gene [12]. In ovarian cancer, miR-138 overexpression repressed cancer metastasis with in vivo evidence via targeting SOX4 and HIF-1α. Also, down-regulation of miR-138 was closely associated with aggressive clinical features [13]. In osteosarcoma, Jiang and colleagues found that miR-138 levels were decreased both in cancer tissues and cell lines. Furthermore, loss of miR-138 promoted cancer cell proliferation, invasion and inhibited cell apoptosis in vitro by regulating differentiated embryonic chondrocyte gene 2 [14]. Similarly, miR-138 expression was remarkably reduced both in hepatocellular carcinoma tissues and cell lines. In addition, up-regulation of miR-138 inhibited tumorigenicity of cancer cells by degrading SOX9 [15]. In gallbladder carcinoma, Ma et al showed that miR-138 expression was underexpressed in cancerous samples. Enforced miR-138 expression greatly restrained cancer cell proliferation via directly targeting Bag-1 [16]. Also, Sun et al confirmed that miR-138 levels were significantly decreased in bladder cancer tissues. Reduced miR-138 expression promoted cancer cell invasion and metastases through up-regulating ZEB2 [17]. In cervical cancer, miR-138 expression was dramatically downregulated in cancer tissues and cell lines. Moreover, restoration of miR-138 suppressed tumorigenicity in vitro and tumor growth in vivo by negatively regulating hTERT [18].
In conclusion, we showed that serum miR-138 expression was significantly downregulated in HNSCC patients. ROC curve analysis revealed that serum miR-138 was an accurate indicator for differentiating HNSCC patients from controls. Moreover, miR-138 underexpression was closely correlated with worse clinical outcome and shorter survival. Therefore, serum miR-138 could serve as a promising marker for the diagnosis and prognosis of HNSCC.
Acknowledgements
This study was supported by the Grants from Science and Technology Commission of Shanghai Municipality (No: 14JC1490600).
Disclosure of conflict of interest
None.
References
- 1.Cui L, Cheng S, Liu X, Messadi D, Yang Y, Hu S. Syntenin-1 is a promoter and prognostic marker of head and neck squamous cell carcinoma invasion and metastasis. Oncotarget. 2016;7:82634–82647. doi: 10.18632/oncotarget.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Xu S, Ma D, Zhuang R, Sun W, Liu Y, Wen J, Cui L. DJ-1 is upregulated in oral squamous cell carcinoma and promotes oral cancer cell proliferation and invasion. J Cancer. 2016;7:1020–1028. doi: 10.7150/jca.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summerer I, Unger K, Braselmann H, Schuettrumpf L, Maihoefer C, Baumeister P, Kirchner T, Niyazi M, Sage E, Specht HM, Multhoff G, Moertl S, Belka C, Zitzelsberger H. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br J Cancer. 2015;113:76–82. doi: 10.1038/bjc.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou B, Ishinaga H, Midorikawa K, Shah SA, Nakamura S, Hiraku Y, Oikawa S, Murata M, Takeuchi K. Circulating microRNAs as novel prognosis biomarkers for head and neck squamous cell carcinoma. Cancer Biol Ther. 2015;16:1042–1046. doi: 10.1080/15384047.2015.1045692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam M, Datta J, Lang JC, Teknos TN. Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol. 2014;50:448–456. doi: 10.1016/j.oraloncology.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao CX, Cui JJ, Zhang W, Zhou HH, Yin JY, Liu ZQ. MicroRNA-138 acts as a tumor suppressor in non small cell lung cancer via targeting YAP1. Oncotarget. 2016;7:40038–40046. doi: 10.18632/oncotarget.9480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Long L, Huang G, Zhu H, Guo Y, Liu Y, Huo J. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J Transl Med. 2013;11:275. doi: 10.1186/1479-5876-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1α. Int J Cancer. 2013;133:867–878. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Mu W, Wang J, Lu J, Jiang S, Li L, Xu H, Tian H. MicroRNA-138 functions as a tumor suppressor in osteosarcoma by targeting differentiated embryonic chondrocyte gene 2. J Exp Clin Cancer Res. 2016;35:69. doi: 10.1186/s13046-016-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang W, Liu K, Liu S, Ji B, Wang Y. MiR-138 suppresses cell proliferation and invasion by inhibiting SOX9 in hepatocellular carcinoma. Am J Transl Res. 2016;8:2159–2168. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ma F, Zhang M, Gong W, Weng M, Quan Z. MiR-138 suppresses cell proliferation by targeting Bag-1 in gallbladder carcinoma. PLoS One. 2015;10:e0126499. doi: 10.1371/journal.pone.0126499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun DK, Wang JM, Zhang P, Wang YQ. MicroRNA-138 regulates metastatic potential of bladder cancer through ZEB2. Cell Physiol Biochem. 2015;37:2366–2374. doi: 10.1159/000438590. [DOI] [PubMed] [Google Scholar]
- 18.Zhou N, Fei D, Zong S, Zhang M, Yue Y. MicroRNA-138 inhibits proliferation, migration and invasion through targeting hTERT in cervical cancer. Oncol Lett. 2016;12:3633–3639. doi: 10.3892/ol.2016.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]


