Abstract
Objective: To evaluate the effect of glycine on regulation of the hepatic toll-like receptor 4 (TLR4) signaling pathway by metabolic endotoxemia in a rat model of non-alcoholic steatohepatitis (NASH). Methods: The NASH rat model was generated by feeding the animals a high-sucrose, high-fat for diet for 12 weeks. We then measured alterations in levels of LPS, TNFα, IL-6, ALT, TG in plasma, and TNFα, and IL-6 in liver. We performed hematoxylin and eosin (HE) staining and immunohistochemical staining to document pathological changes. Expression of TLR4 and IRS-1 in liver was measured by Western Blot and RT-PCR. Results: Compared with control animals, levels of LPS, TNFα, IL-6 in plasma and the levels of TNFα, IL-6 in liver tissues gradually increased. Pathological changes and expression of TLR4 in liver were significantly increased compared with control. mRNA and protein levels of TLR4 and IRS-1 in livers were also upregulated. With concomitant treatment with glycine, endotoxin levels decreased, and TNFα and IL-6 levels in plasma and liver were significantly decreased compared to NASH rats. Pathological changes in liver and immunohistological expression of TLR4 in liver tissues were significantly improved compared to NASH rats. mRNA and protein levels of TLR4 were significantly downregulated while mRNA and protein levels of IRS-1 in liver were markedly upregulated. Progression of NASH appeared to be slowed or limited. Conclusion: These data suggest that hepatic TLR4 signaling pathway is activated in the NASH rat, and oral glycine may reduce the risk of endotoxemia and inflammation of the liver.
Keywords: Glycine, non-alcoholic steatoheptitis, intestinal endotoxin, type 4 toll-like receptors
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a significant health issue, affecting up to 30% of adults and up to 10% of children in developed countries [1,2]. Non-alcoholic steatohepatitis (NASH) is an important precursor of NAFLD, which in its later stages gives rise to hepatic fibrosis, cirrhosis, liver failure, and even liver cancer [3,4]. Currently, the pathogenesis of NASH is not entirely clear. A number of studies suggest that insulin resistance, inflammation, alterations in lipid metabolism, oxidative stress, lipid peroxidation, and mitochondrial dysfunction are all involved in the pathogenesis of NASH [5-7]. The liver plays an important role in presenting innate immunity recognition receptors to pathogens via Kupffer cell surface type 4 Toll-like receptors (TLR4). Endotoxin ligands induce the formation and release of pro-inflammatory cytokines TNF-α and IL-6. The associated signal pathways give rise to liver inflammation and insulin resistance (IR) [8].
In the past 20 years, Han et al have published many studies of intestinal endotoxemia (IETM). These investigators have conducted a series of animal and human studies and have documented that IETM occurs in various types of liver diseases, such as viral hepatitis, alcoholic liver disease, chemical and drug-induced liver injury and hepatic failure [9,10]. Recent data indicate that metabolic diseases such as nonalcoholic fatty liver disease and type 2 diabetes are also associated with increased plasma endotoxin levels [11,12]. Studies have suggested that the amino acid glycine has anti-inflammatory properties, and that it mediates cell protection and immune modulation [13,14]. Our preliminary study suggested that glycine can reduce the level of endotoxin and may thereby attenuate liver injury [15]. Based on these results, sought to determine whether glycine could inhibit liver injury by downregulating the TLR4 signaling pathway in a NASH rat model [16].
Materials and methods
Animals and treatments
Thirty-two male Sprague-Dawley rats, weighing 200-250 g, were obtained from the Animal Center of Shanxi Medical University. Animals were randomly divided into four groups (n=8 for each group). The C (control) group received a regular diet and tap water; the H (high-sucrose/high-fat) group received a diet (HSHF) consisting of 52% calories from carbohydrates, 25% from fat, and 10% from protein; the H+G group received the HSHF diet and tap water with 1% glycine. The G (glycine) group received tap water with 1% glycine and a regular diet [17]. Animals and standard rodent diet were obtained from the Research Animal Center of Shanxi Medical University. The animals were housed under standard laboratory conditions, maintained on a 12 h light and dark cycle, and were afforded unrestricted access to food and water. The experimental protocols were approved by the Shanxi Animal Research Ethics committee.
Rats were sacrificed at 12 weeks. Blood and liver tissues were sampled at the time of sacrifice and were stored at -80°C until processing.
Measurements of serum endotoxin levels from the abdominal aorta
In anesthetized animals, blood samples were collected from the abdominal aorta and centrifuged at 3500 rpm for 10 min. Endotoxin levels in the collected plasma were determined using a Limulus amebocyte lysate regent kit (Clinical Sciences Inc, Xiamen China) according to manufacturer’s instructions (UV-2102C, Shanghai).
Measurement of TNFα, IL-6, ALT, TG in plasma, and TNFα, IL-6 in liver homogenates
TNF-α (Tumor necrosis Factor-α, TNF-α radioimmunoassay kit, Radioimmunity Institute of PLA General Hospital, Beijing China), IL-6 (interleukin-6, IL-6 radioimmunoassay kit, Radioimmunity Institute of PLA General Hospital, Beijing China), ALT (alanine transferase kit, Nanjing Jiancheng Bioengineering Institute, Nanjing China), TG (Triglyceride kit, Nanjing Jiancheng Bioengineering Institute, Nanjing China), and TNFα and IL-6 levels were measured in plasma or liver with appropriate kits according to manufacturers’ instructions.
Liver histology
Liver samples were fixed in 10% formalin, embedded in paraffin, cut into 4 μm thick sections, and stained with hematoxylin and eosin (HE, Hematoxylin and eosin staining kit, Junruishengwu Technology Corporation, Shanghai China).
Immunohistochemical staining
Paraffin-embedded sections were stained with anti-TLR4 protein polyclonal antibodies (1:100 dilution; Zhong Shan-Golden Bridge Biological Technology Co. Beijing, China) to measure quantitative expression levels of TLR4. Specimens were analyzed using a computerized image analysis system (IPP6.0 software, Media Cybernetics Inc., USA). Cells cytoplasm with brown staining was regarded as TLR4-positive. Five slices were examined in each group, with 10 fields of vision observed in each slice. To determine the positivity rate, data were displayed as the number of TLR4-positive cells to total cells (at 200×) for each tissue section. At least 50 cells were observed.
Western blot analysis
Total TLR4, IRS-1 were assessed by Western blot. Aliquots of frozen liver homogenates were further extracted in phosphate-buffered saline containing of 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mM EDTA, 50 mM NaF and 2 mM Na3VPO4 (NaF and Na3VPO4 only for phosphorylation protein; reagents were from Nanjing Jiancheng Biotechnology, Nanjing China) Cells were lysed by 30 min incubation on ice. The lysate was centrifuged at 15,000 rpm for 10 min. 40 µg of protein was loaded in each lane and separated on a 7.5% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), then transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked for 3 h at room temperature with 5% (w/v) non-fat dried milk. After washing 3 times with TBST (Tris 50 mmol/L NaCl 100 mmol/L, pH 7.40), the membrane was incubated at 4°C overnight with specific antibodies. Rabbit polyclonal antibodies against TLR4 (1:500 dilution; Cell Signaling Technology Inc. Danvers, USA), IRS-1 (1:500 dilution; Cell Signaling Technology Inc. Danvers, USA) were employed. The immunoblots were then incubated with the corresponding peroxidase-conjugated goat anti-rabbit secondary antibodies (1:2000 dilution; Zhong Shan-Golden Bridge Biological Technology). Bands were detected with enhanced chemiluminescence. The intensities of the protein bands were analyzed by Quantity One software (Bio-Rad Laboratories, Inc. Hercules, USA).
mRNA expression
We withdrew total RNA from liver and performed the reverse transcription reaction. Primer sequences: TLR4 Upstream: 5’-GCCGGAAAGTTATTGTGGTG-3’; downstream: 5’-CCACTCGAGGTAGGTGTTT-3’ (507 bp); IRS-1 Upstream: 5’-ACGCTCCAGTGAGGATTTAAGCA-3’, downstream: 5’-GGTCCTGGTTGTGAATCGTGAA-3’ (277 bp); β-actin Upstream: 5’-ACGCTCCAGTGAGGATTTAAGCA-3’; downstream: 5’-GGTCCTGGTTGTGAATCGTGAA-3’ (56 bp). For the DNA standard (D12000) to determine the size of the PCR product by 2% agarose gel electrophoresis, we used the Quantity One gel analysis system (Bio-Rad Corporation).
Statistics
All values were displayed as mean ± standard error. Statistical analyses were performed on the SPSS13.0 system (Statistical Product and Service Solutions, USA). Other data were analyzed by one way analysis of variance (ANOVA). Statistical significance level was set at P < 0.05.
Results
Measurements of serum endotoxin level in abdomen aorta and measurement of TNFα, IL-6, ALT, TG in plasma, and TG, TNFα, IL-6 in liver homogenates
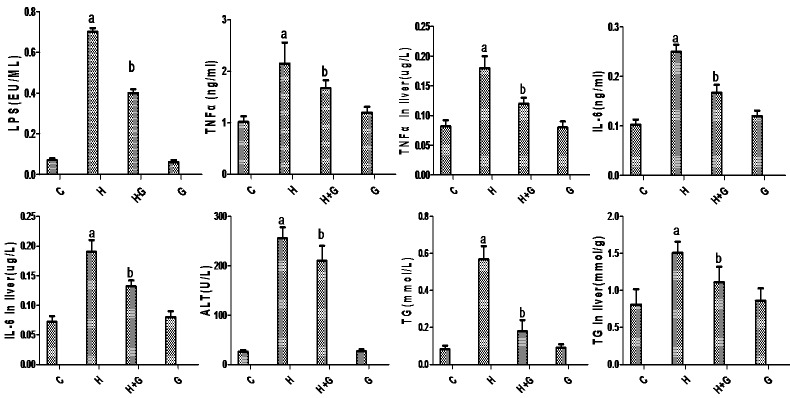
During the experiment, all animals remained in good condition. Plasma levels of endotoxin, TNFα, IL-6, ALT, TG, and liver homogenate levels of TG, TNFα, IL-6 in the HFHS group were significantly higher than those of the SC group at 12 weeks (p < 0.05) (Figure 1). With the addition of glycine, the endotoxin levels and inflammation factors, such as TNFα, IL-6 in serum and in liver significantly decreased compared with the H+G group (Figure 1). Serum levels of ALT, TG and hepatic levels of TG were significantly reduced compared with rats fed the HSHF diet only (Figure 1). These data suggest that glycine treatment might decrease endotoxin levels and inflammation, improve liver function, and may alleviate hyperlipidemia in NASH rats.
Figure 1.
Effect of glycine treatment on LPS, biochemistry and systemic inflammation indices. Control group (C), high-fat/high-sugar group (H), high-fat/high-sugar + glycine group (H+G), glycine group (G). Data represented as means ± standard error (n=8). aP < 0.05 vs control group; bP < 0.05 vs HSHF group.
Pathological observations
In order to investigate liver pathology, we performed HE staining and studied the alterations by light microscopy. There were no significant changes in the livers of normal rats and glycine-treated rats (Figure 2). However, in HFHS group, we observed morphologic changes including fatty degeneration, ballooning degeneration, and inflammatory cell infiltration in lobular and periportal structures (Figure 2). Liver injury was clearly decreased in the liver of glycine-treated rats (Figure 2).
Figure 2.
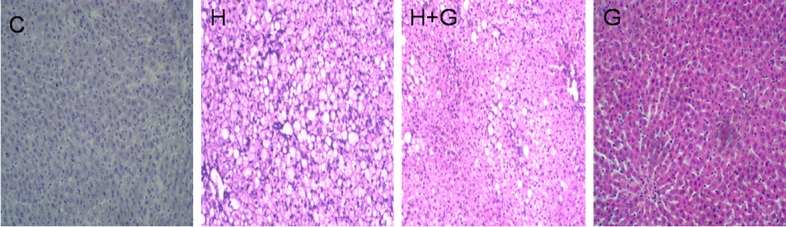
Effect of glycine treatment on liver pathology. Control group (C), high-fat/high-sugar group (H), high-fat/high-sugar + glycine group (H+G), glycine group (G).
Immunohistochemical staining observation of TLR4 in liver
We detected the protein expression of TLR4 in liver tissue by immunohistochemistry. The TLR4 staining assay showed that expression of TLR4 was upregulated by high fat and high sugar diet (Figure 3). Expression of TLR4 was significantly downregulated after treatment of glycine in NASH rats (Figure 3). There were no obvious differences between control and glycine groups (Figure 3). These results suggest that glycine may attenuate TLR4 expression in liver and may downregulate TLR4-mediated inflammation and liver injury.
Figure 3.
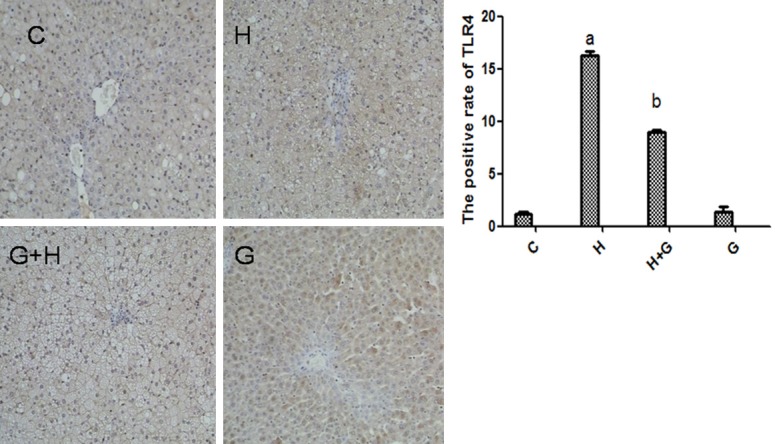
Effect of glycine treatment on the expression of TLR4 by immunohistochemical staining. Control group (C), high-fat/high-sugar group (H), high-fat/high-sugar + glycine group (H+G), glycine group (G).
mRNA and protein expression of TLR4 and IRS-1 in liver
A high-fat/high-sucrose diet led to an approximately 2-fold increase in the mRNA expression ratio of TLR4 in the liver at 12 weeks (P < 0.001; Figure 4). There was no change in expression in control and glycine groups. The mRNA expression ratio of TLR4 was significantly decreased in the liver of glycine-treated rats (Figure 4). Similarly, the protein expression ratio of TLR4 in the liver was also elevated in the H group (Figure 5). The protein expression ratio of TLR4 was significantly decreased in the liver of the glycine group (Figure 5). Alterations were not statistically significant between the C and G groups.
Figure 4.
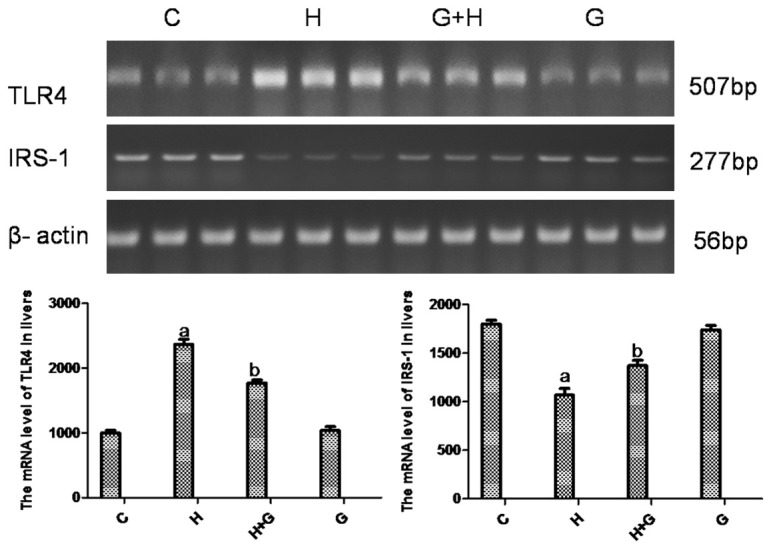
Effect of glycine treatment on the mRNA expression of TLR4 and IRS-1 by PCR. Control group (C), high-fat/high-sugar group (H), high-fat/high-sugar + glycine group (H+G), glycine group (G). Data represented as mean ± standard error (n=8). aP < 0.05 vs control group; bP < 0.05 vs HSHF group.
Figure 5.
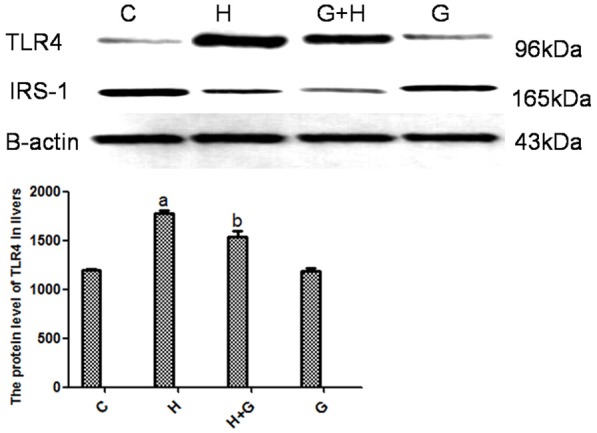
Effect of glycine treatment on the protein expression of TLR4 and IRS-1 by Western blot. Control group (C), high-fat/high-sugar group (H), high-fat/high-sugar + glycine group (H+G), glycine group (G). Data represented as means ± standard error (n=8). aP < 0.05 vs standard control group; bP < 0.05 vs HSHF group.
A high-fat/high-sucrose diet led to an approximately 1.5-fold decrease in the mRNA expression ratio of IRS-1 in the liver by 12 weeks (P < 0.001; Figure 4). There was no change in control and glycine groups. However, the mRNA expression ratio of IRS-1 was significantly increased in the liver of glycine-treated rats (Figure 4). Similarly, the protein expression ratio of IRS-1 in the liver was also decreased in the H group (Figure 5). The protein expression ratio of IRS-1 was significantly increased in the liver of glycine-treated rats (Figure 5). These alterations were not statistically significant between the C and G groups (Figure 5).
Discussion
Obese individuals generated by high-sugar/high-fat diets experience generalized increases in levels of serum endotoxin, defined as “metabolic endotoxemia” [18,19]. This phenomenon is closely correlated with low-grade inflammation and metabolic disorders [20]. Our results revealed that NASH rats displayed increased serum TNFα and IL-6 levels, suggesting an apparent sugar/lipid metabolic disorder. Oral glycine can significantly reduce endotoxin levels, serum inflammatory cytokines and inflammatory lesions in the liver. A high-sugar/high-fat diet was an independent risk factor for changes in the composition of gut bacteria in obese mice [21]. Lipopolysaccharide (LPS), an important TLR4 ligand, may play a role in metabolic diseases induced by high-sugar/high-fat diets [22-25]. TNFα can combine directly with the hepatic insulin receptor, promote IRS -1 tyrosine phosphorylation, and further aggravate the degree of hepatic insulin resistance [26,27]. In one study, TLR4 and TNFα levels in NASH rat liver were increased [28]. The study also found elevated levels of serum endotoxin and TLR4 in the absence of choline left-handed amino acids (MCD) diet and high fructose diet. This suggests a correlation between endotoxin activation of TLR4 signaling pathways and the pathogenesis of NAFLD [29,30].
Glycine is a non-essential amino acid and is the simplest amino acid. Many studies have shown that glycine antagonizes the effect of endotoxin. Young and others found that intravenous injections of glycine in the early stages of sepsis effectively inhibit the rise of TNFα [31]. Studies of endotoxic shock in rats showed that a 5% glycine diet inhibited the rise of TNFα and ALT, and reduced liver necrosis [32]. Liu found that glycine can reduce the level of endotoxin and delay the occurrence and development of cirrhosis in a model of liver fibrosis induced by numerous factors [15]. Han and colleagues showed that glycine can reduce endotoxin and liver damage through following mechanisms: reduction of intestinal permeability; reduction of the absorption of endotoxin; combination with lipid A to reduce the activity of endotoxin; reduction in the expression of lipolysaccharide binding protein (LBP) mRNA and CD14 mRNA, thereby reducing over-activation of Kupffer cell (KC). In addition, glycine is reported to play an important role in inhibition of hepatic apoptosis, [33] and inhibition of the oxidative stress reaction [34]. In preliminary experiments, we also observed that LPS plays an important role in metabolic diseases, such as NASH, and that glycine can effectively reduce enterogenous endotoxin levels. We also found that glycine can attenuate hepatic oxidative stress and endoplasmic reticular stress [15]. The present study suggests that oral glycine can reduce the level of serum endotoxin, significantly reduce liver inflammatory injury, liver TLR4 expression and TNF alpha and IL-6 levels, as well as IRS-1 expression.
There are several questions that remain to be addressed: For example, does glycine affect the proportion of gut bacteria? By what molecular mechanism does glycine reduce the absorption of LPS? In addition, we only studied the role of glycine in a rat model. It is unclear what effect of glycine would have on human patients. Future studies will address these questions.
Acknowledgements
The authors gratefully acknowledge the financial support by the Doctorate Research Starting Foundation of Shanxi Medical University (No.B03201202, No.B03201203), the Youth Science and Technology Research Fund, Basic research projects in Shanxi (Grant No.2014021040-3).
Disclosure of conflict of interest
None.
References
- 1.Organization WH. Obesity and overweight fact sheet. Geneva: World Health Organization; 2012. p. 311. [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 4.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–9. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Köroğlu E, Canbakan B, Atay K, Hatemi İ, Tuncer M, Dobrucalı A, Sonsuz A, Gültepe I, Şentürk H. Role of oxidative stress and insulin resistance in disease severity of non-alcoholic fatty liver disease. Turk J Gastroenterol. 2016;27:361–366. doi: 10.5152/tjg.2016.16106. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1768–76. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day CP. Pathogenesis of steatohepatitis. Balliere’s Best Pract Res Clin Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 10.Zhao LF, Li H, Han DW. Intesinal endotoxemia and liver diseases. World Chin J Digestol. 2000;8:1145–1149. [Google Scholar]
- 11.Li H, Zhao LF, Han DW. Role of endotoxemia in the occurs and development with liver cirrhosis in rats. Chinese Journal of Pathophysiology. 2001;17:353–355. [Google Scholar]
- 12.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, Amin AI, Burt AD, Kumar S, Day CP, McTernan PG. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Koo DJ, Chaudry IH, Wang P. GlyCine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit Care Med. 2001;29:1201–1206. doi: 10.1097/00003246-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Ikejima K, Limuro Y, Forman DT, Thurman RG. A diet containing glycine improve sorvival in endotoxin Shock in the rat. Am J physiol. 1996;271:G97–G103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Han DW, Xu RL, Wu HW, Qu CX, Wang F, Wang XY, Zhao YC. Glycine protects against high sucrose and high fat-induced nonalcoholic steatohepatitis in rats. Oncotarget. 2016;7:80223–80237. doi: 10.18632/oncotarget.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Han DW, Xu RL, Li S, Wu H, Qu C, Wang F, Wang X, Zhao Y. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PLoS One. 2014;9:e115148. doi: 10.1371/journal.pone.0115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Hafidi M, Pérez I, Zamora J, Soto V, Carvajal-Sandoval G, Baños G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1387–93. doi: 10.1152/ajpregu.00159.2004. [DOI] [PubMed] [Google Scholar]
- 19.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 20.Gäbele E, Dostert K, Hofmann C, Wiest R, Schölmerich J, Hellerbrand C, Obermeier F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55:1391–1399. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 21.Rapin JR, Wiernsperger N. Possible links between intestinal permeability and food processing: a potential therapeutic niche for glutamine. Clinics (Sao Paulo) 2010;65:635–643. doi: 10.1590/S1807-59322010000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildebrandt MA, Hoffmann C. High-fat diet determines the composition of the murine gut microbiome independently ofobesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 24.Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van’t Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170:1399–1405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 25.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, areceptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 26.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu ZJ, Fan JG, Wang XP. The expression of liver LPS receptor was upregulated in non-alcoholic fatty hepatitis rat. Chinese liver disease. 2006;14:50–53. [Google Scholar]
- 29.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 30.Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, Choi HS. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51:892–899. doi: 10.1194/jlr.M800626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Koo DJ, Chaudry IH, Wang P. GlyCine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit Care Med. 2001;29:1201–1206. doi: 10.1097/00003246-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Liu JC, Han DW, Xu RL, Zhao Y. The effect of glycine on the expression of CD14 gene and protein of hepatic tissue in the course of developing cirrhosis of rats. Chin J Hepatol. 2002;10:181–184. [PubMed] [Google Scholar]
- 34.Pal PB, Pal S, Das J, Sil PC. Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes. Amino Acids. 2012;42:1669–1683. doi: 10.1007/s00726-011-0869-3. [DOI] [PubMed] [Google Scholar]