Abstract
Objective: Recent studies have shown that understanding the differences between Gleason 3+4 and Gleason 4+3 in PCa patients may improve their treatment. This study aimed to evaluate the different expression levels of glycolytic proteins for Gleason score of 4+3 and 3+4. Methods: A total of 90 PCa patients, including 38 cases with a Gleason score of 7, were included in this study. The expression of glycolytic proteins in both prostate cancer and normal prostate tissues, in GGG2 and GGG3 as well were assessed by immunohistochemical staining. Results: Compared with GGG3, the GGG2 cases displayed significantly lower expression of all proteins (P < 0.05). The correlation among all enzymes showed that the key glycolytic enzyme, HK2, was significantly positively related to another key enzyme, PKM2 (r = 0.550, P < 0.01), and the expression of PFKFB4 was correlated with the expression of HK2 (r = 0.236, P < 0.05) and PKM2 (r = 0.392, P < 0.01). Additionally, neither GLUT1 nor PFKFB3 was correlated with PFKFB4, HK2 or PKM2. Further analysis showed that HK2 (r = 0.297, P < 0.01) and PKM2 (r = 0.431, P < 0.01) were significantly positively related to the Gleason score in PCa tissues. Conclusions: Glycolytic proteins expression levels were upregulated in PCa tissues. Furthermore, GGG3 exhibits a higher level of glycolysis compared with GGG2 in PCa tissues. Additionally, the key glycolytic enzymes, HK2 and PKM2, are overexpressed simultaneously in PCa and significantly correlate with PCa progression as represented by the GS.
Keywords: Glycolysis, key enzymes, prostate cancer, immunohistochemical staining, Gleason grade group
Introduction
Metabolic reprogramming has recently emerged as a new hallmark of cancer [1]. Aerobic glycolysis, in which cancer cells opt for glycolysis rather than mitochondrial respiration even in the presence of oxygen [2], has been described as a promising target for the development of new therapies [3,4].
There are steps of particular importance for the regulation of glycolysis, which is initiated by the cellular uptake of glucose via glucose transporters (GLUTs) on the cell surface [5]. Next, glucose is phosphorylated to glucose-6-phosphate by hexokinase (HKs). Fructose-6-phosphate is again phosphorylated to fructose-1,6-bisphosphate by phosphofructokinase (PFK), the activity of which is allosterically regulated by fructose-2-6-biphosphate (F2,6BP), which is the product of the bifunctional enzyme, phosphofructokinase 2/fructose-bisphosphatase (PFKB). In particular, studies have reported that although PFKBP3 has the highest kinase activity for the production of F2,6BP, the expression of PFKFB4 is essential for the survival of prostate cancer cells but not for normal cells based on unbiased screens [6]. Another key enzyme, pyruvate kinase (PK), catalyzes the conversion of phosphoenolpyruvate to pyruvate. Furthermore, HK, PK and PFK are three key enzymes in the process of glycolysis that regulate the rate of glycolysis as rate-limiting enzymes.
Cancer tissues exhibit a higher level of glycolysis than normal tissues to satisfy their increased needs for energy and biosynthetic precursors. In addition, fluorodeoxyglucose [I8F] enters cancer cells through the same GLUTSs as glucose. Therefore, fluorodeoxyglucose positron emission tomography (FDG-PET) for malignancy has been widely introduced as a metabolic imaging technique [7]. However, there seems to be a consensus that the application of FDG-PET for the detection or grading of prostate cancer (PCa) is limited, which may suggest that glucose consumption in PCa is not directly associated with malignancy [8]. However, Tom Powles et al. [9] concluded that although FDG-PET does not appear to be useful in primary treatment decisions for hormone-sensitive disease, it may have a role as a surrogate index of the response to chemotherapy in hormone-resistant disease. Additionally, some studies have demonstrated that the accumulation of FDG in the prostate is higher in advanced compared with early stages of PCa [10,11]. In summary, PCa, unlike many other neoplasms, may exhibit unique metabolic profiles, and therefore a better understanding of the relationship between PCa and glycolysis is needed.
The current GS system, which ranges from 6-10, has a significant deficiency when used to evaluate the prognosis of cases with a Gleason score of 7. There are clear differences in biological characteristics, such as aggressiveness, and the prognosis of PCa between mostly well-differentiated cancer with a reduced component of more poorly differentiated cancer (Gleason 3+4 = 7) and mostly poorly differentiated with a reduced component of well-differentiated cancer (4+3 = 7) [12]. A new Gleason grade group (GGG) was proposed, in which GGG2 and GGG3 represent 3+4 = 7 and 4+3 = 7, respectively [13]. Therefore, using the new GGG score, the difference between 3+4 = 7 and 4+3 = 7 can be recognized. In addition, increasing numbers of studies suggest that the percentage of Gleason pattern 4 in radical prostatectomy specimens represents the most important prognostic factor among early-stage prostate cancer patients. Thus, the diagnostic distinction between GGG2 and GGG3 is conducive to their corresponding treatment [14]. In conclusion, understanding the differences between GGG2 and GGG3 may provide more effective tools to help patients acquire improved medical benefits.
In this study, glycolysis profiles were studied by evaluating the expression of all key glycolytic enzymes, including GLUT1, HK2, PFKFB3, PFKFB4, and PKM2, using tissue microarrays (TMA). Each dot in the same position of the TMA was derived from the same tissue by radical prostatectomy, making it possible to investigate the relationship among all markers in one tissue. Furthermore, the association of the expression of all markers with the Gleason score of prostate cancer patients, especially GGG2 and GGG3, was analyzed. The current study was conducted to verify the importance of glycolysis in PCa and the association between the key enzymes in glycolysis and the progression of PCa represented by the different Gleason score.
Materials and methods
Patients and tissue samples
All six TMA slides generated from radical prostatectomy, including 90 pairs of prostate cancer and matched adjacent normal tissues, were purchased from BioChip Company (Shanghai, China). None of the patients had received any chemical treatment or physical therapy before surgery. The tumor content of the prostate cancer specimens was defined as prostatic adenocarcinoma. Adjacent normal prostate tissues were completely embedded, step-sectioned at 3-mm intervals and evaluated by pathologists with genitourinary expertise. The mean age of the patients was 70.7±8.1 (50-90) years. The present study was approved by the Ethics Committee of Taizhou Hospital of Zhejiang Province and was conducted in accordance with the Principles of the Declaration of Helsinki. All participants provided signed informed written consent in advance of their participation in the study.
Immunohistochemistry
Immunohistochemical studies were performed using the Leica autostainer XL ST5010 (Leica Biosystems, Wetzlar, Germany). Briefly, the sections were deparaffinized in xylene, dehydrated with ethanol and then subjected to antigen retrieval in citrate buffer (10 mM, pH 6.0) for 30 min. The sections were then blocked by washing the slides in Peroxidase-Blocking Reagent (Dako Cytomation, Glostrup, Denmark) for 15 minutes at room temperature, followed by incubation with the primary antibody (Abcam, Cambridge, UK) overnight at 4°C in a humidified chamber. The sections were then incubated with the secondary antibody (Abcam, Cambridge, UK) for 30 min at RT. The primary antibody dilutions are detailed in Table 1. After complete washing in PBS, the sections were developed in freshly prepared diaminobenzidine solution (DAB), counterstained with hematoxylin, dehydrated through a graded ethanol series, cleared with xylene, and cover-slipped.
Table 1.
Details of the antibody and dilution
| Protein | Location | Antibody | Company | Antibody dilution |
|---|---|---|---|---|
| GLUT1 | Cytoplasm | ab115730 | Abcam | 1:1500 |
| HK2 | Cytoplasm | ab104836 | Abcam | 1:2000 |
| PFKFB3 | Nucleus | ab181861 | Abcam | 1:500 |
| PFKFB4 | Cytoplasm | ab71622 | Abcam | 1:1200 |
| PKM2 | Cytoplasm | ab38237 | Abcam | 1:1000 |
Evaluation of immunohistochemical staining
TMAs were scanned by an Aperio Scanscope XT, and the whole field of each dot was obtained for immunohistochemical evaluation. All sections were examined and scored by two pathologists who were blinded to the patient clinical information. The IHC score was calculated by combining the quantity score (percentage of positively stained cells) with the staining intensity score. The percentage of positively stained cells was scored on a scale from 0 to 4 as follows: 0 (< 1%), 1 (1-24%), 2 (25-49%), 3 (50-74%), and 4 (75-100%). The staining intensity was scored from 0 to 3 as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The final IHC score was determined by multiplying the intensity and the extent of the positivity scores, and the final scores ranged from 0-12. Samples with an IHC score of more than 3 were considered positive, and those with a score less than 3 were considered negative.
Statistical analysis
The difference in protein expression between the tumor tissue and its normal counterpart for each patient was evaluated using the Mann-Whitney U-test. Correlations between the expression of all markers and clinicopathological factors were calculated using Spearman’s correlation. A Cox regression model was used for the multivariate analyses. P < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics Version 20 software.
Results
Detection of the expression of glycolytic enzymes by immunohistochemical staining
Our results showed that prostate cancer cells with a high expression level of GLUT1, HK2, PFKFB3, PFKFB4 and PKM2 could be observed based on a widespread brown color in the cytoplasm or nucleus, whereas the adjacent normal tissues showed almost no staining (Figure 1A-J). The IHC scoring system was used to quantify the data from the TMA arrays, and the results showed that positive staining rates in cancer tissues were significantly higher for GLUT1 (67.8%, 61/90), HK2 (95.6%, 86/90), PFKFB3 (60%, 54/90) and PFKFB4 (57.8%, 52/90) than for the adjacent non-cancerous tissues (56.7%, 51/90; 88.9%, 80/90; 31.1%, 28/90; 35.6%, 32/90, P < 0.05). However, the expression of PKM2 did not differ between PCa (98.9%, 89/90) and adjacent normal (98.9%, 89/90) tissues (P > 0.05). Further evaluation of the IHC scores for all enzymes showed that GLUT1 (5.37±3.73), HK2 (7.40±3.43), PFKFB3 (3.00±2.61), PFKFB4 (3.68±3.61) and PKM2 (7.12±3.30) were all expressed at higher levels in prostate cancer tissues compared with the adjacent normal tissues (3.36±2.27, 5.88±3.10, 1.69±1.52, 2.42±2.84, and 6.12±2.89, respectively), as shown in Figure 1K-O.
Figure 1.
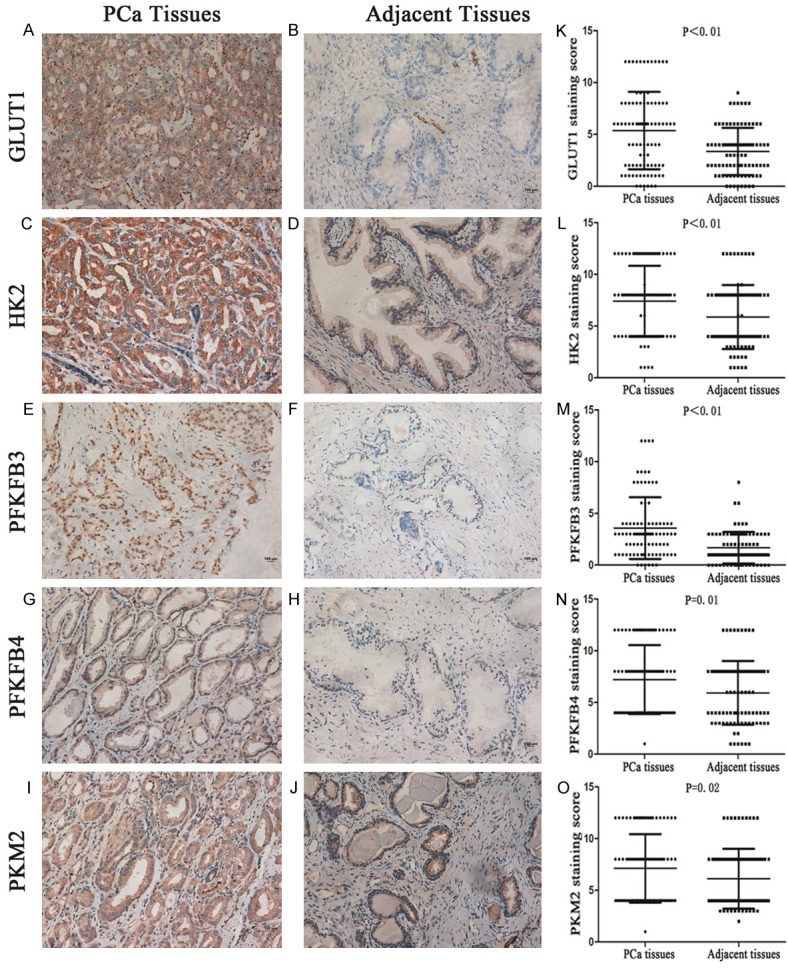
Representative immunohistochemistry demonstrates that all key glycolytic enzymes, GLUT1 (A), HK2 (C), PFKFB3 (E), PFKFB4 (G), and PKM2 (I), are more highly expressed in PCa tissues than in adjacent tissues (B, D, F, H and J). The histograms (K-O) represent the IHC score of glycolytic key enzymes, and the results show that the expression levels of all enzymes were significantly elevated in PCa tissues compared with the adjacent normal tissues, as assessed by the Mann-Whitney U test.
Correlation among glycolytic enzymes in the same PCa tissue
To better understand the correlation among glycolytic enzymes, Spearman’s correlation was used to analyze the expression of all enzymes in the same PCa tissue. Interestingly, as shown in Table 2, we found that one of the key glycolytic enzymes, HK2, was significantly positively related to another key enzyme, PKM2 (r = 0.550, P < 0.01). The expression of PFKFB4 was correlated with that of HK2 (r = 0.236, P < 0.05) and PKM2 (r = 0.392, P < 0.01). Additionally, there was only a weak correlation between the expression of GLUT1 and PFKFB3 (r = 0.270, P < 0.05). Neither GLUT1 nor PFKFB3 was correlated with PFKFB4, HK2 or PKM2.
Table 2.
Spearman’s correlation assay of the correlation between proteins expression in PCa tissue
| PCa tissue | GLUT1 staining | HK2 staining | PFKFB3 staining | PFKFB4 staining | PKM2 staining | |
|---|---|---|---|---|---|---|
| GLUT1 | Correlation | 1 | -0.052 | 0.270 | -0.044 | -0.011 |
| Staining | Sig. (2 tailed) | < 0.01** | 0.627 | 0.010* | 0.682 | 0.919 |
| HK2 | Correlation | -0.052 | 1 | -0.007 | 0.236 | 0.550 |
| Staining | Sig. (2 tailed) | 0.627 | < 0.01** | 0.946 | 0.025* | < 0.01** |
| PFKFB3 | Correlation | 0.270 | -0.007 | 1 | 0.207 | 0.025 |
| Staining | Sig. (2 tailed) | 0.010* | 0.946 | < 0.01** | 0.051 | 0.818 |
| PFKFB4 | Correlation | -0.044 | 0.236 | 0.207 | 1 | 0.392 |
| Staining | Sig. (2 tailed) | 0.682 | 0.025* | 0.051 | < 0.01** | < 0.01** |
| PKM2 | Correlation Coefficient | -0.011 | 0.550 | 0.025 | 0.392 | 1 |
| Staining | Sig. (2 tailed) | 0.919 | < 0.01** | 0.818 | < 0.01** | < 0.01** |
| N | 90 | 90 | 90 | 90 | 90 | 90 |
Correlation is significant at the 0.05 level;
Correlation is significant at the 0.01 level.
Correlation between the expression of glycolytic enzymes and clinical parameters
The correlations between the expression of glycolytic proteins and clinicopathological factors are detailed in Table 3. The Spearman statistics demonstrated that there was no significant correlation between the expression of any enzymes and the age of the patient. Additionally, the expression of GLUT1, PFKFB3, and PFKFB4 in PCa tissues did not correlate with the Gleason score (P > 0.05). However, further analysis showed that HK2 (r = 0.297, P < 0.01) and PKM2 (r = 1, P < 0.01) were significantly positively related to the Gleason score in PCa tissues.
Table 3.
Spearman’s correlation assay of the correlation between protein expression and clinical parameters in PCa tissue
| PCa tissue | Age | Gleason score | |
|---|---|---|---|
| GLUT1 staining score | Correlation | -0.097 | -0.031 |
| Coefficient Sig. (2-tailed) | 0.362 | 0.770 | |
| N | 90 | 90 | |
| HK2 staining score | Correlation | -0.178 | 0.297 |
| Coefficient Sig. (2-tailed) | 0.093 | < 0.01** | |
| N | 90 | 90 | |
| PFKFB3 staining score | Correlation | 0.060 | 0.088 |
| Coefficient Sig. (2-tailed) | 0.573 | 0.410 | |
| N | 90 | 90 | |
| PFKFB4 staining score | Correlation | -0.205 | 0.086 |
| Coefficient Sig. (2-tailed) | 0.053 | 0.421 | |
| N | 90 | 90 | |
| PKM2 staining score | Correlation | -0.052 | 0.431 |
| Coefficient Sig. (2-tailed) | 0.629 | < 0.01** | |
| N | 90 | 90 |
Correlation is significant at the 0.01 level (2-tailed).
Different expression levels of glycolytic enzymes in PCa with Gleason score 3+4 and Gleason score 4+3
To evaluate differences in the expression of glycolytic proteins in PCa with Gleason score 3+4 and Gleason score 4+3, 20 cases with Gleason score 3+4 and 18 cases with 4+3 were studied. Figure 2A-J shows that the degree of staining was clearly diminished in the 3+4 cases compared with the 4+3 cases. Further investigation of the IHC score indicated a significantly lower expression level of all glycolytic proteins (GLUT1 (5.10±3.89), HK2 (6.40±3.07), PFKFB3 (2.65±1.90), PFKFB4 (3.75±3.54) and PKM2 (7.00±3.40)) in Gleason score 3+4 cases compared with Gleason score 4+3 cases (7.2±3.2; 9.00±2.77; 5.28±3.53; 6.72±3.91; 9.33±2.74, P < 0.05), as shown in Figure 2K-O.
Figure 2.
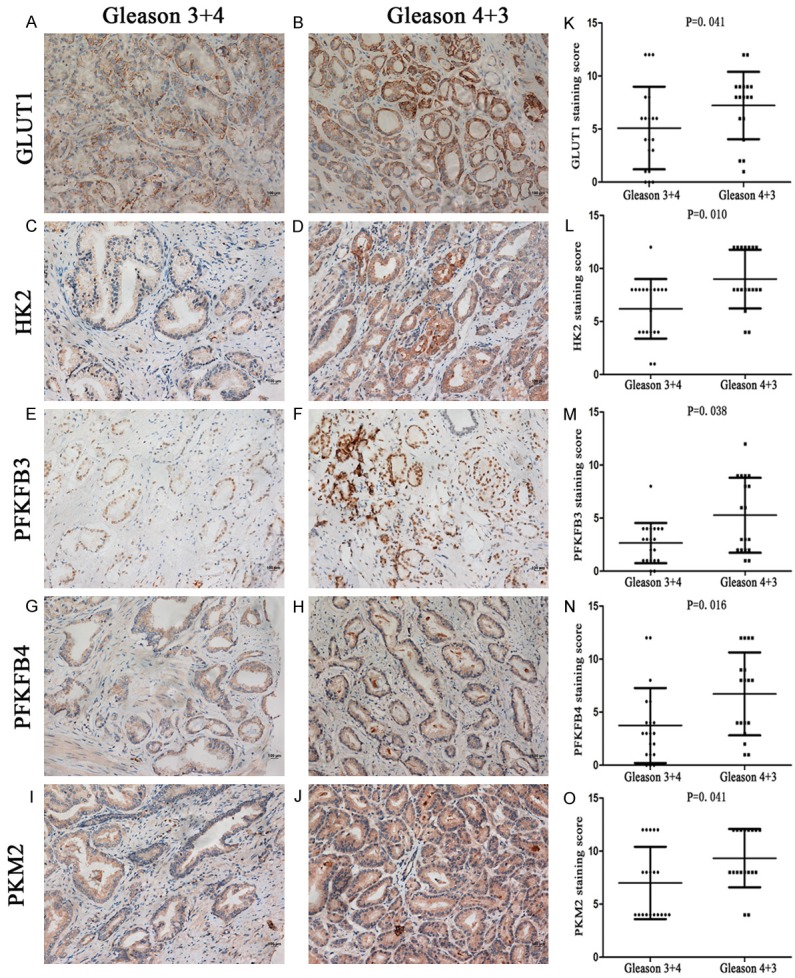
Representative immunohistochemistry showing that compared with Gleason score 3+4, all key glycolytic enzymes, GLUT1 (A), HK2 (C), PFKFB3 (E), PFKFB4 (G), and PKM2 (I), are more highly expressed in Gleason score 4+3 (B, D, F, H and J). The histograms (K-O) show the IHC score of key glycolytic enzymes, as assessed by the Mann-Whitney U test. The expression levels of all enzymes were significantly higher in Gleason score 4+3 than in Gleason score 3+4.
Discussion
PCa seems to exhibit special metabolic characteristics such that glycolysis is not as active in PCa as in liver or lung cancer [6]. However, the correlation between PCa and glycolysis remains largely unknown. Additionally, differences in terms of the expression of key enzymes involved in glycolysis between PCa and adjacent normal tissue and the relationship among these key enzymes have not been fully elucidated. Here, we investigated the expression of five glycolytic enzymes by immunohistochemistry and found that all enzymes were more highly expressed in PCa tissues than in the adjacent normal tissues. Importantly, the key glycolytic enzymes, HK2 and PKM2, were positively stained in almost all 90 cases of PCa tissues, while GLUT1, PFKFB3, and PFKFB4 were positive in approximately 60% of the cases. Although the adjacent normal tissues also showed a high positive staining rate of HK2 and PKM2, there were significant differences between the PCa tissues and the matched normal tissues in the expression of HK2 and PKM2 using the IHC scoring system, which is more precise than using the positive staining rate alone. Further correlations between the expression of enzymes and the Gleason score were studied, and the results showed that the key enzymes of glycolysis, HK2 and PKM2, were highly correlated with the Gleason score, while GLUT1, PFKFB3, and PFKFB4 showed no correlation. Although PFKFB4 showed no correlation with the Gleason score, correlative studies have shown that PFKFB4 plays an essential role in prostate cancer [6]. The PFKFB4 merits further research. Based on these results, we may infer that HK2 and PKM2 play major roles in the progression of PCa, and the levels of all glycolytic enzymes are also important.
We further analyzed the correlation among five glycolytic proteins. Every dot in the same position of each TMA was derived from the same PCa or adjacent normal tissue, allowing us to investigate the internal relationship among glycolytic proteins. We found a weak correlation between the expression of GLUT1 and PFKFB3. However, these issues remain to be clarified in subsequent analyses. In addition, Ando et al. found that both GLUT1 and PFKFB2 were weakly activated by IL-6 in the presence of cycloheximide, which supports a potential association among the glycolytic enzymes [15]. Interestingly, there was a significant correlation between the expression of the key glycolytic enzymes, HK2 and PKM2. HK has been shown to catalyze the first committed step in glucose metabolism and to facilitate all major glucose utilization pathways [16]. Although there are four types of HK enzymes in mammals, HK1, HK2, HK3, and glucokinase (GCK) [17], only HK2 appears to be more highly expressed in cancer compared with normal cells [18] and to contribute to controlling the rate of glycolysis in tumors [19]. Apart from its role in glucose metabolism, HK2 is required for the initiation and maintenance of several tumor types including prostate cancer [20,21]. Recent studies have shown that metabolic reprogramming driven by AKT-associated HK2 functions as one survival mechanism in PI3K-driven prostate cancer after the inhibition of AR [22]. There are four types of PKs-PKM1, PKM2, PKL and PKR-and a large of number studies have shown that PKM2 important for the progression of PCa [23,24]. Additional research has shown that PKM2 shares similar characteristics to HK2. PKM2 regulates the final step of glycolysis during the production of pyruvate [25,26] and contributes to glycolysis by interacting with and activating HIF-1α to elevate the expression of glycolytic genes, such as GLUT1 and lactate dehydrogenase A (LDHA) [27]. In addition to its role in glycolysis, recent studies have found that PKM2 functions as a kinase that promotes cell cycle progression by regulating mitotic checkpoints, chromosome segregation and cytokinesis in cancer cells [28,29]. Recently, Panasyuk et al. identified a far-reaching phenomenon indicating that the expression of HK2 and PKM2 in fatty livers is simultaneously regulated by PPARγ [30], elucidating a possible relationship between HK2 and PKM2. However, the correlation between HK2 and PKM2 in PCa has not yet been elucidated. Our findings revealed a significant correlation between the expression of HK2 and PKM2 in PCa, which may indicate an interaction between HK2 and PKM2. The combination of HK2 and PKM2 may provide promising diagnostic and therapeutic strategies.
PCa patients with Gleason score 3+4 or 4+3 were classified as the same group by the traditional Gleason score system and were deemed to be have the same prognosis. After the proposal of the new GGG system, studies have validated its role in the prediction of prognosis and cancer-related death in PCa [31,32]. Stamey et al. have shown that the percentage of Gleason grade 4/5 among radical prostatectomy specimens represents the most important prognostic factor in early-stage prostate cancer patients [33]. Remarkably, AS may be offered to PCa patients with certain characteristics, such as a Gleason score of 3+4 and a “favorable intermediate-risk (FIR)” (percentage of positive scores < 50%, and only one additional IR factor), according to National Comprehensive Cancer Network guidelines [34]. However, research addressing the risk of unfavorable disease among GS 3+4 FIR PCa patients revealed limitations in predicting a GS upgrade using clinical parameters alone, and new risk assessment tools to improve the prediction models are urgently needed [35]. Hence, the identification of an alternative tool to better distinguish GGG2 and GGG3 may be a promising strategy. In the present study, we investigated, for the first time, differences between the expression of all key enzymes of glycolysis in GGG2 and GGG3. The results showed that compared with GGG2, the expression of glycolytic enzymes was significantly higher in cases with GGG3, which indicated that GGG3 had a higher rate of glycolysis. Our findings suggest that PCa with GGG3 exhibits a higher glycolytic rate than GGG2, which indicates that surveillance at the level of glycolysis may be a potential and effective tool to predict an upgrade in PCa with GGG2.
To summarize, our findings showed that compared with adjacent normal tissues, PCa tissues exhibit high levels of glycolysis. Moreover, PCa with GGG3 displays higher glycolytic levels than GGG2. Additionally, the key glycolytic enzymes, HK2 and PKM2, are overexpressed in PCa and significantly correlate with the Gleason score. Furthermore, there is a significant correlation between HK2 and PKM2, which suggests that combining HK2 and PKM2 may provide an effective diagnosis and therapies for PCa. However, the present study has some limitations. First, although all specimens were examined and scored by fully trained pathologists with genitourinary expertise, the retrospective nature and the lack of pathologist reexamination of all cases represent potential limitations. Second, the sample size is a limitation. However, this is the first study to demonstrate differences between the expression of all key enzymes involved in glycolysis between GGG2 and GGG3. Further analyses with larger sample sizes across multiple centers are needed to confirm the correlation between PCa and glycolysis and between PKM2 and HK2.
Acknowledgements
This study was funded by National Natural Science Foundation of China (81372767 and 81370702); Science and Technology Planning Project of Guangdong Province (2013B021800183, 2014A020212063 and 2013B022000045); Program for New Century Excellent Talents in University, Ministry of Education, China (NCET-11-0541); Clinical Research Launching Program of Southern Medical University (LC2016PY058); Research Foundation of Shenzhen Hospital of Southern Medical University (PT2016LC04). Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Gatenby RA, Gillies RJ. Why do cancer have high aerobic glycosis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 3.Cruys B, Wong BW, Kuchnio A, Verdegem D, Cantelmo AR, Conradi LC, Vandekeere S, Bouché A, Cornelissen I, Vinckier S, Merks RM, Dejana E, Gerhardt H, Dewerchin M, Bentley K, Carmeliet P. Glycolytic regulation of cell rearrangement in angiogenesis. Nat Commun. 2016;7:12240. doi: 10.1038/ncomms12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pusapati RV, Daemen A, Wilson C, Sandoval W, Gao M, Haley B, Baudy AR, Hatzivassiliou G, Evangelista M, Settleman J. mTORC1-dependent metabolic reprogramming underlies escape from glycolysis addiction in cancer cells. Cancer Cell. 2016;29:548–562. doi: 10.1016/j.ccell.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Senyilmaz D, Teleman AA. Chicken or the egg: Warburg effect and mitochondrial dysfunction. F1000 Prime Rep. 2015;7:7–41. doi: 10.12703/P7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Value of FDG-PET scanning in management of lung cancer. Lancet. 2002;359:1361–1362. doi: 10.1016/s0140-6736(02)08388-5. [DOI] [PubMed] [Google Scholar]
- 8.Effert PJ, Bares R, Handt S, Wolff JM, Büll U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18 fluorine-labeled deoxyglucose. J Urol. 1996;155:994–998. [PubMed] [Google Scholar]
- 9.Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1511–1520. doi: 10.1016/j.eururo.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 10.Oyama N, Akino H, Suzuki Y, Kanamaru H, Sadato N, Yonekura Y, Okada K. The increased accumulation of [18F] fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–629. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 11.Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr. 2007;39:223–229. doi: 10.1007/s10863-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guimaraes MS, Quintal MM, Meirelles LR, Magna LA, Ferreira U, Billis A. Gleason score as predictor of clinicopathologic findings and biochemical (PSA) progression following radical prostatectomy. Int Braz J Urol. 2008;34:23–29. doi: 10.1590/s1677-55382008000100005. [DOI] [PubMed] [Google Scholar]
- 14.Loeb S, Folkvaljon Y, Robinson D, Lissbrant IF, Egevad L, Stattin P. Evaluation of the 2015 Gleason grade groups in a nationwide population-based Cohort. Eur Urol. 2016;69:1135–1141. doi: 10.1016/j.eururo.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando M, Uehara I, Kogure K, Asano Y, Nakajima W, Abe Y, Kawauchi K, Tanaka N. Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J Nippon Med Sch. 2010;77:97–105. doi: 10.1272/jnms.77.97. [DOI] [PubMed] [Google Scholar]
- 16.Vander HM, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara Y, Yamamoto K, Kogure K, Ichihara J, Terada H. Steady state transcript levels of the type II hexokinase and type 1 glucose transporter in human tumor cell lines. Cancer Lett. 1994;82:27–32. doi: 10.1016/0304-3835(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 19.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s doubleedged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, Jha AK, Smolen GA, Clasquin MF, Robey B, Hay N. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, Koochekpour S, Saleem M, Huang H, Lu J, Deng Y. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin PL, Yin JJ, Seng V, Casey O, Corey E, Morrissey C, Simpson RM, Kelly K. Androgen deprivation leads to increased carbohydrate metabolism and hexokinase 2-mediated survival in Pten/Tp53-deficient prostate cancer. Oncogene. 2017;36:525–533. doi: 10.1038/onc.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannoni E, Taddei ML, Morandi A, Comito G, Calvani M, Bianchini F, Richichi B, Raugei G, Wong N, Tang D, Chiarugi P. Targeting stromalinduced pyruvate kinase M2 nuclear translocation impairs oxphos and prostate cancer metastatic spread. Oncotarget. 2015;6:24061–24074. doi: 10.18632/oncotarget.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong N, Yan J, Ojo D, De Melo J, Cutz JC, Tang D. Changes in PKM2 associate with prostate cancer progression. Cancer Invest. 2014;32:330–338. doi: 10.3109/07357907.2014.919306. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett. 2013;339:153–158. doi: 10.1016/j.canlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Z. Nonmetabolic functions of pyruvate kinase isoform M2 in controlling cell cycle progression and tumorigenesis. Chin J Cancer. 2012;31:5–7. doi: 10.5732/cjc.011.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, Wei C, Guo F, Chen Y, Lu Z. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 2014;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Wang Y, Wang T, Hawke DH, Zheng Y, Li X, Zhou Q, Majumder S, Bi E, Liu DX, Huang S, Lu Z. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat Commun. 2014;5:5566. doi: 10.1038/ncomms6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, Pontoglio M, Ferré P, Scoazec JY, Birnbaum MJ, Ricci JE, Pende M. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias-Stella JA 3rd, Shah AB, Montoya-Cerrillo D, Williamson SR, Gupta NS. Prostate biopsy and radical prostatectomy Gleason score correlation in heterogenous tumors: proposal for a composite Gleason score. Am J Surg Pathol. 2015;39:1213–1218. doi: 10.1097/PAS.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 32.Berney DM, Beltran L, Fisher G, North BV, Greenberg D, Moller H, Soosay G, Scardino P, Cuzick J. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer. 2016;114:1078–1083. doi: 10.1038/bjc.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 34.NCCN clinical practice guidelines in oncology (NCCN Guidelines1). Prostate cancer. version 3. 2016. National Comprehensive Cancer Network Web site. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 35.Morlacco A, Cheville JC, Rangel LJ, Gearman DJ, Karnes RJ. Adverse disease features in Gleason score 3+4 “favorable intermediaterisk” prostate cancer: implications for active surveillance. Eur Urol. 2017;72:442–447. doi: 10.1016/j.eururo.2016.08.043. [DOI] [PubMed] [Google Scholar]


