Abstract
Acute myocardial infarction (AMI) therapy has not remarkably improved due to delay in the diagnosis to a great extent. Circulating microRNAs have shown some potential for diagnosis of cardiovascular diseases. The aim of this study was to estimate the diagnostic value of circulating miR-126-3p for AMI. In our study, circulating miR-126-3p levels were determined by quantitative polymerase chain reaction and the results showed it was 106-fold higher than that in controls, and elevated miR-126-3p was associated with aging through logistic correlation analyses. Receiver-operator characteristic curve was used to evaluate the sensitivity and specificity of miR-126-3p for diagnosis of AMI, indicating that its diagnostic effect was superior to the current clinical markers such as CK, CK-MB, hs-TnI, and MYO. Our results indicate that miR-126-3p in circulation is a potential novel diagnostic biomarker for AMI.
Keywords: Acute myocardial infarction, biomarker, diagnosis, microRNA, miR-126-3p
Introduction
Acute myocardial infarction (AMI) is a major life threatening with higher rates of mortality and morbidity [1]. World Health Organization (WHO) reported that AMI accounts for 30% of global death annually and will lead to 23 million sufferers just in China by 2030 [2]. Despite great advances in prevention strategies and treatment technologies, the cure rate needs to be promoted once AMI occurs, probably due to delay in the diagnosis [3]. The insights from the French FAST-MI program over 15 years showed that early-use of evidence-based therapy can effectively improve survival (HIR 0.54; 95% CI 0.40 to 0.72) [4]. The current methods, including electrocardiogram, coronary angiography and cardiac indicators, contribute greatly to the clinical diagnosis of AMI; however, they lack enough sensitivity and individuation for early diagnosis [5,6]. Therefore, it is essential to discover and identify the novel markers with high precocity and accuracy.
MicroRNAs (miRNAs), a class of small, non-coding RNAs that target and regulate the expression of complementary mRNAs, play a key role in tissue injury and pathological processes. miRNAs existing in microvesicles, blood and other fluid is stable due to protecting them from endogenous RNase activity [7], and more importantly they can be secreted to the extracellular environment [8,9]. Therefore, miRNA is detectable conveniently in peripheral blood and promises well as an effective disease biomarker. Pathological elevation or decrease of circulating miRNAs can predict the occurrence and prognosis of various diseases such as coronary artery disease (CAD), heart failure and cancer [10-13]. miR-126 is mainly expressed in endothelial cells, plasmacytoid decentric cells and hematopoietic progenitor cells, and plays multiple roles in cardiovascular physiology and pathology. miR-126 is implicated to be involved in the process of vessel growth and inflammatory responses [14-18]. Recently, several reports showed that miR-126 is related to athe-rosclerosis [19], and may be a predictor for vascular events. However, little is known about the association of miR-126 and AMI occurrence [20-22].
In this study, the plasma miR-126-3p level of patients with AMI was compared to that without AMI, and the specificity and sensitivity of miR-126-3p were evaluated to assess the feasibility of using it as the biomarker of AMI.
Materials and methods
Study population
27 patients with AMI and 30 non-AMI control subjects were recruited in this study from Affiliated Hospital of Guangdong Medical University. AMI was diagnosed based on combination of several clinical indices such as high-sensitive troponin (hs-TnI), myoglobin (MYO), creatine kinase (CK), CK-MB, pathological Q wave and coronary angiography. 30 controls attended routine medical examinations, and had no clinical manifestation and family history of cardiovascular disorders. Those patients who had any heart failure, arrhythmia, cardiomyopathies, inflammatory diseases or hematological disorders were excluded. Written consents were obtained from all subjects and the study protocol was approved by the Ethics Committee of Affiliated Hospital of Guangdong Medical University.
Biochemical assays
All subjects underwent the biochemical assay in the cardiovascular medicine center of the hospital. The clinical indices related to cardiovascular events were collected such as lipoprotein (a) (Lp(a)), blood urine nitrogen (BUN), serum creatinine (Scr), serum uric acid (SUA), blood sugar (Glu), total cholesterol (CHOL), triglycerides (TG), high-density lipoprotein (HDL), low density lipoprotein (LDL), apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB).
In addition, 2 mL of peripheral blood was prepared in anticoagulation tube from every subject, and then the plasma was collected after centrifugation at 1000×g for 40 min at 4°C.
Diabetes mellitus were diagnosed according to Glu levels ≥11.1 mmol/L. Hypertension and hyperlipidemia were defined as a systolic/diastolic blood pressure (BP) level ≥140/90 mm Hg and CHOL > 5.17 mmol/L, respectively.
Plasma miR-126-3p determination
miRNAs were extracted from plasma samples using the miRcute miRNA Isolation Kit (TIANGEN BIOTECH CO., Beijing, China) according to the manufacturer’s instruction. Contaminating genomic DNAs were eliminated by use of RNAse free DNase (TAKARA, Dalian, China). Reverse transcription of miRNAs was performed with the miRcute miRNA First-strand cDNA Synthesis Kit (TIANGEN BIOTECH CO., Beijing, China). The resulting cDNA was diluted 10-fold before quantitative polymerase chain reaction (qPCR) was performed by using the miRcute miRNA qPCR Detection Kit (TIANGEN BIOTECH CO., Beijing, China). The qPCR reactions were performed in triplicate. 5 s was selected as the internal control for normalization. The relative expression levels were analyzed using the 2-ΔΔCt method.
Statistical analyses
Before analyses, all data were subjected to normality test (Shapiro-Wilk). Mann-Whitney test was used to compare 2 groups of continuous variables. A Chi-square test was used for categorical variables. A binary logistic regression model was used to evaluate the association between miR-126-3p levels and the risk factors, Sensitivity and specificity of the biomarkers were evaluated with receiver-operator characteristic (ROC), and area under ROC curve (AUC), diagnostic odds ratio (OR) and their 95% confidence interval (CI) of miR-126-3p associated with the diagnostic values were estimated. Statistical analyses were 2-tailed. SPSS 20.0 and GraphPad Prism 6.0 was used for statistical analyses. P values of < 0.05 were considered statistically significant.
Results
Clinical characteristics
Within the cohort, 27 AMI patients (AMI group) were 14 female (46.67%) and 16 male (53.33%) with median age of 59.5 (44 to 75) years. These patients, together with 30 control subjects (Ctl group), were recruited to investigate the association of circulating miR-126-3p with AMI. The baseline clinical characteristics of all subjects are shown in Figure 1.
Figure 1.
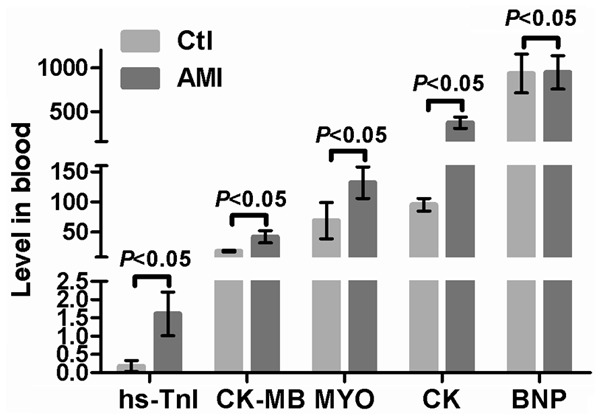
Clinical characteristics of the study population.
Significant differences between AMI group and Ctl group in some risk indicators, such as Scr, SUA, TG and HDL, which are used clinical indices of AMI diagnosis and treatment. In particular, the CAD-related biomarkers CK, CK-MB, MYO and hs-TnI confirmed the diagnosis of AMI. The interferences from hypertension, hyperlipidemia and diabetes mellitus were excluded in view of no significant differences between AMI and Ctl subjects.
Circulating miR-126-3p
The concentration of miR-126-3p was measured in the blood by qPCR, and the results revealed the mean level of miR-126-3p to be 106-fold higher in the AMI patients than that in the controls (Figure 2). This suggested that there was a very close association between miR-126-3p and AMI occurrence, implying that circulating miR-126 may be an effective marker reflecting AMI.
Figure 2.
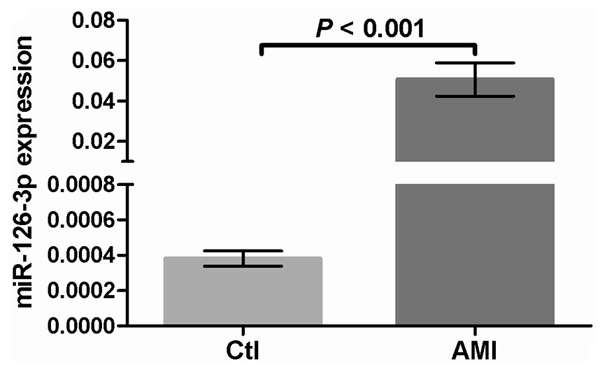
Circulating miR-126-3p level from AMI and control subjects.
The correlation analysis was used to assess the relationship between the clinical indices and AMI occurrence and further obtain the crucial risk factors of AMI. Here, The OR and their 95% CI were calculated for the clinical indices, including gender, age, Lp(a), BUN, Scr, SUA, Glu, TC, TG, HDL, HDL, ApoA1, and ApoB (Table 1). We found that the circulating miR-126-3p expression only had a notable significant correlation with age but not other risk factors (OR (95% CI): 1.316 (1.007, 1.607), P < 0.01). Hence, miR-126-3p may promote ischemic injury of heart by accelerating vascular aging and impacting reducing blood supply; on the other hand, imR-126-3p was more likely to be associated to senile cardiac diseases, and especially applied to aged population.
Table 1.
Correlation analyses between miR-126-3p and the clinic indices of AMI
| Index | P | OR (95% CI) |
|---|---|---|
| BUN | 0.103 | 0.442 (0.166, 1.179) |
| Scr | 0.090 | 1.062 (0.991, 1.139) |
| SUA | 0.129 | 1.018 (0.995, 1.043) |
| Glu | 0.559 | 1.221 (0.625, 2.385) |
| TC | 0.886 | 1.855 (0.000, 8.751×103) |
| TG | 0.306 | 0.251 (0.018, 3.545) |
| LPa | 0.393 | 1.002 (0.997, 1.007) |
| HDL | 0.227 | 0.005 (0.000, 25.458) |
| LDL | 0.480 | 0.45 (0.000, 240.486) |
| ApoA1 | 0.762 | 1.931 (0.027, 136.774) |
| ApoB | 0.340 | 473.811 (0.002, 1.489×108) |
| Gender | 0.234 | 0.151 (0.007, 3.411) |
| Age | 0.007 | 1.316 (1.077, 1.607) |
miR-126-3p as a potential biomarker of AMI
ROC was analyzed to evaluate the diagnostic power and availability of circulating miR-126-3p for AMI. The AUC value of miR-126-3p was 0.992 (95% CI = 1.000-1.000, P < 0.001), indicating that miR-126-3p has potential to become a potent biomarker with high precocity and accuracy. Comparatively, the present clinical biomarkers, such as hs-TnI (AUC 0.787; 95% CI = 0.667-0.906, P < 0.001), MYO (AUC 0.781; 95% CI = 0.660-0.903, P < 0.001), CK (AUC 0.849; 95% CI = 0.747-0.952, P < 0.001) and CK-MB (AUC 0.863; 95% CI = 0.759-0.968, P < 0.001), provided lower efficacy than miR-126-3p. These results showed miR-126-3p is able to accurately discriminate AMI from controls, and the threshold miR-126-3p expression value of 0.0000123 that maximized true-positive and false-negative results (sensitivity 0.967, specificity 0.933) (Figure 3).
Figure 3.
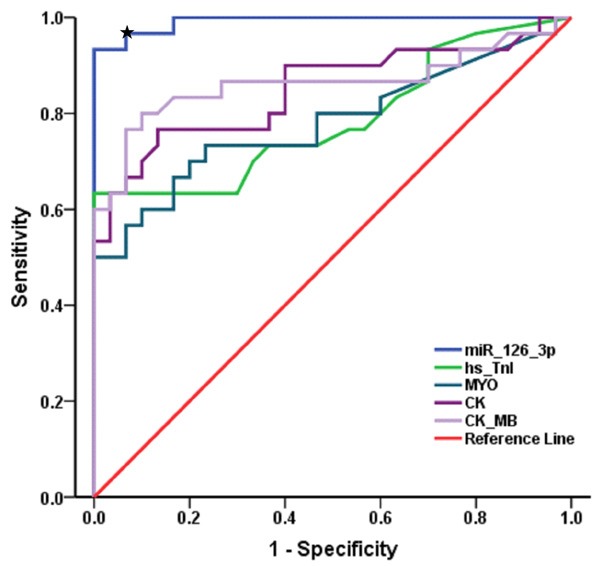
ROC analyses of miR-126-3p and the existing clinical biomarkers. The blue curve indicated miR-126-3p and other curves indicated the existing biomarkers including hs-TnI, MYO, CK and CK-MB. Asterisk indicated the threshold expression of 0.0000123 miR-126-3p maximized true-positive and false-negative results (sensitivity 0.967, specificity 0.933).
Discussion
Besides cardiac myocytes and fibroblasts, vascular endothelial cells are the indispensable elements of heart function. In the previous studies, cardiac-enriched miRNAs such as miR-1, 133, 208 and 499 were indicated to be potential biomarkers for diagnosis of AMI [23-25]. However, little is known about the association of endothelia-enriched miRNAs with AMI. We have reported that miR-19a in circulation is very close related to the occurrence of AMI [26]. Here we also found the circulating miR-126-3p was increased in the patients with AMI.
It was hypothesized that miR-126 may contribute to post-MI cardiac regeneration by affecting the homing ability of circulating hematopoietic progenitor cells [27]. However, miR-126 is also shown to play an indirect role in leukocyte infiltration and vascular inflammation [17,18], which may further exacerbate myocardial ischemic injury. Thus, miR-126 plays a key role in myocardial tissue injury and pathological processes through various means.
This study indicates that miR-126-3p is a sensitive and specific biomarker for AMI. The circulating miR-126-3p level in AMI patients was 106-fold higher than control subjects, which reached the highly detectable and differentiable requirements. Therefore, miR-126-3p in circulation was greatly associated with the occurrence of AMI, and provided high diagnostic accuracy and discriminating ability. To avoid possible sample deviation from selection of clinical subjects, the ages of AMI and controls subjects were limited to 40-80 years, and all cardiovascular events, stroke, tumor, and inflammatory diseases were excluded in this study. Furthermore, the results of logistic regression analyses confirmed that gender and glomerular filtration did not affect the circulating miR-126-3p level, indicating that miR-126-3p was of high diagnostic value for AMI.
miRNAs have been demonstrated to be critical mediators in cardiovascular system responding to injury, such as myocardial infarction caused by ischemia/reperfusion damage [28,29]. Moreover, they can be secreted to the extracellular environment so as to be detected conveniently in a wide range of cell-free serum [8,9]. Thus, changes in serum miRNAs have been proposed as potential biomarkers for a variety of diseases [30]. miR-126-3p has been identified as an effective biomarker of rheumatoid arthritis and acute kidney injury [31,32]. A recent report showed that the level of miR-126 was significantly decreased in patients with CAD and high LDL cholesterol level, while significantly increased when LDL cholesterol was high in patients who had risk factors for CAD but did not have angiographically significant CAD, implying that miR-126 may regulate cholesterol metabolism [33]. However, we found plasma miR-126-3p showed no significant correlation with Lp(a), TG, TC, HDL, LDL, ApoA1 and ApoB, suggesting that miR-126 may influence the occurrence of AMI through other mechanisms.
In the present study, circulating miR-126-3p levels were determined in peripheral blood, and elevated miR-126-3p was considered to be associated with aging based on significant differences and logistic correlation analyses. ROC method was used to evaluate the sensitivity and specificity of miR-126-3p for diagnosis of AMI, its diagnostic effect was superior to all of the current clinical markers such as CK, CK-MB, hs-TnI and MYO. Therefore, miR-126-3p in circulation is a potential biomarker for predicting and monitoring therapy outcome of AMI, and promises well as a novel generation of AMI diagnostic tool, helpful for improvement of AMI patient management in clinical practice.
Acknowledgements
This paper was supported by Collaborative Innovation and Platform Environment Construction Projects of Guangdong Province (2015A050502049), Science and Technology Project of Guangdong Province (2014A020212739 and 2016A020214017), Medical Science Foundation of Guangdong Province (A2015286 and A2016230) and National Natural Science Foundation of China (No. 81270262 and No. 81300035).
Disclosure of conflict of interest
None.
References
- 1.Perricone AJ, Vander Heide RS. Novel therapeutic strategies for ischemic heart disease. Pharmacol Res. 2014;89:36–45. doi: 10.1016/j.phrs.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Cardiovascular diseases. World Health Organization. URL <http://www.who.int/cardiovascular_diseases/en/> (accessed 12.12.-14.). 2014.
- 3.Maznyczka A, Kaier T, Marber M. Troponins and other biomarkers in the early diagnosis of acute myocardial infarction. Postgrad Med J. 2015;91:322–30. doi: 10.1136/postgradmedj-2014-133129. [DOI] [PubMed] [Google Scholar]
- 4.Puymirat E, Schiele F, Steg PG, Blanchard D, Isorni MA, Silvain J, Goldstein P, Guéret P, Mulak G, Berard L, Bataille V, Cattan S, Ferrières J, Simon T, Danchin N FAST-MI investigators. Determinants of improved one-year survival in non-ST segment elevation myocardial infarction: insights from the french FAST-MI program over 15 years. Int J Cardiol. 2014;177:281–6. doi: 10.1016/j.ijcard.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Reddy K, Khaliq A, Henning RJ. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J Cardiol. 2015;7:243–76. doi: 10.4330/wjc.v7.i5.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathil MF, Md Arshad MK, Gopinath SC, Hashim U, Adzhri R, Ayub RM, Ruslinda AR, Nuzaihan M N M, Azman AH, Zaki M, Tang TH. Diagnostics on acute myocardial infarction: cardiac troponin biomarkers. Biosens Bioelectron. 2015;70:209–20. doi: 10.1016/j.bios.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Février B, Raposo G. Exosomes: endosomalderived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–21. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–9. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 11.van Empel VP, De Windt LJ, da Costa Martins PA. Circulating miRNAs: reflecting or affecting cardiovascular disease? Curr Hypertens Rep. 2012;14:498–509. doi: 10.1007/s11906-012-0310-7. [DOI] [PubMed] [Google Scholar]
- 12.Redova M, Sana J, Slaby O. Circulating miRNAs as new blood-based biomarkers for solid cancer. Future Oncol. 2013;9:387–402. doi: 10.2217/fon.12.192. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Wang Q, You W, Chen M, Xia J. MiRNAs as biomarkers of myocardial infarction: a meta analysis. PLoS One. 2014;9:e88566. doi: 10.1371/journal.pone.0088566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 17.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular disease, inflammation and angiogensis. Cadiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shao S, Geng H, Yu Y, Wang C, Liu Z, Yu C, Jiang X, Deng Y, Gao L, Zhao J. Expression profiles of six circulating microRNAs critical to atherosclerosis in patients with subclinical hypothyroidism: a clinical study. J Clin Endocrinol Metab. 2014;99:E766–774. doi: 10.1210/jc.2013-1629. [DOI] [PubMed] [Google Scholar]
- 20.Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3:e001249. doi: 10.1161/JAHA.114.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JM, Jung KH, Chu K, Lee ST, Ban J, Moon J, Kim M, Lee SK, Roh JK. Atherosclerosis-related circulating microRNAs as a predictor of stroke recurrence. Transl Stroke Res. 2015;6:191–197. doi: 10.1007/s12975-015-0390-1. [DOI] [PubMed] [Google Scholar]
- 23.Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, Li K, Yu B, Li Z, Wang R, Wang L, Li Q, Wang N, Shan H, Li Z, Yang B. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 25.Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, Corsten MF, Schroen B, Lair ML, Heymans S, Wagner DR. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem. 2012;58:559–567. doi: 10.1373/clinchem.2011.173823. [DOI] [PubMed] [Google Scholar]
- 26.Zhong J, He Y, Chen W, Shui X, Chen C, Lei W. Circulating microRNA-19a as a potential novel biomarker for diagnosis of acute myocardial infarction. Int J Mol Sci. 2014;15:20355–20364. doi: 10.3390/ijms151120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguado-Fraile E, Ramos E, Sáenz-Morales D, Conde E, Blanco-Sánchez I, Stamatakis K, del Peso L, Cuppen E, Brüne B, Bermejo ML. miR-127 protects proximal tubule cells against ischemia/reperfusion: identification of kinesin family member 3B as miR-127 target. PLoS One. 2012;7:e44305. doi: 10.1371/journal.pone.0044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguado-Fraile E, Ramos E, Conde E, Rodríguez M, Liaño F, García-Bermejo ML. microRNAs in the kidney: novel biomarkers of acute kidney injury. Nefrologia. 2013;33:826–834. doi: 10.3265/Nefrologia.pre2013.Aug.12198. [DOI] [PubMed] [Google Scholar]
- 30.Saikumar J, Ramachandran K, Vaidya VS. Noninvasive micromarkers. Clin Chem. 2014;60:1158–1173. doi: 10.1373/clinchem.2013.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro-Villegas C, Pérez-Sánchez C, Escudero A, Filipescu I, Verdu M, Ruiz-Limón P, Aguirre MA, Jiménez-Gomez Y, Font P, Rodriguez-Ariza A, Peinado JR, Collantes-Estévez E, González-Conejero R, Martinez C, Barbarroja N, López-Pedrera C. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis Res Ther. 2015;17:49. doi: 10.1186/s13075-015-0555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguado-Fraile E, Ramos E, Conde E, Rodríguez M, Martín-Gómez L, Lietor A, Candela Á, Ponte B, Liaño F, García-Bermejo ML. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One. 2015;10:e0127175. doi: 10.1371/journal.pone.0127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, Satoh H, Fujii S. Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J. 2012;10:16. doi: 10.1186/1477-9560-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


