Abstract
Trigeminal neuralgia (TN) is one of the most intense forms of facial pain. It has been reported that the P2X3 receptor plays a crucial role in facilitating pain transmission, and the calcitonin-gene-related peptide (CGRP) from trigeminal ganglia (TGs) might perform differing function in nociceptive afferent input transmission. The present study investigated whether emodin can affect TN pain transmission by suppressing the expression of P2X3 receptors and CGRP in TGs. Chronic constriction injury of the infraorbital branch of the trigeminal nerve (CCI-ION) was used as TN model. The TN rats were randomly divided into the following 4 groups: (1) a sham group (Sham), (2) a sham rats treated with emodin group (TN + E), (3) a TN rats treated with 0.5% sodium carboxymethyl cellulose (CMC) as vehicle group (TN) and (4) a TN rats treated with emodin group (TN + E). The mechanical hyperalgesia threshold of TN rats was tested by Electric Von Frey filaments. The change of the expression of P2X3 receptors and CGRP in rat’s TG was detected with RT-PCR, immunohistochemical staining, and Western blotting. The phosphorylation of p38 and ERK1/2 pathway of TG was detected by Western blotting. After CCI-ION injury, the threshold of mechanical hyperalgesia for the territory of ligated infraorbital nerve in TN group decreased significantly compared with that in sham group. On day 14 after operation of CCI-ION, there was also an evident increase in the expression of P2X3 receptors and CGRP in the TG of TN group. However after treatment with emodin, the response of mechanical hyperalgesia of TN rats was clearly increased while the enhanced expression of P2X3 receptor and CGRP in TN rats was significantly decreased. The phosphorylation of p38 and ERK1/2 in TN group was stronger than that in Sham group. But these phosphorylation changes in the TN rats were much weaker after treatment with emodin. In conclusion, P2X3 receptor may cooperate with CGRP in the pain transmission of TN, and emodin can inhibit the expression and activation of P2X3 receptor and CGRP in TG to relieve TN.
Keywords: Emodin, trigeminal neuralgia, P2X3 receptor, calcitonin-gene-related peptide, trigeminal ganglia
Introduction
Trigeminal neuralgia (TN), one of the most intense forms of facial pain, has been caught for great clinical interest. TN leads to paroxysms of short-lasting but very uncomfortable pain. Typically the pain is severe, lancinating, and activated by cutaneous stimulation [1]. The incidence of TN is 4.3 per 100,000 persons per year, and slightly higher for women (5.9 per 100,000) than men (3.4 per 100,000) [2]. There are some medical and surgical treatments for TN. The preferred medical treatments consist of anticonvulsant drugs, muscle relaxants and neuroleptic agents. Gasserian ganglion percutaneous techniques, gamma-knife surgery and microvascular decompression are used as the invasive treatment options for patients who are refractory to medications [3,4]. Studies have being conducted towards a better understanding of TN, but the underlying pathophysiological mechanism is still unclear.
Evidence indicates that ATP is implicated in peripheral pain signaling by acting on P2X3 receptors, and the P2X3 receptor of sensory neurons plays a crucial role in pain perception [5-7]. Upregulation of P2X3 expression in the dorsal root ganglion (DRG) has been observed under the neuropathic pain and inflammatory pain [6]. P2X3 receptor may play a role in the mediation of abnormal nociception in primary sensory neurons during orthodontic pain and trigeminal neuralgia [8,9]. It was reported that afferent neurons expressing calcitonin-gene-related peptide (CGRP) in trigeminal ganglions might perform differing roles in nociceptive afferent input transmission [10,11]. Cady et al. found that when sensory axon reflexes following activation of primary afferent neurons, P2X3 up-expression or activation by ATP may give rise to release of CGRP, which contributes to inflammatory or pain responses [12]. Therefore, we postulated there is a positive action of P2X3 receptor on CGRP in TN transmission.
Emodin (3-methyl-1, 6, 8-trihydroxyanthraquinone) is an active anthraquinone constituent of rhubarb extract and functions through anti-mutagenic, anti-cancer, anti-diuretic, vasorelaxant, anti-inflammation, anti-apoptosis and immunosuppressive activities [13-18]. Our previous studies showed that emodin relieved peripheral neuropathic pain [19], but it is not clear whether emodin can affect the signal transmission of the TN. Facial neuropathic pain can be experienced after nerve injury of trigeminal nerve [20]. TN is an example of an extreme form of neuropathic pain and remains to be a real therapeutic challenge. It has a significant impact on the quality of life and the socioeconomic functioning of the patients [21]. The present study has investigated the effects of emodin on pain transmission mediated by P2X3 receptor and CGRP in the TN model.
Materials and methods
Ethical approval and animals
Male Sprague-Dawley rats (180-230 g) were provided by the Center of Laboratory Animal Science of Nanchang University. Use of the animals was reviewed and approved by the Animal Care and Use Committee of the Medical College of Nanchang University. The ethical guidelines of the International Association for the Study of Pain (IASP) for pain research in animals were followed.
Experimental design
The rat TN model (a chronic constriction injury of the infraorbital branch of the trigeminal nerve, CCI-ION) was established as described previously [20]. Emodin was dissolved in 0.5% sodium carboxymethyl cellulose (CMC) as vehicle at the concentration of 10 mg/ml. The animals were housed in plastic boxes in a group of three at 21-25°C. Rats (n = 72) were randomly divided into sham operation group (Sham), sham operation group treated with emodin (Sham + E), TN model group (TN), and TN rats treated with emodin (TN + E). Each group had 18 rats. Emodin was administrated once a day at dosage of 50 mg/kg by intraperitoneal injection after sham or CCI-ION operation for 13 days.
Emodin (Batch No: ZL080726) and CMC were obtained from Nanjing Zhelang Medical Company and P2X3 antibody was bought from Chemicon International, Inc. USA. CGRP antibody was bought from ABCAM International, Inc. USA. Antibodies for extracellular signal-regulated kinase1 and 2 (ERK1/2), p-ERK1/2, p38 and p-p38 were bought from Cell Signaling, USA.
Chronic constriction injury of the infraorbital branch of the trigeminal nerve (CCI-ION)
The CCI-ION procedure was performed for establish a TN model. Each rat was anesthetized with penthiobarbital sodium (Shanghai Xingya Medical Company, Batch No: 140601). After skin preparation and disinfection, an arc incision was made upon the brow and achieved a blunt dissection using a glass needle. In this process the skull, frontal bone and nasal bone would be seen gradually until the fossa orbitalis appeared. The orbital contents were pushed aside using the glass needle to expose the infraorbital nerve located at the bottom of the medial orbital. Two ligatures (5-0 chromic gut) were performed loosely with microsurgical techniques. The interval between two ligatures was approximately 1 mm. The same investigator created CCI-ION animals to avoid variation. The standard tightness for ligatures is that the diameter of the nerve fibers should appear slightly thinner, which will not affect the blood circulation. Finally, the incision was sutured routinely and the rats were fed normally after woke up. In the sham-operated rats the nerve was left untouched.
Measurement of mechanical withdrawal threshold (MWT)
Noxious-pressure stimulation was used to evaluate mechanical hyperalgesia. Unrestrained rats were placed in a clear plastic chamber (22×12×22 cm) on a stainless steel mesh floor and allowed to acclimate. The mechanical hyperalgesia threshold of rats was tested using electric von Frey filaments (BME-404 NO.E5489). Nociception was assessed on days 0, 1, 3, 5, 7, 9, 11, and 13 after CCI-ION by measurement of the withdrawal threshold in the innervated area of the infraorbital nerve. The area of stimulation was centered on the rat nasal area, and extended to the vibrissa. Stimulations were administered when the rat was in a sniffing/no locomotion state: with four paws placed on the ground, neither moving nor freezing, but exhibiting sniffing behavior. A new stimulus was applied only when the rat resumed this position and at least 30 s after the preceding stimulation.
Immunohistochemistry
On day 14 after operation of CCI-ION, 6 rats of each group were anesthetized with penthiobarbital sodium and perfused transcardially with 200 ml of normal saline, followed by 200-300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS). The TG of surgery side was dissected, postfixed for 24 h, and transferred into 20% sucrose in 0.1 M PBS at 4°C for overnight. Tissues were sectioned at 15 µm at a cryostat and stored at -20°C. One tissue from six nonadjacent sections of the TG segments was selected randomly for immunohistochemistry examination. Immunohistochemical staining was performed using SP-9001 kit (Beijing Zhongshan Biotech CO.) according to the manufacturer’s instruction. After immunohistochemistry, image scanning analysis system (HMIV-2000, Wuhan) was used to analyze the changes in stain values (average optical density) of P2X3 or CGRP in ganglia. Background was determined by averaging the optical density of 10 random areas.
Reverse transcription-polymerase chain reaction (RT-PCR)
On the day 14 after operation, 6 Sprague-Dawley rats of each group were decapitated after anesthetized with urethane [1.2 g/kg, intraperitoneally (i.p.)]. The TGs were taken out and transferred immediately into PBS. Then total RNA was isolated from TGs using a chloroform procedure with TRIzol Reagent Kits (TIANGEN Co.) according to the manufacturer’s protocols. The cDNA synthesis was performed with Revert Aid First Strand cDNA Synthesis Kit (Fermentas Co.). Then, the cDNA was stored at 4°C after 5 min reaction at 99°C. Afterward, 10 μL cDNA product was used as templates in PCR amplifications together with suitable primers (for P2X3, sense: 5’-CAACTTCAGGTTTGCCAAA-3’, and antisense: 5’-TGAACAGTGAGGGCCTAGAT-3’; for CGRP sense: 5’-GTCATCGCTCACCAGGGAGG-3’, and antisense: 5’-CACACCGCTTAGATCTGGGG-3’; for β-actin, sense: 5’-TAAAGACCTCTATGCCAACACAGT-3’, and antisense: 5’-CACGAT GGAGGGGCCGGACTCATC-3’). The 10 μL PCR products were amplified with the following parameters: 94°C×3 min→94°C×45 sec, 57°C×45 sec, 72°C×45 sec, 30 cycles→72°C×5 min.
Western blotting analysis
On the day 14 after operation, 6 Sprague-Dawley rats of each group were decapitated after anesthetized with urethane [1.2 g/kg, intraperitoneally (i.p.)]. The TGs were isolated and flushed with ice-cold PBS. Ganglia were homogenized by mechanical disruption in lysis buffer (containing phosphatase inhibitors for detection of phosphorylated substrates). Homogenate was then pelleted at 6,000 g for 10 min and supernatant was collected. The quantity of total protein in the supernatant was determined with Lowry method. Samples were diluted with buffer (100 mM TrisCl, 200 mM dithiothreitol, 4% sodium dodecylsulfate (SDS), 0.2% bromophenol Blue, 20% glycerol) and heated to 95°C for 10 min. Equal amounts of protein (20 μg) from each sample were separated by SDS-polyacrylamide gel electrophoresis using Bio-Rad system and 12% gel. The separated proteins were electrophoretically transferred onto PVDF membrane by using the same system. The membrane was blocked with 5% non-fat dry milk in 25 mmol/L Tris buffered saline, pH 7.2, plus 0.1% Tween 20 (TBST) for 3 h at room temperature, followed by incubation with primary antibodies (rabbit anti-P2X3 1:500; rabbit anti-CGRP 1:1000; rabbit polyclonal anti-ERK1/2 and rabbit polyclonal anti-P-ERK1/2 1:1000) in the same buffer for overnight at 4°C. The membrane was then washed in TBST, and incubated with secondary antibody, horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (1:1000, Beijing Zhongshan Biotech. Co.) in the same buffer for 1 h at room temperature. After a final wash in TBST, chemiluminescent signals were collected on autoradiography film with the use of the enhanced chemiluminescence (ECL) kit (Shanghai Pufei Biotech. Co.). The quantification of band intensity was carried out with AlphaImager 2200 software. Band densities were normalized to individual β-actin internal control.
Statistical analysis
All results were expressed by mean ± S.E.M. Differences between treatment groups were analyzed by Student’s t-test or, where appropriate, ANOVA followed by Dunnett’s posthoc test for multiple comparisons. P value<0.05 was considered to be statistically significant.
Results
Effects of emodin reduces the mechanical hyperalgesia in TN rats
After operation, MWT (mechanical withdrawal threshold) of rats in TN group from d3 to d13 was lower than that in Sham group. After treatment of emodin, however, the MWT of TN + E group raised significantly compared with TN group from postoperative d5 to d13 [d3: P<0.01, F (3.44) = 25.031; d5: P<0.01, F (3.44) = 189.068; d7: P<0.01, F (3.44) = 194.039; d9: P<0.01, F (3.44) = 182.762; d11: P<0.01, F (3.44) = 182.238; d13: P<0.01, F (3.44) = 157.061]. Emodin had no significant effect on MWT of rats in Sham group after operation (P>0.05). See Figure 1.
Figure 1.

Effect of emodin on the mechanical withdrawal threshold (MWT) of TN rats. The MWT of rats in the four groups was measured. N = 12 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Effects of emodin attenuates the expression of P2X3 and CGRP in TG
Effects of emodin on the expression of P2X3 and CGRP mRNA within TG neurons were assessed by RT-PCR. The mRNA levels of P2X3 and CGRP were 42% and 38% higher respectively (P<0.01) in TG rats than those in control rats (Figures 2 and 3). There was no difference in the expression of P2X3 receptor or CGRP between Sham group and Sham + E group, while both P2X3 receptor and CGRP mRNA expression in TN + E group was significantly lower than that in TN group [for CGRP: P<0.01, F (3.20) = 8.147; for P2X3: P<0.01, F (3.20) = 11.281]. See Figures 2 and 3.
Figure 2.

Effects of emodin on the expression of P2X3 receptor at mRNA level in TG. The expression of P2X3 receptor mRNA in TG of each group on postoperative d14 was tested by RT-PCR. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Figure 3.

Effects of emodin on the expression of CGRP at mRNA level in TG. The expression of CGRP mRNA in TG of each group on postoperative d14 was tested by RT-PCR. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
The expression of P2X3 receptor and CGRP within the TG neurons was also examined by immunohistochemistry, which was done in the same animals that had gone through the behavioral testing. The immunostaining of P2X3 was significantly increased by 83% in TG rats than that in control rats (Figure 4), emodin had no effect on the intensity of P2X3 receptor staining in Sham group (P>0.05), but significantly decreased the intensity of P2X3 receptor staining in TN group (P<0.01, F (3.20) = 72.79] (Figure 4). Similarly, the density of CGRP staining was 115% higher in TN group than the Sham group and emodin was able to significantly lower CGRP staining in TN groups [P<0.01, F (3.20) = 68.171]. See Figures 4 and 5.
Figure 4.

Effects of emodin on the expression of P2X3 receptor in TG assessed by immunohistochemistry. On postoperative d14, the average optical density of P2X3 staining in TG was measured. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group (arrows indicate the immunostained neurons; scale bars, 100 μm).
Figure 5.
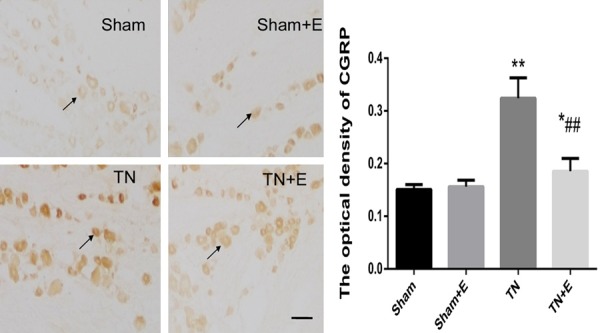
Effects of emodin on the expression of CGRP in TG assessed by immunohistochemistry. On postoperative d14, the average optical density of CGRP in TG was measured. N = 6 per group, *P<0.05 vs. sham group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group (arrows indicate the immunostained neurons; scale bars, 100 μm).
Meanwhile, the effects of emodin on the expression of P2X3 and CGRP at the protein level in TG were detected by Western blotting. The mass of both P2X3 and CGRP was significantly upregulate by 164% and 175% respectively in TN group (Figures 6 and 7). No difference was seen between Sham + E group and the Sham group. However, the increased mass of both P2X3 and CGRP protein in TN rats was effectively diminished by emodin [for CGRP, P<0.01, F (3.20) = 51.817; for P2X3, P<0.01, F (3.20) = 61.492]. See Figures 6 and 7.
Figure 6.

Effects of emodin on the expression of P2X3 receptor at protein level in TG. The expression of P2X3 receptor protein in TG of each group on postoperative d14 was determined by Western blotting. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Figure 7.

Effects of emodin on the expression of CGRP at protein level in TG. The expression of CGRP protein in TG of each group on postoperative d14 was determined by Western blotting. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Effects of emodin suppresses the phosphorylation of p38 and ERK1/2 of TG
The phosphorylation of p38 and ERK1/2 in TG were measured by Western blotting. The results showed that the phosphorylation of p38 and ERK1/2 in TN group was stronger than that in Sham group. However, the enhanced phosphorylation of p38 and ERK1/2 in TN rats was remarkably decreased by emodin treatment [for p38, P<0.01, F (3.20) = 17.066; for ERK, P<0.01, F (3.20) = 20.286]. See Figures 8 and 9.
Figure 8.

Effect of emodin on the phosphorylation of p38 in TG. The expression levels of p38 and p-p38 were detected by Western blotting. Using image analysis, the stain values (integrated optical density) of p-p38 were normalized to individual p38/β-actin. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Figure 9.

Effect of emodin on the phosphorylation of ERK1/2 in TG. The expression levels of ERK1/2 and p-ERK1/2 were detected by Western blotting. Using image analysis, the stain values (integrated optical density) of p-ERK1/2 were normalized to individual ERK1/2/β-actin. N = 6 per group, **P<0.01 vs. sham group; ##P<0.01 vs. TN group.
Discussion
TN is characterized by recurrent episodes of severe, shock-like pain confined to the distribution of one or more of the nerve’s three major branches: the ophthalmic (V1), maxillary (V2), or mandibular (V3) [22]. In this study, a chronic constriction injury of the infraorbital branch of the trigeminal nerve (CCI-ION) was induced as a rat model of the TN [20,23,24]. Responsiveness to mechanical stimulation (vibrissae territories) with von Frey filaments was used to evaluate allodynia after ligature of infraorbital nerve branch. Consequently, lesion and inflammation of the nervous fiber in primary afferent TG occurred after CCI-ION, which were involved in transmission of noxious information. Our studies showed that a hyper-responsiveness of the territory of the ligated infraorbital nerve to light mechanical stimulation with von Frey hairs was developed on day 3 after the injury.
It is reported that activation of P2X3 receptor subtype in primary sensory neurons is involved in neuropathic pain [6,25]. P2X3 receptor, when activated, depolarizes nociceptive neurons to facilitatethe transmission of pain, and blockading the receptor can reduce nociception mediated by sensory neurons in chronic pain states [26,27]. It has been reported that activation of P2X3 and P2X2/3 receptors in the tooth pulp is sufficient to elicit nociceptive behavioral responses and trigeminal brainstem neuronal activity [28]. Neuropathic pain enhances the expression of P2X3 receptor in TG neurons [29,30]. In our lab, we also found that the expression of P2X3 receptor in TG neurons of CCI-ION rats increased significantly compared to that in control group [9]. Thus it is possible that the hyperalgesia in CCI-ION rats is caused by functional up-regulation of P2X3 receptor in TG neuron.
In our study, the immunoreactive staining of P2X3 and CGRP in the TG of TN group is more intense than Sham group, indicating the potential involvement of P2X3 receptor and CGRP of TG in nociceptive responses after nerve injury. Some researchers suggested that therapeutics for craniofacial pain might be more effective if both P2X3 receptor and CGRP in TG are targeted [31]. So we believed that there was the cooperativity between P2X3 receptor and CGRP in TG in the TN pain transmitting.
The CGRP expressed by peripheral sensory neurons is implicated in the underlying pathology of neuropathic pain, and up-regulation of P2X3 receptor stimulated CGRP release in the TG neurons, contributing to pain and allodynia [10,12]. Furthermore, CGRP could enhance P2X3 receptor activity on cultured mouse trigeminal neurons, and CGRP induced by local inflammation in the lower lip could increase P2X3 receptor in TG neuron [30,32]. In response to activation of trigeminal nociceptors, CGRP might facilitate inflammatory injury in the TN. The CGRP-mediated increase in P2X3 expression and ATP release was shown to involve activation of nociceptive signaling pathways. In our study, after operation from d3 to d13, the MWT of rats in TN group was significantly lower than that in Sham group. Thus, positive interaction between P2X3 receptor and CGRP may occur in the pathological changes of TN.
Emodin is an active anthraquinone constituent of rhubarb extract. It was reported that emodin exhibited the anti-inflammatory effect [33,34]. It was demonstrated that emodin was able to relieve the chronic pain by decreasing the expression of P2X2/3 receptors in L4/L5 DRG in CCI rats [19]. Current international guidelines recommend that carbamazepine and oxcarbazepine are the first-line drugs. If there is a decrease in efficacy or tolerability of medication, surgery needs to be considered [35]. However, carbamazepine is reported as efficacious in only 70-80% of patients, and associated with adverse effects such as drowsiness, confusion, nausea, ataxia, nystagmus and hypersensitivity, which may necessitate discontinuation of medication [36]. Therefore it is cried for further investigating the mechanism of pain formation and developing new analgesic drugs of TN. Neuropathic pain, especially TN, is often not adequately controlled by currently available analgesics. The results of our study showed that treatment of emodin increased the threshold of mechanical hypersensitivity and reduced the expression of P2X3 receptor and CGRP of TG in TN rats. Thus emodin would be useful to relieve TN pain by these new mechanisms.
It was reported that CGRP up-regulated the active forms of the MAP kinases (p38 and ERK) and PKA in TG, causing a sustained increase in the expression of P2X3 receptor in spinal neurons [12]. During inflammation and concurrent hypersensitivity to mechanical noxious stimulation, the activated P2X3 receptor in DRG neurons enhanced the phosphorylation of ERK and led to the functional activation of primary afferent neurons [37]. Our results revealed that the phosphorylation of ERK1/2 and p38 of TG in TN group was stronger than that in Sham group. In TN rats treated with emodin, such increased phosphorylation of ERK1/2 and p38 was significantly inhibited. These data suggest that when the nociceptive stimulus was presented to the inflamed site, abundant ATP would leak out from the damaged cells to activate P2X3 receptor and increase CGRP release, resulting in the phosphorylation of ERK and p38, and eventually the induction of mechanical hyperalgesia in TN rats. Therefore we proposed that emodin may decrease the activation of P2X3 receptor and inhibit the release of CGRP to reduce the phosphorylation of ERK1/2 and p38 in TG.
Conclusions
In summary, P2X3 receptor and CGRP of TG are involved in TN of CCI-ION rats. Emodin can decrease the expression of P2X3 receptor and CGRP release in TG and inhibit the primary afferent pain transmission mediated by P2X3 receptor and CGRP during TN. Emodin may be used as a new drug with fewer side effects for management of TN.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81360136, 81460106), the Major Disciplines of Academic and Technical Leaders Project of Jiangxi Province (20142BCB22001), the Jiangxi Science and Technology Support Plan (20142BBG70051) and the Jiangxi Education Project (151470).
Disclosure of conflict of interest
None.
References
- 1.Hodaie M, Coello AF. Advances in the management of trigeminal neuralgia. J Neurosurg Sci. 2013;57:13–21. [PubMed] [Google Scholar]
- 2.Obermann M, Katsarava Z. Update on trigeminal neuralgia. Expert Rev Neurother. 2009;9:323–329. doi: 10.1586/14737175.9.3.323. [DOI] [PubMed] [Google Scholar]
- 3.Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences (Riyadh) 2015;20:107–114. doi: 10.17712/nsj.2015.2.20140501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thapa D, Ahuja V, Dass C, Verma P. Management of refractory trigeminal neuralgia using extended duration pulsed radiofrequency application. Pain Physician. 2015;18:E433–435. [PubMed] [Google Scholar]
- 5.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 6.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Xu C, Yu K, Li G, Wan F, Liu S, Lin J, Liu H, Zhang J, Li X, Liang S. Effect of tetramethylpyrazine on DRG neuron P2X3 receptor involved in transmitting pain after burn. Burns. 2010;36:127–134. doi: 10.1016/j.burns.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Lai WL, Chen YX. [Differential regulation of P2X3 protein expression in the rat trigeminal ganglion after experimental tooth movement] . Hua Xi Kou Qiang Yi Xue Za Zhi. 2006;24:389–392. [PubMed] [Google Scholar]
- 9.Xiong W, Tan M, He L, Ou X, Jin Y, Yang G, Huang L, Shen Y, Guan S, Xu C, Li G, Liu S, Xu H, Liang S, Gao Y. Inhibitory effects of tetramethylpyrazine on pain transmission of trigeminal neuralgia in CCI-ION rats. Brain Res Bull. 2017;134:72–78. doi: 10.1016/j.brainresbull.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9:9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Durham PL, Masterson CG. Two mechanisms involved in trigeminal CGRP release: implications for migraine treatment. Headache. 2013;53:67–80. doi: 10.1111/j.1526-4610.2012.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 14.Tabolacci C, Cordella M, Turcano L, Rossi S, Lentini A, Mariotti S, Nisini R, Sette G, Eramo A, Piredda L, De Maria R, Facchiano F, Beninati S. Aloe-emodin exerts a potent anticancer and immunomodulatory activity on BRAF-mutated human melanoma cells. Eur J Pharmacol. 2015;762:283–292. doi: 10.1016/j.ejphar.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, Sethi G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341:139–149. doi: 10.1016/j.canlet.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Qu K, Shen NY, Xu XS, Su HB, Wei JC, Tai MH, Meng FD, Zhou L, Zhang YL, Liu C. Emodin induces human T cell apoptosis in vitro by ROSmediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacol Sin. 2013;34:1217–1228. doi: 10.1038/aps.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Zhang N, Cao Y, Zhang W, Su G, Sun Y, Liu Z, Li F, Liang D, Liu B, Guo M, Fu Y, Zhang X, Yang Z. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-kappaB and MAPKs signal pathways. Eur J Pharmacol. 2013;705:79–85. doi: 10.1016/j.ejphar.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Zhang J, Li G, Xu H, Yi Y, Wu Q, Song M, Bee YM, Huang L, Tan M, Liang S, Li G. Protection of vascular endothelial cells from high glucose-induced cytotoxicity by emodin. Biochem Pharmacol. 2015;94:39–45. doi: 10.1016/j.bcp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Liu H, Deng L, Zhu G, Xu C, Li G, Liu S, Xie J, Liu J, Kong F, Wu R, Li G, Liang S. Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull. 2011;84:406–413. doi: 10.1016/j.brainresbull.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broggi G, Ferroli P, Franzini A. Treatment strategy for trigeminal neuralgia: a thirty years experience. Neurol Sci. 2008;29(Suppl 1):S79–82. doi: 10.1007/s10072-008-0893-6. [DOI] [PubMed] [Google Scholar]
- 22.Punyani SR, Jasuja VR. Trigeminal neuralgia: an insight into the current treatment modalities. J Oral Biol Craniofac Res. 2012;2:188–197. doi: 10.1016/j.jobcr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idanpaan-Heikkila JJ, Guilbaud G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviour. Pain. 1999;79:281–290. doi: 10.1016/s0304-3959(98)00172-9. [DOI] [PubMed] [Google Scholar]
- 24.Michot B, Bourgoin S, Kayser V, Hamon M. Effects of tapentadol on mechanical hypersensitivity in rats with ligatures of the infraorbital nerve versus the sciatic nerve. Eur J Pain. 2013;17:867–880. doi: 10.1002/j.1532-2149.2012.00259.x. [DOI] [PubMed] [Google Scholar]
- 25.Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2012;8:41–56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp CJ, Reeve AJ, Collins SD, Martindale JC, Summerfield SG, Sargent BS, Bate ST, Chessell IP. Investigation into the role of P2X(3)/P2X(2/3) receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. Br J Pharmacol. 2006;148:845–852. doi: 10.1038/sj.bjp.0706790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou YL, Jiang GQ, Wei J, Zhang HH, Chen W, Zhu H, Hu S, Jiang X, Xu GY. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain. 2015;156:1892–1905. doi: 10.1097/j.pain.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi K, Shimizu K, Hu JW, Suzuki I, Sakagami H, Koshikawa N, Sessle BJ, Shinoda M, Miyamoto M, Honda K, Iwata K. Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain. 2010;6:59. doi: 10.1186/1744-8069-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saloman JL, Chung MK, Ro JY. P2X(3) and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience. 2013;232:226–238. doi: 10.1016/j.neuroscience.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda M, Shinoda M, Kiyomoto M, Honda K, Suzuki A, Tamagawa T, Kaji K, Kimoto S, Iwata K. P2X3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neurosci Lett. 2012;528:67–72. doi: 10.1016/j.neulet.2012.08.067. [DOI] [PubMed] [Google Scholar]
- 31.Ambalavanar R, Dessem D. Emerging peripheral receptor targets for deep-tissue craniofacial pain therapies. J Dent Res. 2009;88:201–211. doi: 10.1177/0022034508330176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, Fabbretti E. Neutralization of nerve growth factor induces plasticity of ATPsensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. 2007;27:8190–8201. doi: 10.1523/JNEUROSCI.0713-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MY, Kwon HJ, Sung MK. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci Biotechnol Biochem. 2009;73:828–832. doi: 10.1271/bbb.80714. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Wu F, Chen C. Design and synthesis of aloe-emodin derivatives as potent anti-tyrosinase, antibacterial and anti-inflammatory agents. Bioorg Med Chem Lett. 2015;25:5142–5146. doi: 10.1016/j.bmcl.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Regis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J, Levivier M. Long-term safety and efficacy of Gamma Knife surgery in classical trigeminal neuralgia: a 497-patient historical cohort study. J Neurosurg. 2016;124:1079–1087. doi: 10.3171/2015.2.JNS142144. [DOI] [PubMed] [Google Scholar]
- 36.Wang QP, Bai M. Topiramate versus carbamazepine for the treatment of classical trigeminal neuralgia: a meta-analysis. CNS Drugs. 2011;25:847–857. doi: 10.2165/11595590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Dai Y, Fukuoka T, Wang H, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Contribution of sensitized P2X receptors in inflamed tissue to the mechanical hypersensitivity revealed by phosphorylated ERK in DRG neurons. Pain. 2004;108:258–266. doi: 10.1016/j.pain.2003.12.034. [DOI] [PubMed] [Google Scholar]


