Abstract
Free fatty acid (FFA)-induced pancreatic β-cell loss is implicated in the pathogenesis of type 2 diabetes mellitus (T2DM). It has been documented that circulating microRNA (miR)-802 levels are significantly greater in T2DM patients than in healthy subjects. However, the role of miR-802 in FFA-induced damage to β cells is still unclear. In the present study, we measured the expression of miR-802 in the INS-1 rat insulinoma cell line after palmitate treatment for 48 h. Gain- and loss-of-function studies were conducted to determine the function of miR-802 in palmitate-induced apoptosis and reactive oxygen species (ROS) production. The target gene(s) of miR-802 was functionally characterized. Compared to control cells, palmitate treatment caused a time- and concentration-dependent induction of miR-802 in INS-1 cells. Knockdown of miR-802 significantly blocked palmitate-induced apoptosis and attenuated ROS formation. Moreover, miR-802 downregulation prevented the reduction of prosurvival proteins Mcl-1 and Bcl-xL by palmitate. In contrast, ectopic expression of miR-802 stimulated apoptosis and ROS generation in INS-1 cells. Sirtuin 6 (SIRT6) was identified to be a direct target gene of miR-802. Overexpression of miR-802 suppressed the expression of SIRT6. Enforced expression of SIRT6 abolished the induction of apoptosis and ROS production by miR-802. Taken together, miR-802 is required for palmitate-induced damage to β cells by targeting SIRT6 and represents a potential therapeutic target for T2DM.
Keywords: Diabetes, free fatty acid, miR-802, SIRT6
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disease associated with substantial morbidity [1]. High levels of circulating free fatty acids (FFAs) are linked to insulin resistance and pancreatic β cell loss in the pathogenesis of T2DM [2]. In vitro studies demonstrate that saturated FFAs such as palmitate can inhibit insulin secretion and trigger apoptosis in pancreatic β cells [3,4]. Induction of oxidative stress is suggested to be an important mechanism for palmitate-mediated deleterious effects on β cells [5]. A previous study has reported that prolonged exposure of MIN6 murine beta cells to palmitate causes the formation of substantial reactive oxygen species (ROS) and mitochondrial dysfunction, consequently leading to apoptotic death [6]. Understanding of the exact mechanism involved in palmitate-induced lipotoxicity is of significance in developing effective therapeutic approaches for T2DM.
microRNAs (miRs) are small, endogenous, non-coding RNAs that regulate target gene expression [7]. Via partial complementary base-pairing to the sequences in 3’-untranslated region (3’-UTR) of target mRNAs, miRs can interfere with protein translation and/or promote the degradation of target mRNAs. Mounting evidence indicates the implication of miRs in the development of metabolic disorders including T2DM [8]. A number of miRs such as miR-7 [9] and miR-122 [10] have been found to be dysregulated in T2DM patients and show a predictive value in this disease. In vitro studies have validated that several miRs including miR-17-92 cluster [11], miR-23a-3p, miR-23b-3p, and miR-149-5p [12] impact the survival and function of pancreatic β cells.
It has been documented that miR-802 is upregulated in obese individuals and inducible transgenic overexpression of miR-802 in mice causes insulin resistance [13]. This miR is also increased in patients with T2DM compared to healthy subjects and serves as a biomarker for T2DM [14]. Despite these reports, little is known about the role of miR-802 in pancreatic β cell function and survival in T2DM.
In this study, we examined the expression of miR-802 in palmitate-treated INS-1 rat insulinoma cells and explored its impact on palmitate-induced apoptosis. The underlying molecular mechanism for the action of miR-802 was further investigated.
Materials and methods
Cell culture
INS-1 and HEK-293T cells were obtained from the Shanghai Institute of Cell Biology (Shanghai, China) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA).
Cell treatment
Palmitate (Sigma-Aldrich) was dissolved in 0.1 M NaOH to yield a 50 mM stock solution. Dilution of the palmitate stock solution to indicated concentrations was performed using RPMI-1640 medium supplemented with 2% fatty acid-free bovine serum albumin (BSA; Sigma-Aldrich). For palmitate treatment, cells were incubated with 0.1, 0.5, and 1.0 mM palmitate for 6-48 h. Afterwards, cells were tested for gene expression, viability, and apoptosis. For inhibitor experiments, cells were pretreated for 2 h with 5 mM n-acetylcysteine (NAC; Sigma-Aldrich) before overexpression of miR-802.
Real-time PCR analysis
Total RNA was extracted from cultured cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using the TaqMan MicroRNA Assay kit (Applied Biosystems, Foster City, CA, USA) in accordance with the manufacturer’s protocol. The level of miR-802 was normalized against that of U6.
miR mimics/inhibitors and plasmids
miR-802 mimic, anti-miR-802, and negative controls were purchased from Dharmacon (Austin, TX, USA). The entire 3’-UTR of SIRT6 mRNA was amplified by PCR and cloned into pGL3-Basic vector (Promega, Madison, WI, USA). Mutation of a putative binding site for miR-802 in SIRT6 3’-UTR was achieved using the QuikChange site-direct mutagenesis kit (Stratagene, La Jolla, CA, USA). SIRT6 coding sequence lacking the 3’-UTR was amplified by PCR and inserted into pcDNA3.1(+) vector. All constructs were confirmed by sequencing.
Cell transfection
INS-1 cells were seeded in 12-well plates (4 × 105 cells/well) and transfected with miR-802 mimics or inhibitors (a final concentration of 40 nM) using FuGene6 (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Twenty-four hours later, transfected cells were exposed to palmitate (1.0 mM) for 48 h. In some experiments, cells at 70% confluence were co-transfected with miR-802 mimics together with the SIRT6-expressing plasmid 24 h before palmitate treatment.
Cell viability assay
INS-1 cells transfected with miR-802 mimic or negative control miR were plated onto 96-well plates (5 × 103 cells/well) and cultured for 48 h. Each well was added with the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium (MTT) solution (0.5 mg/mL; Sigma-Aldrich) and incubated at 37°C for 4 h. Formazan crystals was dissolved in dimethyl sulfoxide, and absorbance at 570 nm was recorded.
Apoptosis analysis
INS-1 cells with miR-802 overexpression or knockdown were plated in 6-well plates (5 × 106 cells/well) and cultured for 48 h. Cells were then suspended in binding buffer and incubated with annexin V-FITC and propidium Iodide (PI; Becton Dickinson Biosciences, San Jose, CA, USA) for 15 min in the dark. Stained cells were analyzed by flow cytometry (Becton Dickinson Biosciences).
Measurement of ROS production
Intracellular ROS levels were measured using a commercially available kit (ab113851, Abcam, Cambridge, UK) with the cell permeant reagent 2’,7’-dichlorofluorescein diacetate (DCF-DA). After diffusion to the cell, DCF-DA can be deacetylated and then oxidized by ROS into a highly fluorescent product 2’,7’-dichlorofluorescein (DCF). The cells were incubated for 30 min with 5 μM DCF-DA, and fluorescence intensity of DCF was measured by flow cytometry.
Western blot analysis
Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer supplemented with Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland). Protein samples (40 μg per lane) were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Membranes were probed with anti-Mcl-1 (#4572; 1:500 dilution), anti-Bcl-xL (#2764; 1:500 dilution), anti-SIRT6 (#12486; 1:500 dilution), and anti-β-actin (#4970; 1:2000 dilution) antibodies (all from Cell Signaling Technology, Danvers, MA, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. Protein bands were visualized by enhanced chemiluminescence (Millipore, Billerica, MA, USA) and quantitated by densitometry using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
Luciferase reporter assay
HEK-293T cells were seeded in 24-well plates (2 × 104 cells/well) and allowed to attach overnight. The cells were then transfected with wild-type or mutant SIRT6 3’-UTR reporters (0.1 μg) together with pRL-TK (0.02 μg; Promega, Madison, WI, USA) as well as miR-802 mimic or control miR (50 nM) using FuGene6. The pRL-TK vector encoding Renilla luciferase was used to control for transfection efficiency. Forty-eight hours later, luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
Data are expressed as means ± standard deviation (SD) and analyzed using the Student’s t test or one-way analysis of variance (ANOVA) followed by the Tukey’s test. Differences were considered statistically significant when P values were < 0.05.
Results
Upregulation of miR-802 in INS-1 cells by palmitate
Compared to control cells, palmitate-treated INS-1 cells displayed a significant upregulation of miR-802 after treatment with 24 h (P < 0.05; Figure 1A). The level of miR-802 was increased by 2.2-, 2.6-, and 3.3-fold by palmitate at the concentrations of 0.1, 0.5, and 1.0 mM, respectively. INS-1 cells were then treated with palmitate (1.0 mM) for indicated times (4, 8, 16, 30, and 48 h) and analyzed for miR-802 expression (Figure 1B). It was found that miR-802 expression was significantly elevated from 8 h (2.5-fold of control) up to 30 h (3.8-fold of control) after palmitate treatment. The level of miR-802 began to decline at 48 h, but was still greater than control values (P < 0.05).
Figure 1.
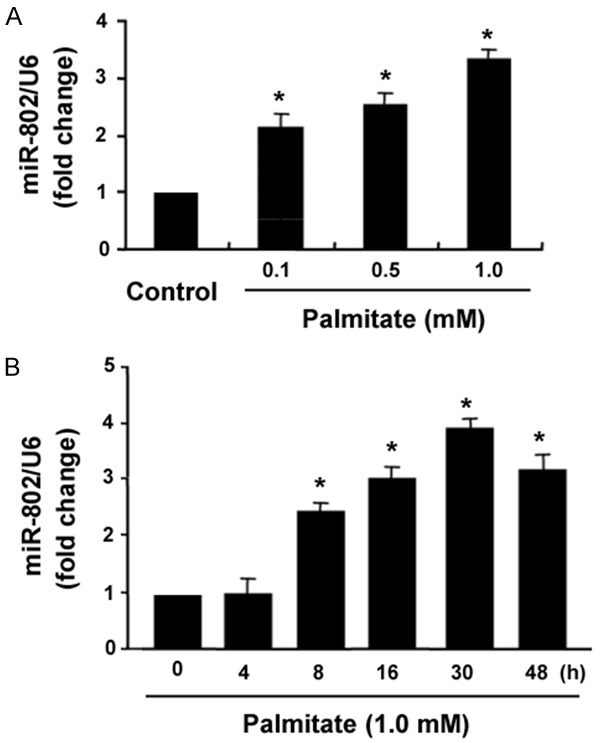
Upregulation of miR-802 in INS-1 cells by palmitate. A. INS-1 cells were treated with indicated concentrations of palmitate for 24 h, and miR-802 levels were measured by real-time PCR analysis. B. INS-1 cells were treated with palmitate (1.0 mM) for indicated times and measured for miR-802 expression. *P < 0.05 vs. untreated control cells.
Overexpression of miR-802 evokes oxidative damage to INS-1 cells
Next, we investigated the effect of miR-802 overexpression on the survival of INS-1 cells. Ectopic expression of miR-802 reduced the viability by 59% after culturing for 48 h, compared to INS-1 cells transfected with control miR (P < 0.05; Figure 2A). Moreover, overexpression of miR-802 caused a significant induction of apoptosis in INS-1 cells, increasing the percentage of apoptosis from 5.6 ± 1.2% (control levels) to 36.5 ± 2.8% (P < 0.05; Figure 2B). In addition, miR-802 overexpression led to a 3-fold increase in the ROS level in INS-1 cells (P < 0.05; Figure 2C). To confirm whether miR-802-induced apoptosis is causally linked to excessive ROS production, we pretreated INS-1 cells with a ROS scavenger, NAC. Of note, miR-802-mediated apoptotic response was almost completely blocked by NAC pretreatment (Figure 2D), suggesting that accumulation of ROS contributes to the induction of apoptosis by miR-802.
Figure 2.
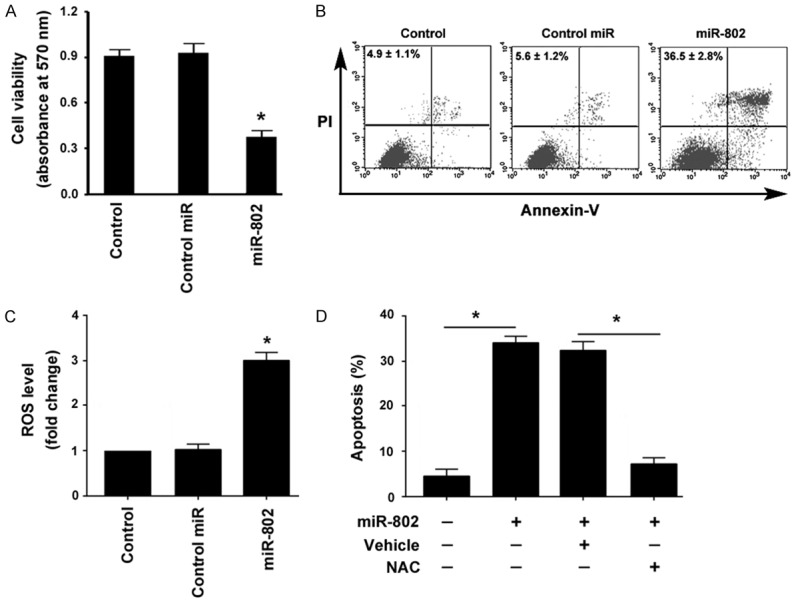
miR-802 overexpression causes oxidative damage to INS-1 cells. A. Cell viability assay. INS-1 cells were transfected with control miR or miR-802 mimic and tested for viability after 48 h. *P < 0.05 vs. nontransfected control cells. B. Apoptosis detected by annexin-V/PI staining. Representative dot plots of flow cytometry are shown. Numbers inserted indicate the results from three independent experiments. C. Measurement of ROS levels using the cell permeant reagent 2’,7’-dichlorofluorescein diacetate. *P < 0.05 vs. nontransfected control cells. D. INS-1 cells were pretreated with NAC or DMSO (as vehicle control) before transfection with miR-802 mimic and tested for apoptosis. *P < 0.05.
Depletion of miR-802 attenuates palmitate-induced apoptosis and oxidative stress
Next, we checked whether knockdown of miR-802 abolishes the induction of apoptosis by palmitate. As illustrated in Figure 3A, transfection with anti-miR-802 inhibitors rendered INS-1 cells more resistant to palmitate-induced apoptosis, decreasing the percentage of apoptosis by 3-fold (Figure 3A). Consistently, palmitate-mediated ROS production was significantly inhibited by depletion of miR-802 (P < 0.05, compared to control cells; Figure 3B). At the molecular level, miR-802 knockdown prevented the reduction of prosurvival proteins Mcl-1 and Bcl-xL by palmitate (Figure 3C).
Figure 3.
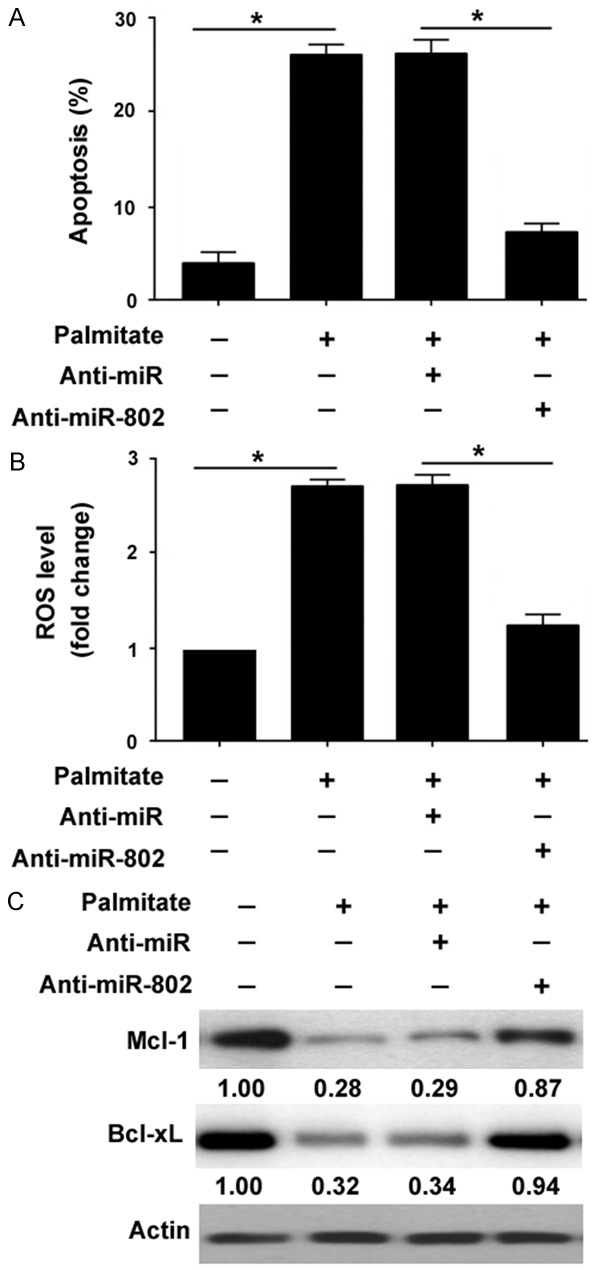
Depletion of miR-802 attenuates palmitate- induced apoptosis and oxidative stress. INS-1 cells were transfected with anti-miR-802 or control (Anti-miR) and treated with palmitate (1.0 mM) for 30 h. A. Apoptosis analysis by flow cytometry after annexin-V/PI staining. *P < 0.05. B. Measurement of ROS production in INS-1 cells. *P < 0.05. C. Western blot analysis of Mcl-1 and Bcl-xL protein levels. Numbers indicate fold change in protein levels relative to control.
SIRT6 serves as a direct target of miR-802
Bioinformatic analysis using miRDB software (http://mirdb.org/) suggested that miR-802 could target a large number of genes including SIRT6 (Figure 4A). Since SIRT6 plays a prosurvival role in different biological processes [15-17], in this study we focused on the potential involvement of SIRT6 in the pro-apoptotic activity of miR-802. Western blot analysis revealed that miR-802 overexpression remarkably downregulated the protein expression of SIRT6 in INS-1 cells (Figure 4B). To confirm the direct repression of SIRT6 by miR-802, we constructed a luciferase reporter gene harboring wild-type or mutated 3’-UTR of SIRT6. It was found that miR-802 overexpression significantly inhibited the expression of the reporter carrying wild-type SIRT6 3’-UTR, but not the mutant one (Figure 4C). Therefore, miR-802 shows the capacity to target SIRT6 in INS-1 cells.
Figure 4.
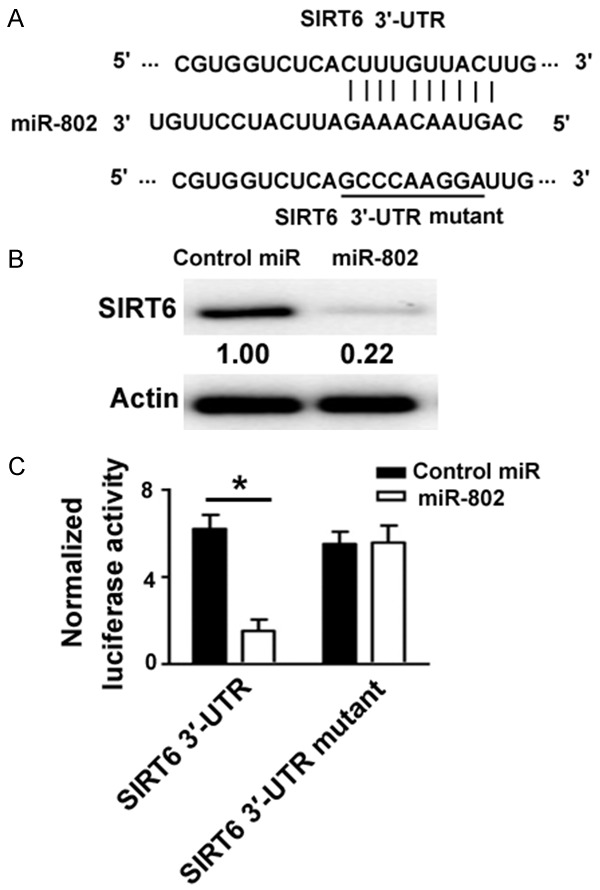
SIRT6 serves as a direct target of miR-802. A. Bioinformatic analysis using miRDB software (http://mirdb.org/) showed that the 3’-UTR of SIRT6 mRNA carried a putative binding site for miR-802. Mutation of the binding site (underlined) was achieved with the QuikChange site-direct mutagenesis kit. B. Western blot analysis of SIRT6 protein levels in INS-1 cells transfected with control miR or miR-802 mimic. Numbers indicate fold change in protein levels relative to control. C. Luciferase reporter assay. HEK-293T cells were transfected with wild-type or mutant SIRT6 3’-UTR reporters (0.1 μg) together with pRL-TK (0.02 μg) as well as miR-802 mimic or control miR (50 nM). Luciferase activities were measured 48 h posttransfection. *P < 0.05.
Enforced expression of SIRT6 rescues INS-1 cells from miR-802-induced apoptosis
A previous study has reported that SIRT6 protects against palmitate-induced apoptosis in pancreatic β cells [17]. Therefore, we performed rescue experiments using a miR-resistant SIRT6. Western blot analysis revealed that transfection with the miR-resistant SIRT6 resulted in an overexpression of SIRT6 in the presence of miR-802 (Figure 5A). Of note, enforced expression of SIRT6 suppressed miR-802-induced apoptosis in INS-1 cells (Figure 5B). In addition, miR-802-induced ROS formation was compromised by overexpression of SIRT6 (Figure 5C).
Figure 5.
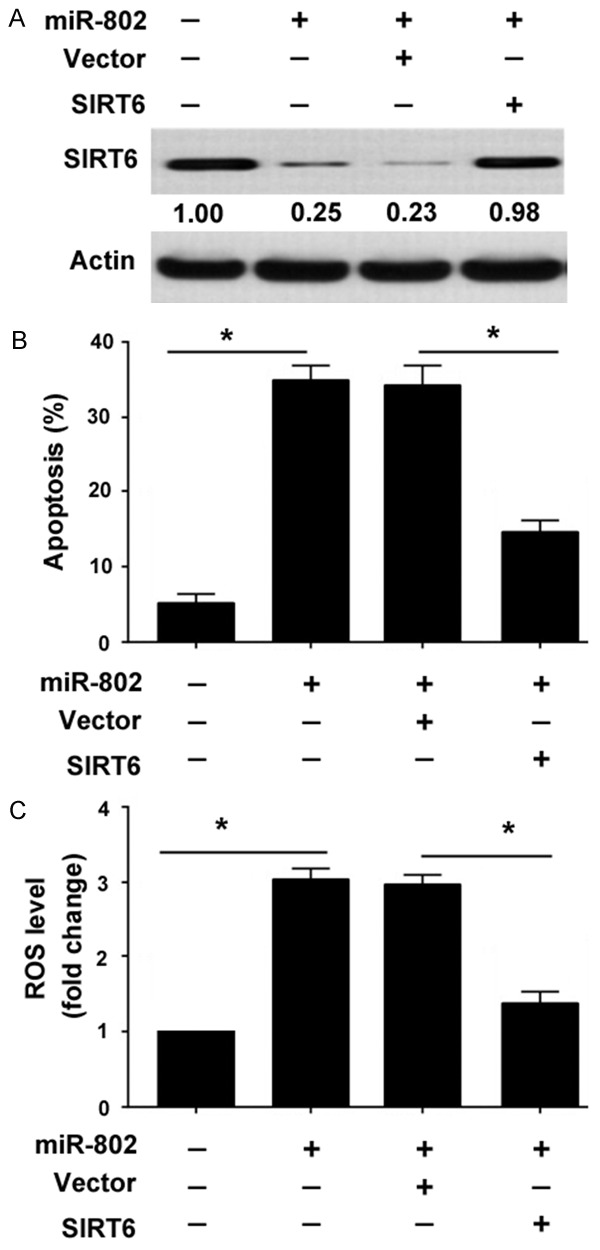
Enforced expression of SIRT6 rescues INS-1 cells from miR-802-induced apoptosis. (A) Western blot analysis of SIRT6 protein levels in INS-1 cells transfected with indicated constructs. Numbers indicate fold change in SIRT6 protein levels. (B) INS-1 cells transfected with indicated constructs and tested for apoptosis 48 h after transfection. (C) Measurement of ROS production in INS-1 cells treated as in (A). *P < 0.05.
Discussion
In this report, we showed that miR-802 was upregulated in INS-1 cells in response to palmitate treatment. Such upregulation was in a concentration- and time-dependent manner. In agreement with our observations, several previous studies have documented the dysregulation of miRs by palmitate in different cell types [18-21]. For instance, palmitate treatment led to an upregulation of miR-96 [18], miR-1271 [19], and miR-195 [20] in HepG2 hepatocytes. In a transgenic mouse model, overexpression of miR-802 reduced insulin sensitivity and impaired glucose tolerance, indicating its implication in glucose metabolism [13]. To explore the role of miR-802 in INS-1 cells, we performed miR-802 overexpression experiments. Our data demonstrated that ectopic expression of miR-802 significantly suppressed the viability of INS-1 cells. Moreover, there was a significant induction of apoptosis after overexpression of miR-802. Consistently, miR-802 overexpression inhibited cell proliferation and promoted apoptosis in prostate cancer cells [22]. Another study described the inhibitory activity of miR-802 on tongue squamous cell carcinoma cell viability [23]. These data indicate that miR-802 exerts cytotoxic activity against many distinct types of cells.
Since palmitate can induce apoptotic death in pancreatic β cells [4,6], we hypothesized that miR-802 may mediate the pro-apoptotic activity of palmitate in INS-1 cells. In support of this hypothesis, our data showed that depletion of miR-802 protected INS-1 cells from palmitate-induced apoptosis. At the molecular level, miR-802 knockdown abolished the reduction of Mcl-1 and Bcl-xL proteins in palmitate-treated cells. Both Mcl-1 and Bcl-xL are well-defined anti-apoptotic proteins and contribute to the survival of pancreatic β cells in response to apoptotic stimuli [24,25]. Induction of ROS production is causally linked to palmitate-elicited apoptosis in INS-1 cells [6]. Therefore, we investigated the impact of miR-802 overexpression on ROS formation in INS-1 cells. It was found that enforced expression of miR-802 significantly promoted ROS production in INS-1 cells. Depletion of miR-802 significantly counteracted palmitate-mediated ROS production. Pretreatment of INS-1 cells with the ROS scavenger NAC almost completely blocked miR-802-induced apoptosis. Collectively, these data indicate that miR-802 mediates palmitate-induced apoptosis in INS-1 cells through promotion of ROS formation. It has been documented that Bcl-xL overexpression interferes with ROS generation in SH-SY5Y human neuroblastoma cells [26]. Mcl-1 also shows the ability to prevent ROS formation [27]. Therefore, downregulation of both Mcl-1 and Bcl-xL may provide an explanation for miR-802-induced ROS generation. However, the exact mechanism needs to be further clarified.
We provided evidence that SIRT6 was a direct target of miR-802 and overexpression of miR-802 significantly downregulated the endogenous expression of SIRT6 in INS-1 cells. SIRT6 is a class III histone deacetylase implicated in the transcriptional control of a large number of genes [28]. SIRT6 acts as a stress responsive protein and protects against oxidative injury and DNA damage [28,29]. It has been documented that SIRT6 renders hepatocellular carcinoma cells more resistant to Bax-mediated apoptosis through deacetylation of Ku70 [30]. Similarly, SIRT6 reduces palmitate-mediated apoptosis in pancreatic β cells [17]. Consistently, we found that enforced expression of SIRT6 confers protection against miR-802-induced apoptosis in INS-1 cells. Taken together, we suggest that the pro-apoptotic activity of miR-802 is partially ascribed to downregulation of SIRT6. However, it should be mentioned that miR-802 can modulate multiple genes other than SIRT6. For example, miR-802 exerts a suppressive activity in tongue squamous cell carcinoma through targeting MAP2K4 [23]. Therefore, additional studies are required to fully uncover the mechanism by which miR-802 regulates apoptosis of pancreatic β cells.
In conclusion, our data show that miR-802 is stimulated by palmitate and mediates palmitate-induced apoptosis and oxidative stress in INS-1 cells. Repression of SIRT6 is an important mechanism for miR-802-induced apoptosis in INS-1 cells. Targeting miR-802 may represent a promising therapeutic strategy for prevention of FFA-induced oxidative damage in T2DM.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81400804).
Disclosure of conflict of interest
None.
References
- 1.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167:228–256. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Cui C, Nie A, Wang Y, Ni Q, Liu Y, Yin Q, Zhang H, Li Y, Wang Q, Gu Y, Ning G. The mTORC2/PKC pathway sustains compensatory insulin secretion of pancreatic β cells in response to metabolic stress. Biochim Biophys Acta. 2017;1861:2039–2047. doi: 10.1016/j.bbagen.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Yin Y, Yong W, Yu J, Zhang X, Lin H, Zhu Y, Han X. Pdcd2l promotes palmitate-induced pancreatic beta-cell apoptosis as a foxO1 target gene. PLoS One. 2016;11:e0166692. doi: 10.1371/journal.pone.0166692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassel R, Ducreux S, Alam MR, Dingreville F, Berlé C, Burda-Jacob K, Chauvin MA, Chikh K, Païta L, Al-Mawla R, Crola Da Silva C, Rieusset J, Thivolet C, Van Coppenolle F, Madec AM. Protection of human pancreatic islets from lipotoxicity by modulation of the translocon. PLoS One. 2016;11:e0148686. doi: 10.1371/journal.pone.0148686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehinger S, Ortiz R, Díaz MI, Aguirre A, Valenzuela M, Llanos P, Mc Master C, Leyton L, Quest AF. Phosphorylation of caveolin-1 on tyrosine-14 induced by ROS enhances palmitateinduced death of beta-pancreatic cells. Biochim Biophys Acta. 2015;1852:693–708. doi: 10.1016/j.bbadis.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Long B, Han W, Yuan S, Wang K. microRNAs: important regulators of stem cells. Stem Cell Res Ther. 2017;8:110. doi: 10.1186/s13287-017-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto N, Tanaka T. Role of miRNAs in the pathogenesis and susceptibility of diabetes mellitus. J Hum Genet. 2017;62:141–150. doi: 10.1038/jhg.2016.150. [DOI] [PubMed] [Google Scholar]
- 9.Wan S, Wang J, Wang J, Wu J, Song J, Zhang CY, Zhang C, Wang C, Wang JJ. Increased serum miR-7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res Clin Pract. 2017;130:171–179. doi: 10.1016/j.diabres.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Willeit P, Skroblin P, Moschen AR, Yin X, Kaudewitz D, Zampetaki A, Barwari T, Whitehead M, Ramírez CM, Goedeke L, Rotllan N, Bonora E, Hughes AD, Santer P, Fernández-Hernando C, Tilg H, Willeit J, Kiechl S, Mayr M. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes. 2017;66:347–357. doi: 10.2337/db16-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Tian L, Wan S, Xie Y, Chen X, Ji X, Zhao Q, Wang C, Zhang K, Hock JM, Tian H, Yu X. MicroRNA-17-92 cluster regulates pancreatic beta-cell proliferation and adaptation. Mol Cell Endocrinol. 2016;437:213–223. doi: 10.1016/j.mce.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Grieco FA, Sebastiani G, Juan-Mateu J, Villate O, Marroqui L, Ladrière L, Tugay K, Regazzi R, Bugliani M, Marchetti P, Dotta F, Eizirik DL. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p regulate the expression of proapoptotic BH3-only proteins DP5 and PUMA in human pancreatic β-cells. Diabetes. 2017;66:100–112. doi: 10.2337/db16-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornfeld JW, Baitzel C, Könner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, Seibler J, Cereghini S, Heeren J, Stoffel M, Brüning JC. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi C, Nakatsuka A, Eguchi J, Teshigawara S, Kanzaki M, Katayama A, Yamaguchi S, Takahashi N, Murakami K, Ogawa D, Sasaki S, Makino H, Wada J. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism. 2015;64:489–497. doi: 10.1016/j.metabol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res. 2015;330:81–90. doi: 10.1016/j.yexcr.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Ran LK, Chen Y, Zhang ZZ, Tao NN, Ren JH, Zhou L, Tang H, Chen X, Chen K, Li WY, Huang AL, Chen J. SIRT6 overexpression potentiates apoptosis evasion in hepatocellular carcinoma via BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer Res. 2016;22:3372–3382. doi: 10.1158/1078-0432.CCR-15-1638. [DOI] [PubMed] [Google Scholar]
- 17.Xiong X, Sun X, Wang Q, Qian X, Zhang Y, Pan X, Dong XC. SIRT6 protects against palmitate-induced pancreatic β-cell dysfunction and apoptosis. J Endocrinol. 2016;231:159–165. doi: 10.1530/JOE-16-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang WM, Min KH, Lee W. Induction of miR-96 by dietary saturated fatty acids exacerbates hepatic insulin resistance through the suppression of INSR and IRS-1. PLoS One. 2016;11:e0169039. doi: 10.1371/journal.pone.0169039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WM, Min KH, Lee W. MiR-1271 upregulated by saturated fatty acid palmitate provokes impaired insulin signaling by repressing INSR and IRS-1 expression in HepG2 cells. Biochem Biophys Res Commun. 2016;478:1786–1791. doi: 10.1016/j.bbrc.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Yang WM, Jeong HJ, Park SY, Lee W. Saturated fatty acid-induced miR-195 impairs insulin signaling and glycogen metabolism in HepG2 cells. FEBS Lett. 2014;588:3939–3946. doi: 10.1016/j.febslet.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Wang F, Wu Y, Zuo L, Zhang S, Zhou Q, Wei W, Wang Y, Zhu H. MicroRNA-126 attenuates palmitate-induced apoptosis by targeting TRAF7 in HUVECs. Mol Cell Biochem. 2015;399:123–130. doi: 10.1007/s11010-014-2239-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Lu G, Shao Y, Xu D. microRNA-802 inhibits epithelial-mesenchymal transition through targeting flotillin-2 in human prostate cancer. Biosci Rep. 2017:37. doi: 10.1042/BSR20160521. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Wu X, Gong Z, Sun L, Ma L, Wang Q. MicroRNA-802 plays a tumour suppressive role in tongue squamous cell carcinoma through directly targeting MAP2K4. Cell Prolif. 2017:50. doi: 10.1111/cpr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Tong Y, Gong M, Lu Y, Wang C, Zhou M, Yang Q, Mao T, Tong N. Activation of PPARβ/δ protects pancreatic β cells from palmitate-induced apoptosis by upregulating the expression of GLP-1 receptor. Cell Signal. 2014;26:268–278. doi: 10.1016/j.cellsig.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Allagnat F, Klee P, Cardozo AK, Meda P, Haefliger JA. Connexin36 contributes to INS-1E cells survival through modulation of cytokineinduced oxidative stress, ER stress and AMPK activity. Cell Death Differ. 2013;20:1742–1752. doi: 10.1038/cdd.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saez-Atienzar S, Bonet-Ponce L, da Casa C, Perez-Dolz L, Blesa JR, Nava E, Galindo MF, Jordan J. Bcl-xL-mediated antioxidant function abrogates the disruption of mitochondrial dynamics induced by LRRK2 inhibition. Biochim Biophys Acta. 2016;1862:20–31. doi: 10.1016/j.bbadis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Demelash A, Pfannenstiel LW, Liu L, Gastman BR. Mcl-1 regulates reactive oxygen species via NOX4 during chemotherapy-induced senescence. Oncotarget. 2017;8:28154–28168. doi: 10.18632/oncotarget.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309:H1375–H1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, Zhu Y, Li YX, He JH, Wang M, Jiang W. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016;111:13. doi: 10.1007/s00395-016-0531-z. [DOI] [PubMed] [Google Scholar]
- 30.Tao NN, Ren JH, Tang H, Ran LK, Zhou HZ, Liu B, Huang AL, Chen J. Deacetylation of Ku70 by SIRT6 attenuates Bax-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;485:713–719. doi: 10.1016/j.bbrc.2017.02.111. [DOI] [PubMed] [Google Scholar]


