Abstract
Sevoflurane is a commonly used inhalation anesthesia, which has been previously demonstrated to impair long-term emotional memory consolidation and induce learning dysfunction through inducing hippocampal dysfunction. However, the underlying molecular mechanisms remain largely unknown. MicroRNAs (miRNAs) play critical roles in multiple cells apoptosis, including hippocampal neural. The present study, therefore, was aimed to investigate the miR-145 function on the hippocampal neural apoptosis induced by sevoflurane exposure. A hippocampal neural cell line HT22 was used, and treated with 4.1% sevoflurane. Cell viability and apoptosis were determined by MTT assay and flow cytometry, respectively. microRNA microarray was used to select differentially expression miRNAs in cells with sevoflurane exposure and controls. Results showed that sevoflurane could significantly induce the hippocampal neural cell apoptosis via mitochondria apoptotic pathway. Then, miR-145 was selected as a significantly down expression microRNA in sevoflurane treated HT22 cell lines, by microarray analysis and real-time PCR verification. Furthermore, we found that miR-145 overexpression could protect HT22 cells against apoptosis caused by sevoflurane. Bioinformatics analysis and dual-luciferase reporter assays indicated that Bnip3, which has a key role in the mitochondrial dysfunction, is a novel target of miR-145. Finally, we found that over expression of Bnip3 by pcDNA-Bnip3 transfection significantly induced apoptosis in HT22 cells, which was inhibited by miR-145 mimic. Therefore, it concluded that miR-145 could protect against sevoflurane-induced hippocampal apoptosis through the mitochondrial pathway at least by directly inhibiting its target gene-Bnip3 expression in hippocampal neural cell lines.
Keywords: Sevoflurane, hippocampal neurons, apoptosis, miR-145, Binp3
Introduction
Sevoflurane is one of the most commonly used volatile anesthetics, which can prevent patients suffer from pain due to the surgery. However, studies have showed that sevoflurane inhalation might impair long-term emotional memory consolidation [1], induce learning dysfunction [2], and cause spatial cognitive dysfunction [3]in human and model animals research. In addition to that, studies have showed that sevofluranecould induce the dysfunction of hippocampal neurons. Nie et al. found that sevoflurane anesthesia in infant rats can result in long-term cognitive impairment, possibly by inhibiting neurogenesis [4]. Chen et al. reported that sevoflurane exposure during early time could cause hippocampal neural apoptosis [5]. Despite that, the precise underlying molecular mechanism still remains unclear.
MicroRNAs (miRNA) are a large class of noncoding RNAs, with the nucleotides length ranging from 19 to 24, which are evolutionarily conserved and can regulatea large number of gene expression by targeting mRNAs for cleavage or translational repression [6]. Aberrant expression of miRNA is associated with a variety of human diseases, including dysfunction of hippocampal neurons. For instance, miR-195 participates in the mitochondrial dysfunction of hippocampal neurons in mice [7]. Jin et al. reported that miR-17-92 could regulate adult mice hippocampal neurogenesis, anxiety, and depression [8]. Shao et al. found that miR-124 can protect neuron apoptosis in thyroid hypofunction rat [9].
It has been demonstrated that miR-145 can regulate cell apoptosis, proliferation, neural development and stem cell differentiation [10]. Additional study showed that miR-145 protects cardiomyocytes against hydrogen peroxide (H2O2)-induced apoptosis through targeting the mitochondria apoptotic pathway [11]. However, limited studies have been published on miR-145a participating in hippocampal neural apoptosis.
The present study, therefore, was designed to investigate the miR-145 function on the hippocampal neural apoptosis induced by sevoflurane exposure. By using HT22 cell lines, we firstly found that sevoflurane could significantly induce the hippocampal neural cell apoptosis via mitochondria apoptotic pathway. Furthermore studies suggested that miR-145 was down-regulated in sevoflurane treated HT22 cells and overexpression of miR-145 protect against sevoflurane-induced hippocampal apoptosis by directly inhibiting its target gene-Bnip3 expression. MiR-145 may represent a novel therapeutic target in sevoflurane associated with hippocampus neural dysfunction.
Materials and methods
Chemicals and cell lines
Sevoflurane was obtained from AbbVie Inc., (North Chicago, IL, USA). HT22 cell lines were purchased from Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China).
Cell culture and sevoflurane exposure
HT22 hippocampal neuronal cells were cultured as previously described by Tamaki et al. [12]. The method of HT22 cells exposed to sevoflurane was according to the description of Zhou et al. [13]. Briefly, cell culture plates were put into an airtight plastic chamber with inlet and outlet connectors. To adjust the sevoflurane concentration, the inlet port of the chamber was connected to a sevoflurane vaporizer. And then, the chamber was gassed with 4.1% sevoflurane in the carrier gas (95% air/) for 15 min. The chamber was then sealed and incubated at 37°C for 6 h. The control HT22 cells underwent the same procedure but with 5% CO2 air.
Cell viability assay
Cell viability was analyzed with Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China), followed by manufacturer’s instructions. Briefly, the HT22 cells were seeded into the 96-well plate at a cellular density of 5×103 cells/well. After exposure to sevoflurane, the cell monolayer was rinsed with phosphate-buffered saline (PBS) three times. And then, 10 μL of Cell Counting Kit-8 solution was added into each well. The OD450 values were detected by using the Spectra Microplate Reader (BIOTEC). The absorbance was measured by a microplate reader at 450 nm and expressed as percentages of the control values. Each treatment was performed in triplicate.
Apoptosis assay
The apoptosis of HT22 cell lines, either with sevoflurane exposure, miR-145 mimic/inhibitor treatment, or pcDNA-Bnip transfection, were detected by Annexin V/APC and propidium iodide (PI) apoptosis detection kit I (BD Pharmingen, San Diego, CA, USA), following the manufacturer’s recommendations. The cells were analyzed with a flow cytometry (FACScan; BD Biosciences) equipped with a Cell Quest software. The apoptosis ratio was measured by BD FACS Accuri C6 (Becton-Dickinson, San Jose, USA).
RNA extraction and miRNA microarray analysis
HT22 cells were plated in a cell dish at a density of 5×103 cells per plate. Cells for the sevoflurane exposure were treated as described above. Total RNA, including miRNA from HT22 cells was extracted using TRIzol reagent (Invitrogen, USA). cDNA was synthesized from RNA, using a PrimeScript™ RT reagent Kit purchased from TAKARA (Takara, Dalian, China), according to the manufacturer’s instructions. RNA quality was assessed using the Agilent 2100 bioanalyzer system (Agilent Technologies, USA). MiRNA expression profiles of the groups were compared using mice miRNA microarray (Agilent Technologies). Each group consisted of three assays. The data obtained were analyzed with the Gene Spring GX software (Agilent Technologies). Uncorrected P-values for fold change data from microarrays were generated.
RNA extraction and real-time PCR
The mature miRNA was reverse transcribed by miRNA-specific primers for quantification of miR-145, U6 served as a control. The primers used were as follows: miR-145 RT primer: 5’-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACagggatt-3’, miR-145 forward, 5’-CAGTGCGTGTCGTGGAGT-3’ and reverse, 5’-AGGTCCAGTTTTCCCAGG-3’; and U6 forward, 5’-GCTTCGGCAGCACATATACTAAAAT-3’ and reverse, 5’-CGCTTCACGAATTTGCGTGTCAT-3’. Relative quantification of mRNA expression levels were performed using SYBR Premix Ex Taq II on an ABI Applied Biosystems 7500 Real Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.), according to the manufacturer’s instructions. U6 and GAPDH were used to normalize mRNA and miRNA level respectively. The relative expression of miR-145 and U6 was calculated using the 2-ΔΔCt method.
Western blot analysis
The harvested HT22 cells, either with sevoflurane exposure, miR-145 mimic/inhibitor treatment, or pcDNA-Bnip transfection, were subjected to Western blot analyses. The concentration of the protein was measured by BCA protein assay kit (Beyotime, Shanghai, China), according to manufacturer’s instruction. Ten percentages SDS-PAGE were used to electrophorese samples. The protein was then transferred onto a PVDF (polyvinylidene fluoride) membrane (Bio-Rad, USA). The membranes were incubated with specific antibodies after blocking in skim milk. Autoradiograms were quantified by densitometry (Quantity One software; Bio-Rad). β-actin was used as internal reference, and we presented changes in levels of Bax, Bcl-2, and caspase-3 in treated HT22 cells as percentages of those in controls. Goat anti-, Caspase-3, Bax, and Bcl-2 (1:1000) were purchased from Sigma.
Prediction of miR-145 target and luciferase assay
TargetScan (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/) programs were used to investigate the target gene of miR-145 and the conserved sites bound by the seed region of miR-145.
HT22 cells were transiently transfected with the firefly luciferase reporter plasmids and the Renilla luciferase-expressing plasmid (pRLSV40; Promega, Madison, USA), the latter as a transfection control, were transiently transfected into HT22 cells using Lipofectamine 2000 reagent (Life Technologies, USA). After transfection for 24 h, cells were harvested and lysed with 100 μl of cell culture lysis buffer (CLB, Promega). Lysates of the transfected cells were analyzed using the dual luciferase assay kit (Promega) according to the manufacturer’s protocol. The luciferase activities were measured on a Spectra Max L (Molecular Devices, Sunnyvale, CA). Firefly luciferase activity of WT-Bnip 3’UTR was normalized to Renilla luciferase in each sample. Each experiment was carried out at least three times with triplicate samples.
Statistical analysis
Data are presented as mean ± SD. Comparisons were made using a two-tailed t test or one-way ANOVA for experiments with more than two subgroups. P<0.05 was considered statistically significant.
Results
Sevoflurane-induced HT22 cells apoptosis
HT22 cells were treated with 4.1% sevoflurane for 6 h. The cells viability was significantly decreased in cells exposed to sevoflurane than that of controls (P<0.01, Figure 1A). The activity of caspase-3 was dramatically enhanced in HT22 cells exposed to sevoflurane, when compared with non-exposure controls (P<0.01, Figure 1B). The expression levels of key mediators of mitochondrial apoptotic pathway, including cleaved caspase-3, cleaved PARP, Bax and Bcl-2 were determined by western blot assay. Results showed that expression of the pro-apoptotic protein cleaved caspase-3, cleaved PARP, Bax were all significantly increased while the anti-apoptotic protein Bcl-2 was significantly decreased in HT22 cells exposed to 4.1% sevoflurane (P<0.01 for all, Figure 1C). The apoptosis portion assayed by flow cytometry showed that sevoflurane induced HT22 cells apoptosis significantly (P<0.01, Figure 1D). Summarizing, it indicated that sevoflurane could inhibit hippocampal neural cells viability and also induce apoptosis.
Figure 1.
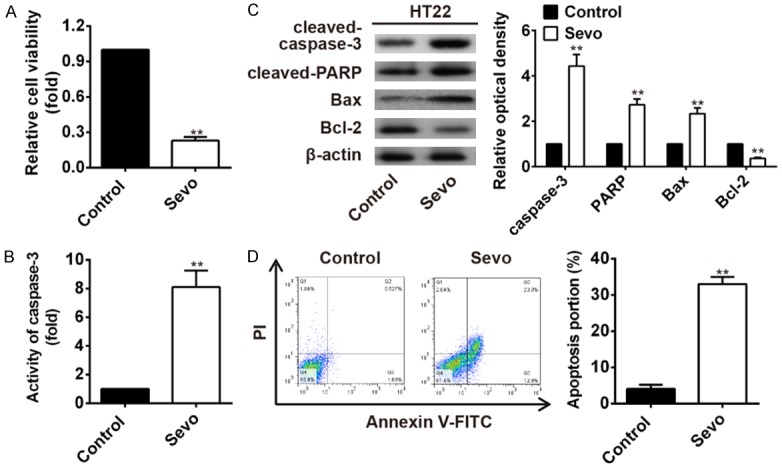
Sevoflurane-induced HT22 cells apoptosis. A. HT22 cells viability was significantly inhibited when treated with 4.1% sevoflurane (**P<0.01 vs. control). B. Caspase 3 activity was significantly increased in HT22 cells exposed to 4.1% sevoflurane than that of controls (**P<0.01 vs. control). C. The cleaved caspase-3, cleaved PARP, Bax, and Bcl-2 protein levels were detected by western blot. The expression former three indicators were all significantly increased while the latter Bcl-2 was significantly decreased in HT22 cells treated with sevoflurane (**P<0.01 vs. control). D. The cells apoptosis portion detected by flow cytometry showed that sevoflurane-induced HT22 cells apoptosis significantly (**P<0.01 vs. control).
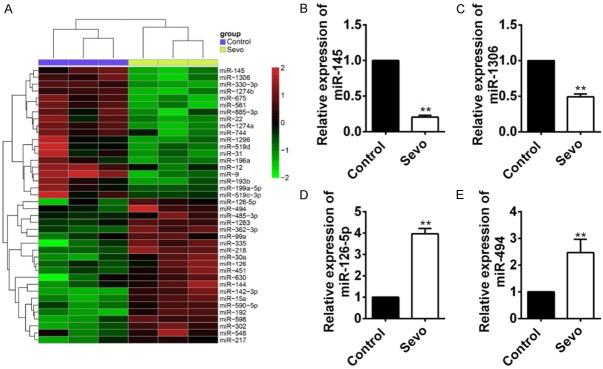
miRNAs profiles and selection of miR-145 in sevoflurane-treated HT22 cells
HT22 cells were treated with 4.1% sevoflurane for 6 h, and microarray analysis was used to select differentially expressed miRNAs in sevoflurane-treated HT22 cells and compared with non-treated controls. Results showed that there were totally 40 differentially expressed miRNAs in the two groups, with 21 upregulated and 19 downregualted (Figure 2A). Real-time PCR was used to verify the four miRNAs (miR-145, miR-1306, miR-126-5p, and miR-494), with the most significant up/down expression pattern. The expression levels detected by Real-time PCR were consistent with microarray analysis. MiR-145 and miR-1306 were both significantly down-regulated in sevoflurane treated HT22 cells than that of controls (P<0.01 for both, Figure 2B and 2C), while miR-126-5p and miR-494 were both significantly up-regulated in sevoflurane treated HT22 cells than that of controls (P<0.01 for both, Figure 2D and 2E).
Figure 2.
microRNA profiles in HT22 cells treated with sevoflurane and non-treated controls. (A) Microarray analysis was used to detect microRNA profiles in HT22 cells treated with sevoflurane and non-treated controls. (B-E) The four significantly up/down-regulated miRNAs: MiR-145 (B), miR-1306 (C), miR-126-5p (D) and miR-494 (E), were verified by real-time PCR in the two groups (**P<0.01 vs. control).
MiR-145 has been reported to involve in the regulation of the mitochondrial apoptotic pathway through its ability of targeting various anti-apoptotic molecules [11]. Herein, we were curious to see whether miR-145 participated in the hippocampal neural apoptosis induced by sevoflurane. If it is, what is the mechanism? Therefore, the miR-145 was selected to use in the following study.
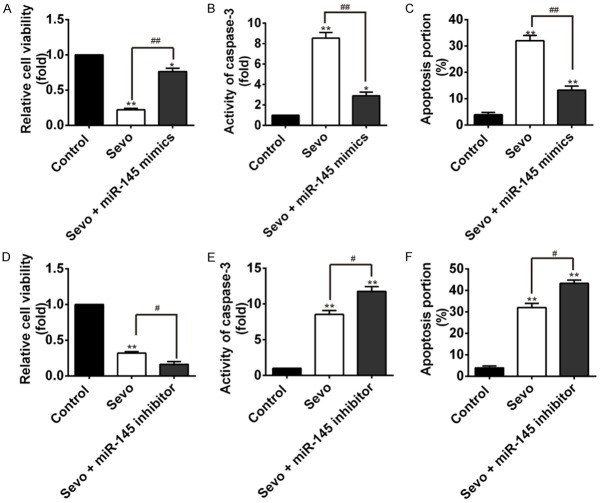
miR-145 overexpression attenuated sevoflurane-induced apoptosis in HT22 cells
To determine whether miR-145 plays a role in the regulation of sevoflurane-induced apoptosis in hippocampal neural cells, HT22 cells were pretreated with miR-145 mimic/inhibitor for 12 h before sevoflurane pretreatment. Cell viability was significantly inhibited in sevoflurane group (**P<0.01) and sevoflurane+miR-145 mimic group (*P<0.05) when compared with the controls. However, miR-145 overexpression significantly reversed sevoflurane-induced cell viability inhibition (##P<0.01, sevoflurane+miR-145 mimic vs. sevoflurane alone, Figure 3A). Notably, overexpression of miR-145 substantially inhibited sevoflurane-induced caspase-3 activity increase (##P<0.01, Figure 3B) and apoptosis rate (##P<0.01, Figure 3C) in HT22 cells. Whereas, treatment of miR-145 inhibitor augmented sevoflurane induced HT22 cells viability inhibition (#P<0.05, Figure 3D), and significantly increased the activity of caspase-3, and the rate of HT22 cells apoptosis induced by sevoflurane (#P<0.05 for both, Figure 3E and 3F). It is, thus, suggested that miR-145a overexpression could protect the HT22 cells from apoptosis induced by sevoflurane.
Figure 3.
Effects of miR-145 on sevoflurane induced apoptosis. HT22 cells were pretreated with miR-145 mimic/inhibitor for 12 h before sevoflurane exposure. (A) HT22 cells were pretreated with miR-145 mimic. The cell viability was assayed by CCK-8 kit in different groups with different treatments: controls, sevoflurane group, and sevoflurane+miR-145 mimic group. (*P<0.05, **P<0.01 vs. control; ##P<0.01, sevoflurane+miR-145 mimic vs. sevoflurane alone). (B, C) miR-145 overexpression dramatically inhibited sevoflurane-induced caspase-3 activity, which was assayed by Caspase 3 activity assay Kit (B) and apoptosis (C) of HT22 cells. The cell apoptosis was detected by flow cytometry. (*P<0.05, **P<0.01, sevoflurane vs. control; ##P<0.01, sevoflurane+miR-145 mimic vs. sevoflurane alone). (D) The HT22 cells were treated with miR-145a inhibitor, and the cell viability was assayed by CCK-8 kit. (*P<0.05, **P<0.01 vs. control; #P<0.05 vs. sevoflurane alone). (E, F) miR-181a inhibitor augmented sevoflurane-induced caspase-3 activity when assayed by a caspase-3 activity kit (E) and apoptosis detected by flow cytometry (F) (*P<0.05, **P<0.01 vs. control; #P<0.05 vs. sevoflurane alone).
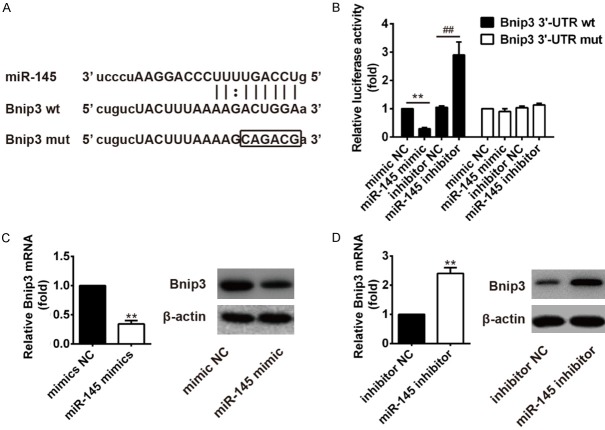
miR-145 directly targets Bnip3 mRNA
In the present study, the bioinformatic tools (Targetscan and miRBase) were used to identify the target gene of miR-145. As shown in Figure 4A, Bnip3, playing a key role in the mitochondrial dysfunction [14], is the theoretical target gene of miR-145. To further validate whether Bnip3 is a direct target gene, we fused the 3’-UTR region of Bnip3 to a luciferase system. As shown in Figure 4B, miR-145 obviously suppressed the luciferase activities of the 3’-UTR segment of Bnip3, while miR-145 inhibitor significantly increased the luciferase activities of the 3’-UTR segment of Bnip3, but not those of the construct containing a mutant binding site (Bnip3 3’-UTR-mut), compared to the NC group.
Figure 4.
Bnip3 is the direct target of miR-145. A. Putative miR-145-binding site exists in the 3’-UTR of Bnip3 mRNA and point mutations were generated in the binding site. B. The luciferase reporter plasmid containing wild-type or mutant Bnip3 3’-UTR was cotransfected into HT22 cells with miR-145 mimic/inhibitor or miR-NC. Luciferase activity was determined by the dual luciferase assay and shown as the relative firefly activity normalized to Renilla activity. Each bar represents the mean and s.d. of three independent experiments. **P<0.05 vs. mimics NC, ##P<0.01 vs. inhibitor NC. C. mRNA expression of Bnip3 was detected by real-time PCR after treatment with miR-145 mimics. The expression of Bnip3 protein was analyzed by Western blotting (n=3). β-actin was used as control. **P<0.01 vs. mimic NC. D. mRNA expression of Bnip3 was detected by real-time PCR after treatment with miR-145 inhibitor. The expression of Bnip3 protein was analyzed by Western blotting (n=3). β-actin was used as control. **P<0.01 vs. inhibitor NC.
Real-time PCR and western blot analysis showed that the expression of Bnip3 was significantly decreased after miR-145 mimic treatment both at mRNA and protein levels, while expression of Bnip3 both at mRNA and protein levels were significantly increased after miR-145 inhibitor treatment, compared to the NC group (Figure 4C, 4D).
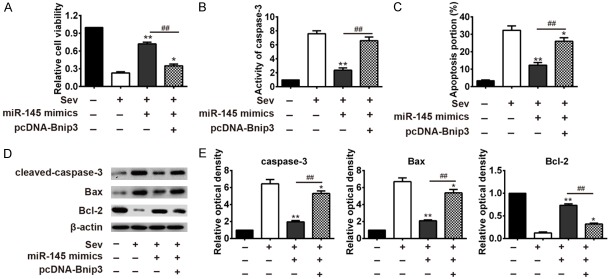
Bnip3 diminished the protection function of miR-145 against apoptosis induced by sevoflurane in HT22 cells
Since miR-145 protected HT22 cells against apoptosis induced by sevoflurane, and it has been estimated that miR-145 directly targeted Bnip3 gene. Herein, we overexpressed Bnip3 by transfecting pcDNA-Bnip3 plasmid to evaluate its function in the sevoflurane-induced cell viability, apoptosis, and other key indicators. We found that the Bnip3 reversed the miR-145 mimic effect on the HT22 cell viability, when compared with non-transfection treatment group (##P<0.01, pcDNA-Bnip3+miR-145 mimic +sevoflurane vs. miR-145 mimic+sevoflurane group, Figure 5A). In the above experiment, we found that miR-145 could rescue the increased apoptosis rate and caspase-3 activity caused by sevoflurane treatment. However, when transfected with pcDNA-Bnip3, the protection effectthat miR-145a against HT22 cells apoptosis diminished, as can be observed from the increased caspase-3 activation (pcDNA-Bnip3+miR-145a mimic+sevoflurane vs. miR-145 mimic+sevoflurane group, Figure 5B), and increased level of apoptosis portion (##P<0.01, pcDNA-Bnip3+miR-145a mimic+sevoflurane vs. miR-145 mimic+sevoflurane group, Figure 5C). In addition, western blot assay showed that expression of the key indicators in the mitochondrial apoptotic pathway were also changed by pcDNA-Bnip3 transfection. The pro-apoptotic protein, including cleaved-caspase 3 and Bax were significantly increased in HT22 cells transfected with pcDNA-Bnip3, while the anti-apoptosis Bcl-2 was substantially decreased in HT22 cells with pcDNA-Bnip3 transfection (##P<0.01, pcDNA-Bnip3+miR-145a mimic+sevoflurane vs. miR-145 mimic+sevoflurane group, Figure 5D, 5E). These results suggested miR-145a protected sevoflurane induced hippocampal neural apoptosis by suppressing its target gene Bnip3.
Figure 5.
Bnip3 diminished the protection function of miR-145 against apoptosis HT22 cells against apoptosis induced by sevoflurane. The pcDNA-Bnip3 plasmid was transfected in HT22 cells with different treatments to overexpresses Bnip3. The cell viability, apoptosis and key indicators of the mitochondrial apoptotic pathway were examined, and compared in different groups, including sevoflurane group, miR-145 mimics+sevoflurane group and pcDNA-Bnip3+miR-145 mimic+sevoflurane group. A. The cell viability was assayed by CCK-8 kit and compared in different groups. pcDNA-Bnip3 reversed the miR-145 mimic effect on the HT22 cell viability. *P<0.05, **P<0.01 vs. Sev group. ##P<0.01 vs. miR-145 mimic+sevoflurane group. B. The caspase-3 activity was detected by caspase-3 activity kit, and the value was compared among different groups. **P<0.01 vs. Sev group. ##P<0.01 vs. miR-145 mimics+sevoflurane group. C. The cell apoptosis was detected by flow cytometry. pcDNA-Bnip3 significantly reversed the protection effect of miR-145 mimics against HT22 cells apoptosis. *P<0.05, **P<0.01 vs. Sev group. ##P<0.01 vs. miR-145 mimic+sevoflurane group. D. Protein expression of the pro-apoptotic protein, including cleaved-caspase 3 and Bax, as well as the anti-apoptosis Bcl-2 in different groups were detected by western blot assay. E. The relative optical density of cleaved-caspase 3, Bax and Bcl-2 were compared in different groups. *P<0.05, **P<0.01 vs. Sev group. ##P<0.01 vs. miR-145 mimic+sevoflurane group.
Discussion
Sevoflurane is a commonly used volatile anesthetic, which is very helpful for reduction of perioperative mortality [15,16]. But studies demonstrated that sevoflurane exposure in hippocampus also affects neurogenesis, neurodegeneration and neurocognitive function through cells apoptosis, which contributes to memory impairment and cognitive dysfunction in hippocampus [17-20]. However, the molecular mechanisms underlying sevoflurane-induced hippocampal neural apoptosis remain largely unknown.
The present study, therefore, was designed to investigate the molecular mechanism of sevoflurane-induced hippocampal neural apoptosis. At the beginning, sevoflurane exposure significantly inhibited cell viability and induced cell apoptosis. From the expression of the key mediators of mitochondrial apoptotic pathway, including increased expression of caspase-3 and Bax, as well as decreased Bcl-2 expression, it is indicated that sevoflurane could induce the hippocampus HT22 cells apoptosis via the mitochondrial pathway. This finding is consistent with the reports published by Zhou et al., who found that sevoflurane exposure in rat could induce the brain neural cell apoptosis via the mitochondrial pathway [21].
miRNAs serve important roles in cell apoptosis, as well as gene expression regulation, which occurs primarily at the translational level [22]. Some studies showed that miRNAs involved in the hippocampal neural apoptosis process. Hu et al. found that miR-34a-targeted neuroprotection against hippocampal neurone cell apo-ptosis post-status epilepticus [23]. Sun et al. demonstrated that miR-665 could target BCL-2L1 and thus influence apoptosis in rodent developing hippocampal astrocytes [24]. Herein, in order to explore the precise molecular mechanism of sevoflurane exposure caused hippocampal neural apoptosis, we used microarray analysis to select differential miRNAs involved in the apoptosis process of HT22 cells with sevoflurane exposure. Among the 40 significantly up/downregulation micoRNAs, miR-145 was specially selected.
miR-145 has been reported to involve in the cell apoptosis process. Cho et al. found that miR-145 could induce the apoptosis of human lung adenocarcinoma by the negative regulation of epidermal growth factor receptor (EGFR) expression [25]. Qi et al. reported that inhibition of miR-145 could exacerbate neuron cell damage [26]. Zheng et al. found that miR-145 could promote TNF-alpha-induced apoptosis in triple-negative breast cancer [27]. In this study, we found that miR-145 was significantly downregulated in HT22 cells with sevoflurane exposure, and furthermore studies showed that miR-145a overexpression could protect the HT22 cells from apoptosis induced by sevoflurane.
Bnip3 is an apoptosis associated protein which could transduce the apoptotic signal in a caspase-independent manner [28,29]. In the present study, we identified Bnip3 as a direct target of miR-145 in HT22 cell lines. In order to verify whether Bnip3 as a target gene of miR-145 involve in the apoptosis of HT22 cells induced by sevoflurane, we transfected pcDNA-Bnip3 plasmid to the HT22 cell lines. Indeed, the protection function of miR-145 against apoptosis induced by sevoflurane in HT22 cells was inhibited by Bnip3 expression, as can be evidenced from the cell viability, the rate of apoptosis, and the expression of key mediators in the mitochondrial apoptotic pathway, such as: cleaved-caspase 3, Bax, and Bcl-2. It is, therefore, suggested that miR-145 protect HT22 cells against sevoflurane caused mitochondrial apoptosis by suppressing its target gene Bnip3 expression.
In conclusion, we have demonstrated that sevoflurane could induce hippocampal neural cells apoptosis through the mitochondrial pathway. miR-145 confers protection against sevoflurane-induced hippocampal apoptosis by directly inhibiting its target gene-Bnip3 expression in hippocampal neural cell lines. Therefore, identification of miR-145 might be a novel protective molecule may potentially enable us to develop additional therapeutic strategies for prevention and treatment of hippocampal dysfunction caused by sevoflurane.
Disclosure of conflict of interest
None.
References
- 1.Zhang C, Zhang Y, Shen Y, Zhao G, Xie Z, Dong Y. Anesthesia/surgery induces cognitive impairment in female Alzheimer’s disease transgenic mice. J Alzheimers Dis. 2017;57:505–518. doi: 10.3233/JAD-161268. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Wang J, Zhang Q, Duan X, Chen Z, Zhang Y. Postconditioning with sevoflurane ameliorates spatial learning and memory deficit after hemorrhage shock and resuscitation in rats. J Surg Res. 2016;206:307–315. doi: 10.1016/j.jss.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Zheng JW, Meng B, Li XY, Lu B, Wu GR, Chen JP. NF-kappaB/P65 signaling pathway: a potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:394–407. [PubMed] [Google Scholar]
- 4.Nie H, Peng Z, Lao N, Dong H, Xiong L. Effects of sevoflurane on self-renewal capacity and differentiation of cultured neural stem cells. Neurochem Res. 2013;38:1758–1767. doi: 10.1007/s11064-013-1074-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Zhou X, Lu D, Yang X, Zhou Z, Chen X, Chen Y, He W, Feng X. Aberrantly expressed long noncoding RNAs are involved in sevoflurane-induced developing hippocampal neuronal apoptosis: a microarray related study. Metab Brain Dis. 2016;31:1031–1040. doi: 10.1007/s11011-016-9838-6. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Zhou H, Jiang L, Mao Y, Cui X, Xie B, Cui D, Wang H, Zhang Q, Xu S. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res. 2016;1652:135–143. doi: 10.1016/j.brainres.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Kim SN, Liu X, Zhang H, Zhang C, Seo JS, Kim Y, Sun T. miR-17-92 cluster regulates adult hippocampal neurogenesis, anxiety, and depression. Cell Rep. 2016;16:1653–1663. doi: 10.1016/j.celrep.2016.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Q, Jiang W, Jin Y. MiR-124 effect in neurons apoptosis in newborn rat with thyroid hypofunction. Int J Clin Exp Pathol. 2015;8:14465–14471. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Yan G, Li Q, Sun H, Hu Y, Sun J, Xu B. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One. 2012;7:e44907. doi: 10.1371/journal.pone.0044907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaki Y, Tabuchi T, Takahashi T, Kosaka K, Satoh T. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–688. doi: 10.1055/s-0029-1240622. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YF, Wang QX, Zhou HY, Chen G. Autophagy activation prevents sevoflurane-induced neurotoxicity in H4 human neuroglioma cells. Acta Pharmacol Sin. 2016;37:580–588. doi: 10.1038/aps.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sassone F, Margulets V, Maraschi A, Rodighiero S, Passafaro M, Silani V, Ciammola A, Kirshenbaum LA, Sassone J. Bcl-2/adenovirus E1B 19-kDa interacting protein (BNip3) has a key role in the mitochondrial dysfunction induced by mutant huntingtin. Hum Mol Genet. 2015;24:6530–6539. doi: 10.1093/hmg/ddv362. [DOI] [PubMed] [Google Scholar]
- 15.Landoni G, Rodseth RN, Santini F, Ponschab M, Ruggeri L, Szekely A, Pasero D, Augoustides JG, Del Sarto PA, Krzych LJ, Corcione A, Slullitel A, Cabrini L, Le Manach Y, Almeida RM, Bignami E, Biondi-Zoccai G, Bove T, Caramelli F, Cariello C, Carpanese A, Clarizia L, Comis M, Conte M, Covello RD, De Santis V, Feltracco P, Giordano G, Pittarello D, Gottin L, Guarracino F, Morelli A, Musu M, Pala G, Pasin L, Pezzoli I, Paternoster G, Remedi R, Roasio A, Zucchetti M, Petrini F, Finco G, Ranieri M, Zangrillo A. Randomized evidence for reduction of perioperative mortality. J Cardiothorac Vasc Anesth. 2012;26:764–772. doi: 10.1053/j.jvca.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Bignami E, Biondi-Zoccai G, Landoni G, Fochi O, Testa V, Sheiban I, Giunta F, Zangrillo A. Volatile anesthetics reduce mortality in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:594–599. doi: 10.1053/j.jvca.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Song FH, He W, Yang XY, Zhou ZB, Feng X, Zhou LH. Neonatal exposure to sevoflurane causes apoptosis and reduces nNOS protein expression in rat hippocampus. Mol Med Rep. 2012;6:543–546. doi: 10.3892/mmr.2012.976. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, She GM, Wang C, Zhang LY, Liu CF. The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci. 2015;19:647–657. [PubMed] [Google Scholar]
- 19.Zhou X, Li W, Chen X, Yang X, Zhou Z, Lu D, Feng X. Dose-dependent effects of sevoflurane exposure during early lifetime on apoptosis in hippocampus and neurocognitive outcomes in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol. 2016;8:111–119. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, da Li W, Yuan BL, Niu LJ, Yang XY, Zhou ZB, Chen XH, Feng X. Lithium treatment prevents apoptosis in neonatal rat hippocampus resulting from sevoflurane exposure. Neurochem Res. 2016;41:1993–2005. doi: 10.1007/s11064-016-1909-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Xian D, Xia J, Tang Y, Li W, Chen X, Zhou Z, Lu D, Feng X. MicroRNA-34c is regulated by p53 and is involved in sevoflurane-induced apoptosis in the developing rat brain potentially via the mitochondrial pathway. Mol Med Rep. 2017;15:2204–2212. doi: 10.3892/mmr.2017.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu K, Xie YY, Zhang C, Ouyang DS, Long HY, Sun DN, Long LL, Feng L, Li Y, Xiao B. MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neurosci. 2012;13:115. doi: 10.1186/1471-2202-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sun WC, Liang ZD, Pei L. Propofol-induced rno-miR-665 targets BCL2L1 and influences apoptosis in rodent developing hippocampal astrocytes. Neurotoxicology. 2015;51:87–95. doi: 10.1016/j.neuro.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011;8:125–131. doi: 10.4161/rna.8.1.14259. [DOI] [PubMed] [Google Scholar]
- 26.Qi X, Shao M, Sun H, Shen Y, Meng D, Huo W. Long non-coding RNA SNHG14 promotes microglia activation by regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience. 2017;348:98–106. doi: 10.1016/j.neuroscience.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Wu Z, Wu A, Huang Z, He N, Xie X. MiR-145 promotes TNF-alpha-induced apoptosis by facilitating the formation of RIP1-FADDcaspase-8 complex in triple-negative breast cancer. Tumour Biol. 2016;37:8599–8607. doi: 10.1007/s13277-015-4631-4. [DOI] [PubMed] [Google Scholar]
- 28.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2025–2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinnadurai G, Vijayalingam S, Gibson SB. BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene. 2008;27(Suppl 1):S114–127. doi: 10.1038/onc.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]