Abstract
Diabetic retinopathy is major cause of vision loss during working age. Breakdown of blood-retinal barrier is an early event in pathogenesis of DR. RPE is the major part of outer BRB. Apelin, an endogenous ligand of APJ, mediates angiogenesis. Our previous study showed that apelin induced proliferation, migration, and collagen I mRNA expression in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways. Now we investigate the connection between apelin and RPE in vascular permeability of diabetic retinopathy and its working mechanism. Our study showed that apelin promotes the proliferation, migration and expression of cytoskeleton and tight junction proteins in human RPE cells using MTS and transwell chamber assay. Apelin also activated the expression of PI-3K/Akt and MAPK/Erk signaling pathways proteins, such as PLCγ1, p38, Akt and Erk phosphorylation in RPE cells using laser scanning confocal detection, PCR and western blot. Pretreatment with the inhibitor of apelin receptor APJ, F13A, abolished the apelin-induced activations of the proliferation, migration and expression of cytoskeleton, tight junction and PI-3K/Akt and MAPK/Erk signaling pathways proteins in human RPE cells. It suggested that apelin as a promoter in retinal vascular permeability during early stage of DR, provides further evidence for neurovascular crosstalk in pathogenesis of DR, which may offer a new target in early prevention and treatment of DR.
Keywords: Apelin, RPE, cytoskeleton, tight junction, PI-3K/Akt, MAPK/Erk
Introduction
Diabetic retinopathy (DR) is one of the most serious and highly specific microvascular complications of diabetes mellitus [1] and the leading cause of new emerging blindness in adults between 20 to 74 ages worldwide [2]. The prevalence of diabetic macular edema (DME), the early stage of DR, was between 1.4-12.8% [3], remains the predominant cause of vision loss in the highly prevalent type 2 diabetes mellitus (T2DM) [4] and is definitely present in patients with T2DM with proliferative diabetic retinopathy (PDR) [5]. In addition to vision loss, DR and DME have also been shown to contribute to the development of other diabetes-related complications including peripheral neuropathy, nephropathy and cardiovascular events [1].
Anatomical and functional changes present in the retina and prior to clinical symptoms of disease and retinal pigment epithelium (RPE), as the key part of the outer blood retinal barrier (BRB), plays a critical role in the pathogenesis of DR [6]. The outer BRB is responsible for transport of nutrients, ions, and water; absorption of light and protection against photo-oxidation; the visual cycle; phagocytosis of shed photoreceptor membranes; and secretion of essential factors for preservation of the structural integrity of the retina. It also contributes to the immune-privileged status of the eye [7]. The BRB leakage and the extravasation of blood constituents into the retina have been attributed in part to direct injury to the tight junction structure. The tight junctions are important structural and functional components for maintenance of BRB integrity. The tight junction proteins zonular occluden-1 (ZO-1) and occluding [8] have been identified as marker proteins. Changes in their distribution and expression have direct impacts on the state of the tight junction, and thus influence BRB permeability. The actual mechanism of DR is not yet completely clarified. So far, the primary research of signaling pathways, including PI-3K/Akt, p38 MAPK, Akt/eNOS and MAPK/Erk. Since the therapeutic methods are under distinct restriction, it becomes urgent to explore an efficient intervention for DR.
Apelin is a recently isolated bioactive peptide growth factor from bovine gastric extract regarding as an endogenous ligand for APJ, and able to mediate angiogenesis and vascular formation [6]. Current investigations demonstrated that Apelin played critical roles in the occurrence and development of diabetes mellitus and its complications. A sequence of apelin cDNA encodes a preproprotein of 77 amino acids, which can generate several active polypeptides: the long (42-77) and the short (65-77) forms of apelin, apelin-36, apelin-17, and apelin-13 [9]. The relative potency of the apelin peptides varies between experimental systems, (Pyr1) apelin-13 and apelin-13 being the most potent activators of apelin receptors expressed in cell lines [6,9], whereas apelin-36 is the most potent inhibitor of HIV infection of cells in vitro [10]. However, (Pyr1) apelin-13, apelin-13, and apelin-36 are equipotent mediators of vascular tone and cardiac contractility in human tissues in vitro [11]. Our previous study has already demonstrated that apelin-13 could promote proliferation and migration of retinal Müller cells [12,13], human RPE cells [14], pericytes [15] and retinal microglial BV2 cells [16]. Apelin has been reported to promote the phosphorylation of extracellular signal-regulated kinases (ERKs), protein kinase B (Akt), and p70S6 kinase in umbilical endothelial cells [17]. However, there is no information about the role of apelin in the expression of cytoskeleton and tight junction proteins in human RPE cells.
In this study, we researched the response and effect of apelin-13 to cytoskeleton and tight junction proteins under high glucose conditions in human RPE cells and found that apelin-13 might be an early protector of vascular permeability in diabetic retinopathy. By the analysis of downstream signaling pathways, we also demonstrated that apelin was upregulated by PI-3K/Akt and MAPKs/Erk stimulation of human RPE cells, which suggested the mechanism of apelin involved in the process of early stage of diabetic retinopathy.
Materials and methods
Reagents
Human exogenous recombinant apelin peptide (No. 057-30, St. Louis, MO, USA) and the special antagonist of apelin receptor APJ, F13A (No. 427197, St. Louis, MO, USA), were purchased from Sigma. Antibodies used in the present study were shown in Table 1. All experiments were performed in accordance with the Research Ethics Committees of People’s Hospital Peking University and with the approval of People’s Hospital Eye Institute.
Table 1.
Antibodies used in the present study
| Antibody | Cat. NO | Source | Manufacturer | Western dilution | Immunofluorescence dilution | |
|---|---|---|---|---|---|---|
|
| ||||||
| HRMECs | Retina | |||||
| Anti-actin | #4970S | Rabbit* | CST | 1:1000 | ||
| Anti-FAK | #3285S | Rabbit* | CST | 1:1000 | ||
| Anti-p-FAK | #8556S | Rabbit* | CST | 1:1000 | ||
| Anti-Src | #2108S | Rabbit* | CST | 1:1000 | 1:100 | |
| Anti-p-Src | #6943S | Rabbit* | CST | 1:1000 | ||
| Anti-PLCγ1 | #5690S | Rabbit* | CST | 1:1000 | ||
| Anti-p-PLCγ1 | #8713S | Rabbit* | CST | 1:1000 | ||
| Anti-p38 | #8690S | Rabbit* | CST | 1:1000 | ||
| Anti-p-p38 | #4511S | Rabbit* | CST | 1:1000 | ||
| Anti-p-Akt | #4060S | Rabbit* | CST | 1:1000 | ||
| Anti-Akt | #4691S | Rabbit* | CST | 1:1000 | ||
| Anti-VE-Cadherin | ab33168 | Rabbit* | Abcam | 1:1000 | 1:200 | |
| Anti-Occludin | No. 331588 | Mouse* | Invitrogen | 1:1000 | 1:1000 | 1:1000 |
| Anti-ZO-1 | ab150266 | Mouse* | Abcam | 1:1000 | 1:1000 | 1:1000 |
| FITC-conjugated donkey anti-rabbit-tetramethyl rhodamine isothiocyanate | ab6881 | Abcam | 1:5000 | 1:1000 | 1:1000 | |
| TRITC-conjugated donkey anti-rabbit-tetramethyl rhodamine isothiocyanate | ab6799 | Abcam | 1:5000 | 1:1000 | 1:1000 | |
CST: Cell signaling technology (Danvers, MA, USA); Abcam (Cambridge, MA, USA); Invitrogen (Carlsbad, CA, USA); FAK: focal adhesion kinase; Akt: protein kinase B; Erk: extracellular signal-regulated kinase; VE-Cadherin: vascular endothelial (VE)-Cadherin; ZO-1: zonula occludens-1; PLCγ1: phospholipaseCγ1;
monoclonal;
#: polyclonal.
Cells culture and treatments
The human retinal pigment epithelial cells (ARPE-19; CRL-2302) were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in Dulbecco’s Modified Eagle Media (DMEM; Gibco, Invitrogen, Grand Island, NY) with 10% fetal bovine serum (FBS, Gibco, Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma) at 37°C under 5% CO2 and 95% ambient air. Exogenous recombinant apelin (0, 1, 10, 100 and 1000 ng/mL) [13] stimulated ARPE-19 for 24 h before determination. ARPE-19 were pretreated with high glucose (25 mmol/L) [18] for 48 h and then stimulated with F13A (0, 0.2, 2, 20 and 200 ng/mL) for another 48 h before test. The ARPE-19 was used for each experiment at 80%-90% confluence and was passaged after being grown for 3 days in cultured media. The cells were used between passages 3 and 6 and were seeded in a 25 cm2 flask at a density of 3 × 106.
Cell viability/cell proliferation
Cell proliferation was assessed using MTS dye according to manufacturer’s instructions (CellTiter96 Aqueous One Solution Assay; Promega, Madison, WI, USA). 1 × 104 ARPE-19 were subcultured into 96-well plates per well. Cells were pretreated in DMEM with 10% FBS for 12 h, before incubated in DMEM (with apelin or F13A) with 10% FBS, and 1% P/S in T25 flask for 24 h. MTS reagent (10 μl/per well) was added to the culture medium, and the cells were incubated for an additional 24 h. The absorbance was measured at 490 nm. Values of the treated cells were compared with the control cells, reported as percentage cell viability.
Cell migration/transwell assay
Transwell assay, also known as cell migration assay, was undoubtedly used for evaluating cell migration capabilities. Briefly, ARPE-19 starved for 12 h were released from T25 culture flask and resuspended in DMEM with 10% FBS and 1% P/S at the density of 5 × 104/ml. 100 μL of ARPE-19 suspension was added to the upper chamber and 600 μL medium containing apelin or F13A, high glucose DMEM, or DMEM (control) to the lower chamber, respectively. The chambers were incubated for 6 h at 37°C and 5% CO2 in the humidified incubator. The filters were fixed within 4% paraformaldehyde (PFA) for 30 min and we subjected the nuclei to DAPI (1:5000; No. D9542, Sigma, USA) staining for 5-10 min. The remaining cells on the upper surface of the filter were removed by wiping with a cotton swab gently and washed in PBS three times. The number of migrated cells were quantified by counting in five random fields (200 × magnification), using a Nikon 50i fluorescence microscope.
Laser scanning confocal detection
The cells cultured on cover glasses (Fisher, US) were washed in 1 × phosphate-buffered saline (PBS, 5 min × 3) and then fixed with 100% acetone [19] for 5 min at room temperature (RT), washed in 1 × PBS (5 min × 3). Blocking was performed with 1% bovine serum albumin (BSA, 30 min-1 h, RT). Primary antibodies were diluted into 1% BSA according to the instruction and incubated 3 h at RT or overnight at 4°C. The primary antibodies used for immunofluorescence staining were rabbit anti-VE-Cadherin, FITC-conjugated anti-ZO-1 and FITC-conjugated anti-occludin. After blocking, the sections were washed (1 × PBS, 5 min × 3) and then incubated with secondary antibodies (2 h RT). Secondary antibodies were used the relevant FITC-conjugated donkey anti-rabbit-tetramethyl rhodamine isothiocyanate. The samples were counterstained with DAPI (1:5000, D9542, Sigma, USA) and then washed (1 × PBS, 5 min × 3), at last they were covered by a non-fluorescent sealant. The cells were viewed using a Laser scanning confocal microscope (DS-Ri1-U2, Nikon, Japan) under 400 × magnifications.
Quantitative real-time PCR
Total RNA was extracted from the RPE cells with an extraction reagent, Trizol reagent (Invitrogen, CA, US), and we determined the concentration and integrity of total RNA with UV spectrophotometry (NANODROP 2000C, Thermo, US). We used the Thermo Revert Aid TM First Strand cDNA Synthesis Kit (K1622, Thermo, US) to reverse RNA (2 μg) into first strand cDNA, using a real-time PCR system (PikoReal 96 PCR system, Thermo Scientific). We converted 2 μg of RPE cells RNA into cDNA in a total reaction volume of 20 μl, containing 1 μg Oligo (dT), 4 μl M-MLV 5 × reaction buffer, 2 μl dNTP, 1 μl Recombinant RNasin Ribonuclease Inhibitor, and 1 μl M-MLV reverse transcriptase. The mixture was incubated for 60 min at 42°C and terminated by heating at 95°C for 5 min. The PCR solution system contained 1 μL of cDNA (1:20 diluted), specific primers 1 μL (10 pmol), 3 μL DEPC water, and 5 μL of 2 × SYBR Select Master Mix (Invitrogen), with a final volume of 10 μL. Each sample was measured in triplicate. The standard PCR conditions included 2 min at 50°C and 10 min at 95°C, followed by 35 cycles of extension at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The target gene primers are shown in the Table 2. The mRNA expression was normalized to the expression level of ACTB. Each run included one synthetic template control and one no-template control for each target. Apelin, APJ, VE-Cadherin, FAK, Src, ZO-1 and occludin were normalized to GAPDH expression. We calculated the changes in mRNA expression according to the 2-ΔΔCT method, with ΔCT = CTarget gene-CTACTB and ΔΔCT = ΔCTreatment-ΔCTControl.
Table 2.
Gene subtype oligonucleotide primers
| Gene ID | Protein Name | Gene Name | Resource | Oligonucleotide Primers, 5’-3’ | Size, base pairs (bp) |
|---|---|---|---|---|---|
| 1003 | VE-Cadherin | CDH5/CD144 | Human | F: GGACATAACACCACGAAACG | 120 |
| R: CGGTCAAACTGCCCATACTT | |||||
| 5747 | FAK | PTK2 | Human | F: GGCACCATCCCTAACCATT | 118 |
| R: AGCCCGTTCACCTTCTTTCT | |||||
| 6714 | Src | SRC | Human | F: GCTTGTGGGTGATGTTTGAC | 105 |
| R: CCTGGACTCTTGGCTCTTCT | |||||
| 100506658 | Occludin | OCLN | Human | F: TGCTCATTATTGTGATGTG | 173 |
| R: GCCATAGCCATAACCATA | |||||
| 7082 | ZO-1 | TJP1 | Human | F: GTAGGAGATTCTTTCTATATTAGA | 199 |
| R: CAGCTCTGTTCTTATTAGG | |||||
| 2597 | GAPDH | GAPDH | Human | F: TTG ACG CTG GGG CTG GCA TT | 117 |
| R: TGG AGG CCA TGT GGG CCA TGA |
Western blot analysis
Briefly, ARPE-19 cells were harvested, washed once with ice-cold PBS and lysed in 100 μL RIPA lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) including 15 μL protease inhibitor and gently shaken on the platform for 30 min and then centrifuged at 12,000 rpm for 20 min at 4°C. ARPE-19 cells were homogenized in 300 μL RIPA lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) including 45 μL protease inhibitor, lysis with gently shaken on the platform for 30 min and then centrifuged at 12,000 rpm for 20 min at 4°C. Protein concentrations determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Samples were analyzed on 10% and 8% NuPAGE Bis-Tris gels according to the manufacturer’s protocol, then transferred to PVDF membranes (Millipore, Billerica, MA, USA), and blocked with 5% BSA or fat-free milk for 1 h and incubated with primary antibodies overnight on ice on the platform. The primary antibodies were used at the following dilutions in Table 2. The PVDF membranes were incubated with goat anti-rabbit horse-radish peroxidase- (HRP-) conjugated secondary antibody for 1 h at room temperature or 4 h on ice on the platform and processed for analysis using an enhanced chemiluminescence (ECL) substrate (PerkinElmer, Inc., MA, USA). The density of each band was analyzed with Image J software. Relative changes in protein expression were calculated in relation to normal group and expressed as a fold-change.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS for Windows, version 17.0, USA). The results were expressed as mean ± SD. Differences among groups were assessed using one-way analysis of variance (ANOVA), followed by Dunnett’s test. A value of P < 0.05 was considered statistically significant. Experiments were performed at least three times and representative experiments are shown.
Results
Apelin induces cell proliferation and migration in human rpe cells
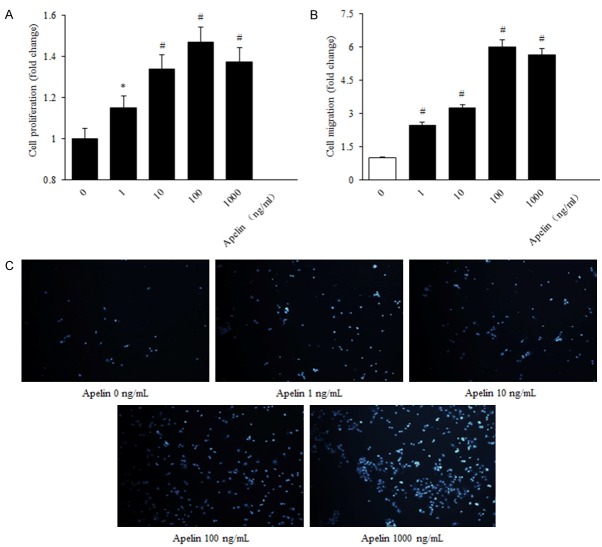
Our previous study has already demonstrated that apelin-13 could induce the proliferation, migration, and expression of collagen I mRNA in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways [14] and played a role in progression of DR to a proliferative phase [20]. Experiments were performed to evaluate whether apelin-13 produced an effect on human RPE cells proliferation and migration. According to the MTS assay results. The human RPE cells proliferation capability was increased in a dose-dependent manner, significantly higher in the apelin group (1, 10, 100, 1000 ng/mL), compared with the control one, and the optimum concentration was 100 ng/mL (control versus apelin, P < 0.05, 100 ng/mL versus control, P < 0.01, 1000 ng/mL versus control, P < 0.05) (Figure 1A-C).
Figure 1.
Apelin induced proliferation and migration expression in human RPE cells. A: RPE cell proliferation was determined with MTS after 24 h incubation with concentrations of apelin (0, 1, 10, 100, 1000 ng/mL). B, C: RPE cell migration in response to apelin treatment was measured using a transwell assay (200 × magnification). The values were assessed by the mean number of migrated cells. The number of migrated cells per high power field (HPF) is shown. *P < 0.05, #P < 0.01 versus untreated control.
Apelin promotes the expression of cytoskeleton and tight junction proteins in human RPE cells
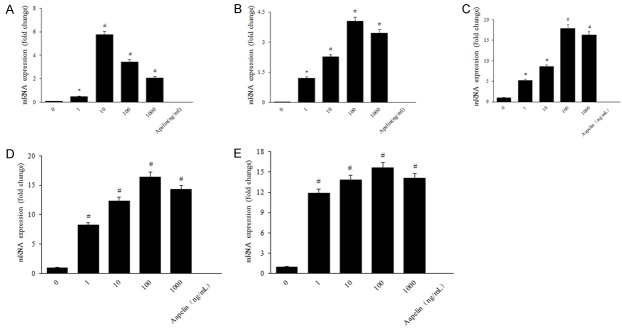
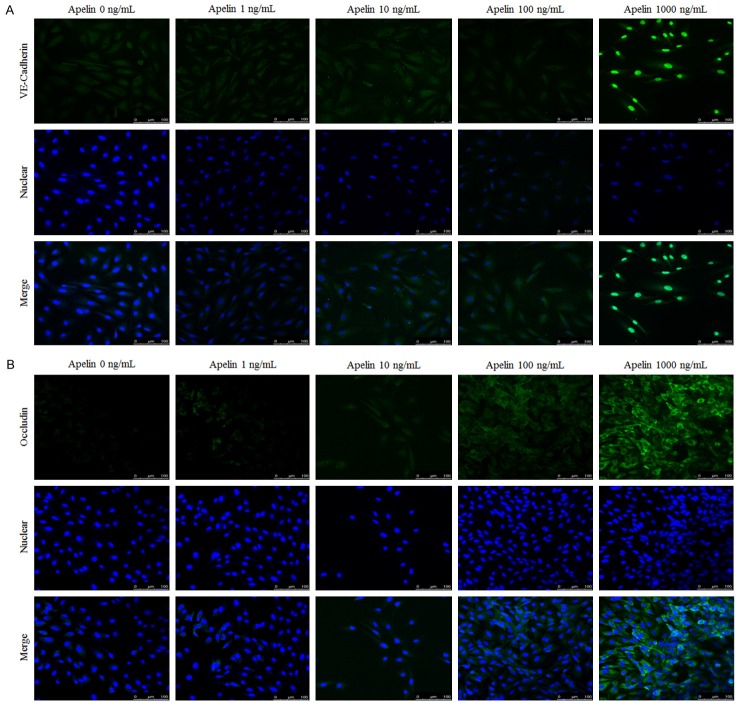
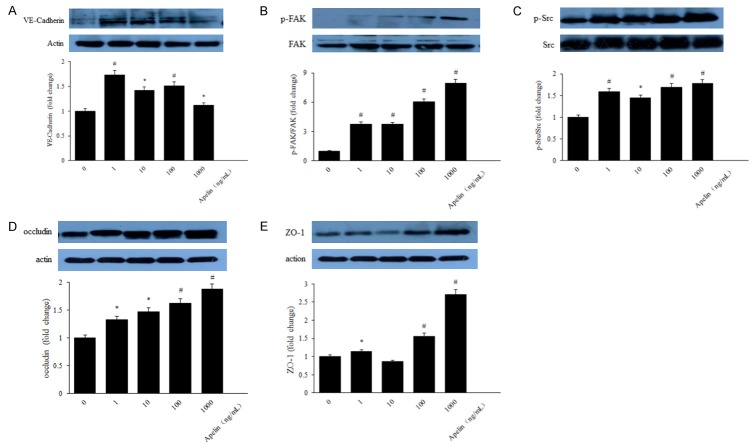
Lots of studies performed cytoskeleton and tight junction on RPE cells in diabetes [21-23]. However, research of apelin on the effect of RPE cytoskeleton and tight junction is relatively rare. In order to indicate the relationship between apelin and cytoskeleton and tight junction proteins, we performed the detection to verify the expression of cytoskeleton and tight junction proteins using QRT-PCR, laser scanning confocal staining and western blot with apelin of different concentrations (1, 10, 100, 1000 ng/mL). PCR results revealed that the mRNA expression of VE-Cadherin, FAK, Src, occludin and ZO-1 were increased significantly with apelin treated group, compared with the control ones (Figure 2A-E). Our laser scanning confocal detection results showed that VE-Cadherin and occluding was increased after treated with apelin in human RPE cells (Figure 3A, 3B). Western blot results showed that the protein expression of cytoskeleton and tight junction were also up-regulated in a dose-dependent way after stimulated with apelin (Figure 4A-E).
Figure 2.
The mRNA expression of cytoskeleton (VE-Cadherin, FAK and Src) and tight junction proteins (ZO-1 and occludin) in human RPE cells by RT-PCR. The mRNA expression of VE-Cadherin (A), FAK (B), Src (C), ZO-1 (D) and occluding (E) were increased significantly in apelin-treated groups, compared with the normal ones. *P < 0.05, #P < 0.01 versus untreated control.
Figure 3.
The expression of cytoskeleton (VE-Cadherin) (A) and tight junction (occludin) (B) in human RPE cells by laser scanning confocal detection. Staining intensities of apelin in RPE cells were stronger significantly, compared with control ones (A and B). *P < 0.05, #P < 0.01 versus untreated control.
Figure 4.
Western blot evaluation of cytoskeleton and tight junction proteins in human RPE cells. VE-Cadherin (A), FAK (B), Src (C), occludin (D) and ZO-1 (E) proteins expression were increased with apelin treatment, compared with the control ones. *P < 0.05, #P < 0.01 versus untreated control.
F13A reduces cell proliferation and migration in human RPE cells under high glucose condition
High glucose was added to the normal glucose DMEM medium with 10% FBS for 48 h after human RPE cells were starved for 12 h, and then cells were incubated with F13A of different concentrations (0.2, 2, 20, 200 ng/mL) for another 24 h. According to the MTS and transwell assay results, the HRMECs proliferation and migration capability were decreased in a dose-dependent manner, significantly lower in the F13A group, compared with the control one, and the optimum concentration was 20 ng/ml (Figure 5A-C).
Figure 5.
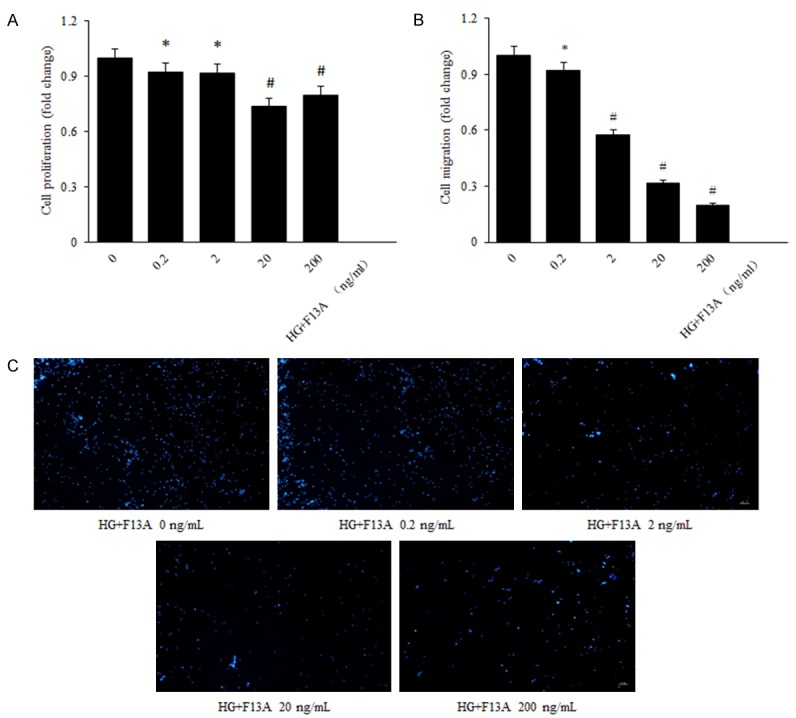
F13A reduces cell proliferation and migration in human RPE cells under high glucose condition. A: RPE cell proliferation was determined with MTS after 24 h incubation with concentrations of F13A (0, 0.2, 2, 20, 200 ng/mL). B, C: RPE cell migration in response to apelin treatment was measured using a transwell assay (200 × magnification). The values were assessed by the mean number of migrated cells. The number of migrated cells per high power field (HPF) is shown. *P < 0.05, #P < 0.01 versus untreated control.
F13A inhibits the expression of cytoskeleton and tight junction proteins in human RPE cells under high glucose condition
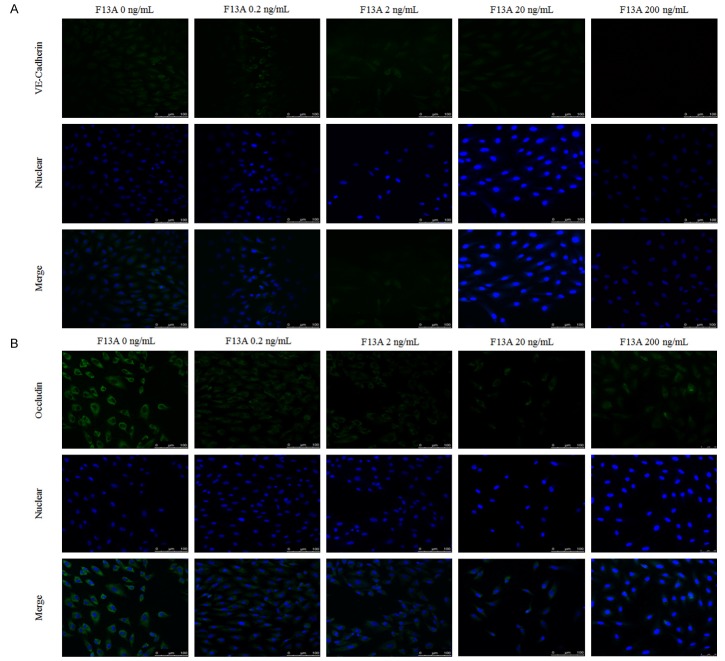
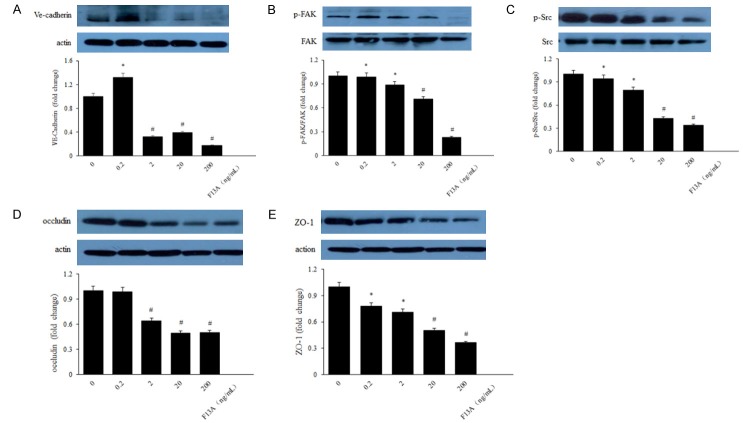
Our laser scanning confocal staining showed that occludin and VE-Cadherin was down-regulated after treated with F13A in HRMECs under high glucose conditions (Figure 6A, 6B). Western blot results showed that the protein expression of cytoskeleton and tight junction were also decreased in a dose-dependent way after stimulated with F13A (Figure 7A-E).
Figure 6.
F13A inhibits the expression of cytoskeleton and tight junction proteins in human RPE cells under high glucose condition. The expression of cytoskeleton, VE-Cadherin (A) and tight junction, occluding (B) in human RPE cells by laser scanning confocal detection. Staining intensities of apelin in RPE cells were decreased significantly, compared with control ones (B). *P < 0.05, #P < 0.01 versus untreated control.
Figure 7.
Western blot evaluation of cytoskeleton and tight junction proteins in human RPE cells under high glucose condition. VE-Cadherin (A), FAK (B), Src (C), occludin (D) and ZO-1 (E) proteins expression were decreased with F13A treatment, compared with the control ones. *P < 0.05, #P < 0.01 versus untreated control.
Apelin plays role on human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways
The PI-3K/Akt and Erks are the most significant signaling factors involved in stimulated endothelial cells and also appear to play critical roles in angiogenesis [18]. Phosphorylation of Akt and Erk is involved in proliferation, migration, vascular remolding, and angiogenesis [24]. In order to illuminate the mechanism of apelin to human RPE cells and retinal vascular permeability in the genesis and development of diabetic retinopathy, we detected the protein expression of the PI-3K/Akt and MAPK/Erk signaling pathways using western blot. The results suggested that elevated expression of phosphorylated PLCγ1, p38, Akt and Erk in apelin-treated human RPE cells (Figure 8A-D), meanwhile F13A inhibited the phosphorylation of PLCγ1, p38, Akt and Erk (Figure 9A-D). Pretreatment with the PI3K inhibitor LY294002 (10 μM) and MAPK inhibitor PD98059 (20 μM) for 30 min blocked the activation of Akt and Erk in human RPE cells (Figure 10A, 10B), indicating that the phosphorylation of Akt and Erk depends on PI3K and MAPK. After treatment with apelin (100 ng/mL) for another 30 min increased the expression of Akt and Erk (Figure 10A, 10B), suggested that apelin played roles on human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways.
Figure 8.
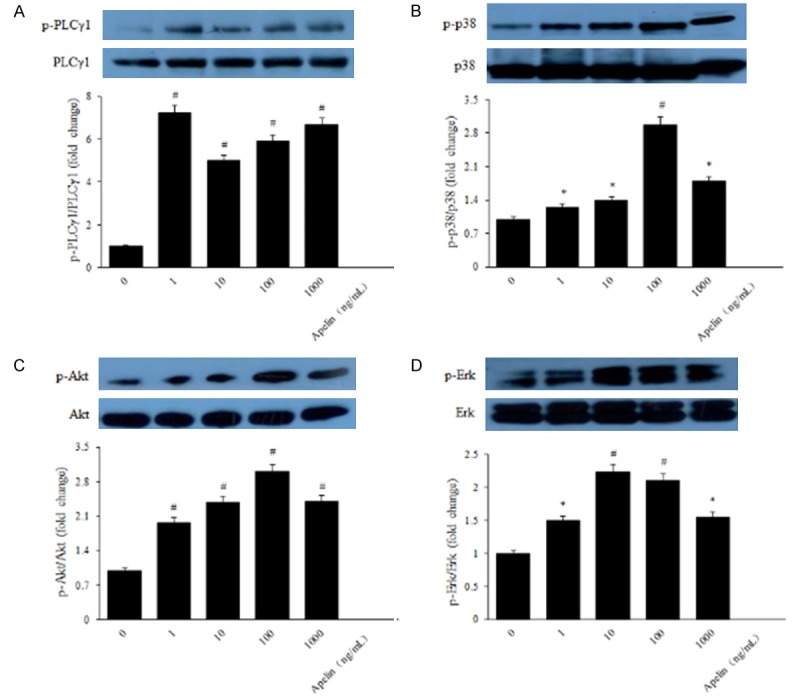
Apelin induced phosphorylation of PI-3K/Akt and MAPK/Erk signaling pathways. Levels of phosphorylated and total PLCγ1, p38, Akt and Erk were determined with western blot analysis, respectively. *P < 0.05, #P < 0.01 versus untreated control.
Figure 9.
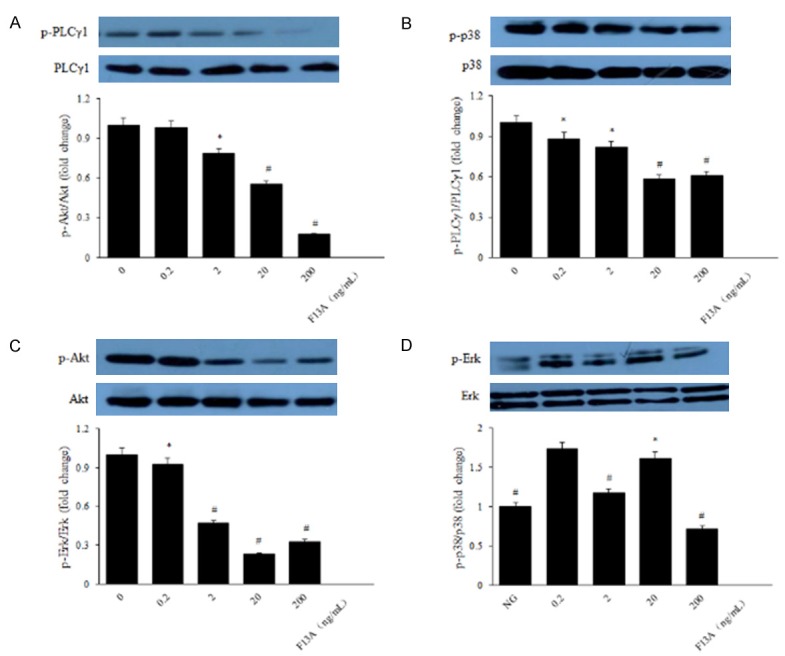
f13A inhibited the phosphorylation of PI-3K/Akt and MAPK/Erk signaling pathways. Levels of phosphorylated and total PLCγ1, p38, Akt and Erk were determined with western blot analysis, respectively. *P < 0.05, #P < 0.01 versus untreated control.
Figure 10.
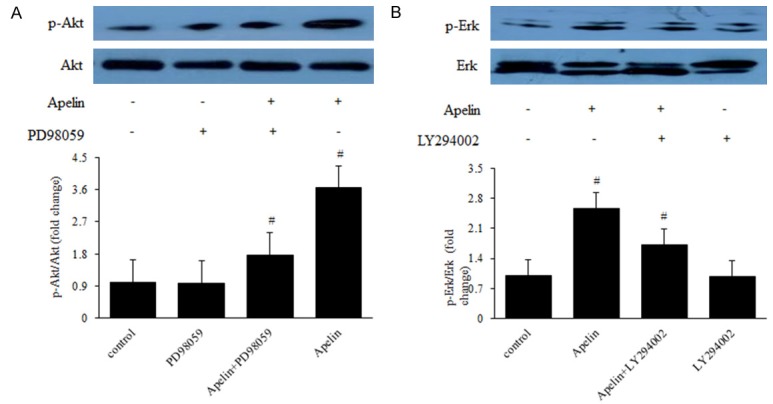
Apelin Plays Role on Human RPE Cells via PI-3K/Akt and MAPK/Erk Signaling Pathways. RPE cells were pretreated with 10 μM LY294002 or 20 μM PD98059 for 30 min and then incubated with 100 ng/mL apelin for 30 min for assay of Akt and Erk phosphorylation. Levels of phosphorylated Akt and Erk were determined with western blot analysis. *P < 0.05, #P < 0.01 versus untreated control.
Discussion
To the best of our knowledge, the current study is the first to investigate the impact of apelin expression on RPE cell barrier function. There are three main findings in the present study. First, we detected that apelin induced the proliferation and migration of human RPE cells; Second, we found that apelin promoted the protein expression of cytoskeleton and tight junction under both normal and high glucose condition; Finally, we demonstrated that apelin activated the PI-3K/Akt and MAPK/Erk signaling pathways in playing role on human RPE cells. Above all, apelin/APJ system involved in the pathology of DR, and apelin is an early promoter of vascular permeability in DME via PI-3K/Akt and MAPK/Erk signaling pathways.
As stated before, DME is derived from breakdown of blood-retinal barrier (BRB). The BRB has two components, the retinal vascular endothelium (inner BRB) and the retinal pigment epithelium (RPE) (outer BRB). This physiological barrier has tight junctions which can prevent the circulation component leak into the eye. It is strongly suggested that the inner BRB is the primary site of the vascular leak that results in DME in both human and animal studies [25,26]. Thus, the relation between apelin/APJ in DME is further studied on human RPE cells in the present study.
The blood-retina barrier (BRB) is composed of both an inner and an outer barrier. The outer BRB refers to the barrier formed at the retinal pigment epithelial (RPE) cell layer and functions, in part, to regulate the movement of solutes and nutrients from the choroid to the sub-retinal space. The tight junctions located between these cells mediate highly selective diffusion of molecules from the blood to the retina and the barrier is essential in maintaining retinal homeostasis. BRB capillary endothelial and RPE cells exhibit regulated transcytotic activity and a tight junctional network that, aided by the cytoskeleton, restricts paracellular permeability.
Apelin is a recently isolated bioactive peptide growth factor from bovine gastric extract regarding as an endogenous ligand for the oligo G protein coupled receptor APJ, and able to mediate angiogenesis and vascular formation [9]. Its localization expression in tissues was mostly in vascular endothelial cells (ECs), adipose tissue and epithelial cells. Likewise, the location of APJ is critical for Apelin/APJ signaling pathway to exert function in various cells and tissues [27]. In ocular diseases, Kasai first reported that apelin is an angiogenic factor in retinal endothelial cells [28], and suggested that spatiotemporally regulated apelin/APJ signaling participates in retinal vascularization in a cooperative manner with vascular endothelial growth factor (VEGF) or fibroblast growth factor 2 (FGF2), and contributes to normal ocular development [29]. Yonem A et al. demonstrated that apelin is upregulated at the leading edge of retinal vessel formation [30]. Our previous study demonstrated that apelin is normally expressed in the retina and various retinal cells including RPE cells and was upregulated in human and mouse retina during diabetes [12-16,20]. Additionally, the overexpression of apelin demonstrated proliferation and migration effect in cultured retinal endothelial cells [31], RPE cells [14] , Muller cells [12,13] and pericytes [15] suggesting apelin as a potential player in the pathogenesis of DR. Here we investigated whether apelin also contributes to hyperglycemia-induced RPE barrier dysfunction, which constitutes the outer retinal barrier. However, the function of apelin in DME, which is the most common complication and early event of DR, is not fully understood.
VE-cadherin is an endothelial-specific cell-cell adhesion protein of the adherens junction complex, plays a critical role on endothelial barrier function and angiogenesis, and also involves in intracellular signaling pathways that control cell cycle progression and cell dynamics directly and indirectly [32]. VE-cadherin is expressed definitely by only endothelial cells to sustain the stability of adherens junction of the endothelial cells and also suppresses their apoptosis [33]. Focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, is the primary enzyme participated in the link up of integrins and installation of FA (focal adhesions) via the catalyzing of several downstream signals and is mediated primarily through Src regulated tyrosine phosphorylation [34,35]. In particular, FAK is a pivotal mediator of angiogenesis during the development engineered to subsist the endothelial-specific deletion of FAK proved by the lethality of the early embryonic mice [36]. Increased vascular permeability and endothelial apoptosis has been determined as a critical mechanism underlying lethality in these embryos [37]. Src kinase (Src) is a proto-oncogene encoding a protein tyrosine kinase that regulates cellular morphology, motility, metabolism, survival, migration and proliferation [38].
In the present study, through immunofluorescence, RT-QPCR and western blot, we first verified that APJ was positively expressed in RPE cells and play a critical role on vascular permeability of diabetic retinopathy. Our findings showed that the expression of apelin was lower under high glucose DMEM medium, compared with normal glucose medium, which suggested the harmful role of high glucose on human RPE cells, and the dissociation of apelin in RPE. At the same time, we found that the protein expression of cytoskeleton and tight junction was decreased either. After added apelin (ng/mL), the protein expression increased significantly under high glucose condition, which suggested the positive effect on vascular permeability. Our study also found that the mRNA and protein expression of apelin and APJ in human RPE cells was lower in high glucose medium, compared with the normal one. It might indicate that high glucose could destroy the cell structure. After added apelin, the protein expression of cytoskeleton and tight junction increased with statistically significance, which indicated that it was apelin that might protect the cells against the destruction of high glucose.
Furthermore, we investigated the underlying mechanisms of vascular permeability by apelin in human RPE cells. The phosphorylation and activation of Akt has been shown to be a downstream effector of apelin signaling, this was first shown to occur via a PTX-sensitive G-protein and PKC [17]. Apelin-13- and apelin-36-mediated Akt phosphorylation in CHO cells, HUVECs [39] and osteoblasts [40]. Additionally, apelin activates Akt in rat hippocampal neuronal cultures [41], mouse cortical neurons [42], rat and human VSMCs respectively [43,44]. Studies have shown the role of apelin in cardioprotection [45] and ischemia/reperfusion may be independent of Akt signaling [46]. Apelin induces the dual phosphorylation of the S6 ribosomal protein kinase (p70S6K) in HUVECs, where apelin promotes cell proliferation via PTX-sensitive, ERK1/2-, mammalian target of rapamycin (mTOR)-, and Akt-dependent intracellular cascades [17]. In pluripotent embryonic stem cells [47] and mouse embryonic endothelial cells [48], apelin induces the phosphorylation of ERK1/2. Our western blot results showed that the protein expression of PI-3K/Akt and MAPK/Erk signaling pathway were significantly higher with apelin-treated group, compared with the control group. Treatment with the PI3K inhibitor LY294002 and MAPK inhibitor PD98059 blocked the activation of Akt and Erk in RPE cells, indicating that the phosphorylation of Akt and Erk depends on PI3K and MAPK. While the biological functions of apelin have been studied for the past nearly two decades [6], novel physiological roles on different kinds of cells [12-16] and its strict signaling pathways continue to emerge and investigate.
In summary, our data indicate that apelin plays a positive role on vascular permeability during the pathogenesis of diabetic retinopathy through the PI-3K/Akt and MAPK/Erk signaling pathways. Although our study has some limitations, including apelin/APJ knockout mice should be used to verify the protection effects of apelin in DR, the present results provided the rational that apelin as an early promoter of vascular permeability which offer a new perspective strategy in the early prevention and treatment of DR.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81271027) and the EFSD/CDS/Lilly grant (2127000043).
Disclosure of conflict of interest
None.
References
- 1.Hagg S, Thorn LM, Putaala J, Liebkind R, Harjutsalo V, Forsblom CM, Gordin D, Tatlisumak T, Groop PH FinnDiane Study Group. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes care. 2013;36:4140–6. doi: 10.2337/dc13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightman S, Towler HM. Diabetic retinopathy. Clin cornerstone. 2003;5:12–21. doi: 10.1016/s1098-3597(03)90015-9. [DOI] [PubMed] [Google Scholar]
- 5.Tong L, Vernon SA, Kiel W, Sung V, Orr GM. Association of macular involvement with proliferative retinopathy in Type 2 diabetes. Diabetic med. 2001;18:388–94. doi: 10.1046/j.1464-5491.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 6.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 7.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Sun T, Wang P, Liu YH, Zhang LW, Xue YX. Effects of erythropoietin on blood-brain barrier tight junctions in ischemia-reperfusion rats. J Mol Neurosci. 2013;49:369–79. doi: 10.1007/s12031-012-9883-5. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–71. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 10.Zou MX, Liu HY, Haraguchi Y, Soda Y, Tatemoto K, Hoshino H. Apelin peptides block the entry of human immunodeficiency virus (HIV) FEBS lett. 2000;473:15–8. doi: 10.1016/s0014-5793(00)01487-3. [DOI] [PubMed] [Google Scholar]
- 11.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1] apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, Jiang YR, Qian J, Tao Y. Apelin-13 regulates proliferation, migration and survival of retinal muller cells under hypoxia. Diabetes Res Clin Pract. 2013;99:158–67. doi: 10.1016/j.diabres.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Wang XL, Tao Y, Lu Q, Jiang YR. Apelin supports primary rat retinal muller cells under chemical hypoxia and glucose deprivation. Peptides. 2012;33:298–306. doi: 10.1016/j.peptides.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Qin D, Zheng XX, Jiang YR. Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol Vis. 2013;19:2227–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Tao Y, Feng J, Jiang YR. Apelin protects primary rat retinal pericytes from chemical hypoxia-induced apoptosis. J Ophthalmol. 2015;2015:186946. doi: 10.1155/2015/186946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Tao Y, Jiang Y. Apelin activates the expression of inflammatory cytokines in microglial BV2 cells via PI-3K/Akt and MEK/Erk pathways. Sci China Life Sci. 2015;58:531–40. doi: 10.1007/s11427-015-4861-0. [DOI] [PubMed] [Google Scholar]
- 17.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–11. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 18.Ye EA, Steinle JJ. miR-146a Attenuates inflammatory pathways mediated by TLR4/NFkappaB and TNFalpha to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediators Inflamm. 2016;2016:3958453. doi: 10.1155/2016/3958453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goossens K, Vandaele L, Wydooghe E, Thys M, Dewulf J, Peelman LJ, Van Soom A. The importance of adequate fixation for immunofluorescent staining of bovine embryos. Reprod Domest Anim. 2011;46:1098–103. doi: 10.1111/j.1439-0531.2011.01770.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu Q, Ma Y, Xu YS, Jiang YR. Apelin in epiretinal membranes of patients with proliferative diabetic retinopathy. Mol Vis. 2014;20:1122–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Beasley S, El-Sherbiny M, Megyerdi S, El-Shafey S, Choksi K, Kaddour-Djebbar I, Sheibani N, Hsu S, Al-Shabrawey M. Caspase-14 expression impairs retinal pigment epithelium barrier function: potential role in diabetic macular edema. Biomed Res Int. 2014;2014:417986. doi: 10.1155/2014/417986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Du S, Wu Q, Hu J, Li T. Decorin prevents retinal pigment epithelial barrier breakdown under diabetic conditions by suppressing p38 MAPK activation. Invest Ophthalmol Vis Sci. 2015;56:2971–9. doi: 10.1167/iovs.14-15874. [DOI] [PubMed] [Google Scholar]
- 23.Rosales MA, Silva KC, Duarte DA, Rossato FA, Lopes de Faria JB, Lopes de Faria JM. Endocytosis of tight junctions caveolin nitrosylation dependent is improved by cocoa via opioid receptor on RPE cells in diabetic conditions. Invest Ophthalmol Vis Sci. 2014;55:6090–100. doi: 10.1167/iovs.14-14234. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, Chung HT, Kwon YG, Kim YM. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol. 2006;291:H2836–46. doi: 10.1152/ajpheart.00113.2006. [DOI] [PubMed] [Google Scholar]
- 25.Sander B, Larsen M, Moldow B, Lund-Andersen H. Diabetic macular edema: passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci. 2001;42:433–8. [PubMed] [Google Scholar]
- 26.Vinores SA, McGehee R, Lee A, Gadegbeku C, Campochiaro PA. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem. 1990;38:1341–52. doi: 10.1177/38.9.2117624. [DOI] [PubMed] [Google Scholar]
- 27.Chu J, Zhang H, Huang X, Lin Y, Shen T, Chen B, Man Y, Wang S, Li J. Apelin ameliorates TNFalpha-induced reduction of glycogen synthesis in the hepatocytes through G protein-coupled receptor APJ. PLoS One. 2013;8:e57231. doi: 10.1371/journal.pone.0057231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Kasai A, Shintani N, Kato H, Matsuda S, Gomi F, Haba R, Hashimoto H, Kakuda M, Tano Y, Baba A. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1717–22. doi: 10.1161/ATVBAHA.108.163402. [DOI] [PubMed] [Google Scholar]
- 30.Duran C, Yonem A, Ustun I, Ozcan O, Ipcioglu OM, Basekim CC. Plasma ghrelin levels in males with idiopathic hypogonadotropic hypogonadism. Endocrine. 2008;34:81–6. doi: 10.1007/s12020-008-9102-x. [DOI] [PubMed] [Google Scholar]
- 31.Kasai A, Ishimaru Y, Higashino K, Yamamuro A, Yoshioka Y, Maeda S. Inhibition of apelin expression switches endothelial cells from proliferative to mature state in pathological retinal angiogenesis. Angiogenesis. 2013;16:723–34. doi: 10.1007/s10456-013-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010;22:651–8. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez D, Herrera B, Beltran A, Otero K, Quintero G, Rojas A. Nitric oxide disrupts VEcadherin complex in murine microvascular endothelial cells. Biochem Biophys Res Commun. 2003;304:113–8. doi: 10.1016/s0006-291x(03)00546-1. [DOI] [PubMed] [Google Scholar]
- 34.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–86. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–23. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 36.Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189:955–65. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roskoski R Jr. Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–64. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 39.Masri B, Morin N, Pedebernade L, Knibiehler B, Audigier Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J Biol Chem. 2006;281:18317–26. doi: 10.1074/jbc.M600606200. [DOI] [PubMed] [Google Scholar]
- 40.Tang SY, Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Zhou HD, Wu XP, Liao EY. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28:708–18. doi: 10.1016/j.peptides.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102:1905–17. doi: 10.1111/j.1471-4159.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- 42.Zeng XJ, Yu SP, Zhang L, Wei L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res. 2010;316:1773–83. doi: 10.1016/j.yexcr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui RR, Mao DA, Yi L, Wang C, Zhang XX, Xie H, Wu XP, Liao XB, Zhou H, Meng JC, Yuan LQ, Liao EY. Apelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt signaling pathways. Amino Acids. 2010;39:1193–200. doi: 10.1007/s00726-010-0555-x. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Su T, Li F, Li L, Qin X, Pan W, Feng F, Chen F, Liao D, Chen L. PI3K/Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin-13. Acta Biochim Biophys Sin (Shanghai) 2010;42:396–402. doi: 10.1093/abbs/gmq035. [DOI] [PubMed] [Google Scholar]
- 45.Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol. 2007;102:518–28. doi: 10.1007/s00395-007-0671-2. [DOI] [PubMed] [Google Scholar]
- 46.Kleinz MJ, Baxter GF. Apelin reduces myocardial reperfusion injury independently of PI3K/Akt and P70S6 kinase. Regul Pept. 2008;146:271–7. doi: 10.1016/j.regpep.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.D’Aniello C, Lonardo E, Iaconis S, Guardiola O, Liguoro AM, Liguori GL, Autiero M, Carmeliet P, Minchiotti G. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ Res. 2009;105:231–8. doi: 10.1161/CIRCRESAHA.109.201186. [DOI] [PubMed] [Google Scholar]
- 48.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. Hypoxiainduced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–40. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]