Abstract
Cancer cells have developed anoikis resistance and thereby survive after detachment from their primary site and while traveling through the circulation. However, the mechanisms underlying resistance to anoikis in osteosarcoma (OS) remain largely unknown. MicroRNAs (miRNA) have been reported to contribute to malignant phenotypes of cancer cells. To investigate the roles of miRNAs in anoikis resistance of OS cells, the implications of 9 well-characterized miRNAs that dysregulated in OS on cell anoikis were screened. As a result, miR-451 was identified as a crucial factor involved in anoikis resistance and anchorage-independent growth of OS cell. MiR-451 was down-regulated in OS cells, re-expression of miR-451 significantly promoted cell anoikis of three OS cell lines and inhibition of miR-451 protected HOS cells from anoikis under anoikis condition. Subsequently, bioinformatics prediction and luciferase reporter assay indicated that Rab14 was a direct target of miR-451, and Rab14 could be down-regulated by miR-451 at both mRNA and protein levels. Genetic silencing of Rab14 recapitulated the role of miR-451 on anoikis resistance and restoration of Rab14 largely abrogated the tumor suppressor function of miR-451. Finally, overexpression of miR-451 remarkably suppressed the lung metastasis of OS cells. Collectively, our findings suggest that the miR-451/Rab14 axis might serve as a novel mechanism of resistance to anoikis in OS.
Keywords: MicroRNA-451, anoikis resistance, Rab14, osteosarcoma
Introduction
Osteosarcoma (OS) is an aggressive bone malignancies derived from osteoblast progenitor cells of mesenchymal origin [1]. The majority of OS patients present with localized disease, and 80%-90% will die from their disease, generally as a result of the development of lung metastases. Despite great achievements in neoadjuvant therapy and surgical treatment, the survival rate of OS has remained at 60% for the past decade [2,3]. The lack of improvement in clinical outcome may be due to the inability to effectively target tumor-propagating cells in OS.
Therefore, the identification of the underlying molecular mechanisms by which OS metastasis is of paramount importance for OS treatment.
Distant metastasis of cancer cells is a multistep process that involves the cancer cells becoming disseminated from the primary site, intravasating into and surviving in the circulation, and extravasating and growing in distant organs [4]. Anoikis is a type of programmed cell death induced by cell detachment from extracellular matrix, behaving as a critical mechanism in preventing anchor-independent cell growth and inappropriate attachment, thus avoiding colonizing of distant organs [5,6]. The ability of cancer cells to develop anoikis resistance is a vital step during cancer progression and metastatic colonization [4]. In OS, the molecular basis underlying the regulation of anoikis resistance is poorly understood, although several investigations have provided new insights into the development of resistance to anoikis, including activation of Src [7], c-Met [8], Ezrin/β4 integrin interaction [9], PI3K/Akt signaling pathway [7], and down-regulation of Caveolin-1 [8]. Dysregulation of microRNAs (miRNAs) has been widely implicated in almost every known malignant phenotype of cancer cells [10]. However, the potential regulatory roles of microRNA in the anoikis resistance of OS cells remain largely unexplored.
MicroRNA (miRNA) is a short strand of RNA molecules, usually ranging between 17 and 27 nucleotides, and plays an integral role in regulating gene expression by directly binding with 3’-untranslated region (UTR) of target messenger RNAs (mRNAs) [11,12]. Accumulating studies showed that miRNAs are dysregulated in various cancer types and implicated in the initiation, maintenance, and progression of cancers [13,14]. As such, it is no surprise to hypotheses that several miRNAs might be dysregulated and play a role in regulating anoikis, to either confer anoikis resistance or anoikis sensitivity. To test this hypothesis, in this study, we screened a repertoire of dysregulated miRNAs and identified miR-451 as a crucial modulator of anoikis resistance in OS cells. Furthermore, the functional target in the regulation of anoikis by miRNA-451 was highlighted to elucidate the underlying mechanisms.
Materials and methods
Cell culture
Osteosarcoma cell lines (U2OS, Saos-2, MG-63, and HOS cells) were from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS). Normal hFOB1.19 cells were grown in DMEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (HyClone, Logan, Utah, USA) and 1% penicillin/streptomycin. Cultures were maintained in a humidified atmosphere at 37°C with 5% CO2.
Cell transfections
The miR-451 mimics, inhibitor, and their respective controls were chemically synthesized by Genechem (Shanghai, China). Small interfering RNA (siRNA) specifically targeting human Rab14 was designed to silence Rab14 expression. The CDS region of the human Rab14 was chemically synthesized and then cloned into the pcDNA3.1 vector (Promega, Madison, Wisconsin, USA). OS cells were transiently transfected with miR-451 mimics, miR-451 inhibitor sequences using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
RNA extraction and quantitative real-time PCR
Total cellular RNA was isolated from the cultured cells using Trizol reagent (Invitrogen, CA, USA). Total miRNA from cultured cells was extracted using the mirVana miRNA Isolation Kit (# AM1561, Ambion, USA). Complimentary DNA was synthesized from 5 ng of total RNA using the TaqMan miRNA reverse transcription kit (Applied Biosystems, USA) according to the manufacturer’s protocol. Quantitative RT-PCR was performed through the SYBR green assay (Invitrogen) with the Applied Biosystems 7500. The qRT-PCR primers of Rab14 and β-actin were obtained from the Harvard Primer Bank. The U6 and β-actin were used as controls. The relative expression levels of miRNA or mRNA was calculated using the 2^-ΔΔCt methods.
Dual luciferase reporter assay
The U2OS and Saos-2 cells at 5 × 104 density per well were seeded in triplicate in 24-well plates and allowed to settle overnight. Next, 100 ng of pGL3-Rab14-3’UTR (WT/Mut), or control-luciferase plasmid plus 1 ng of pRL-TK renilla plasmid (# E2810. Promega, USA) were transfected into the cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocols. After incubation for 48 h, luciferase and renilla signals were detected after transfection using the Dual-Luciferase Reporter assay system (Promega, USA) according to the manufacturer’s protocol. The experiment was performed in triplicate and the data are presented as the mean ± SD.
Cell anoikisassay
To induce anoikis, cells at 5 × 104 density per well were cultured with 6-well plate coated with poly (2-hydroxyethyl methacrylate) (poly-HEMA; Sigma, USA). Poly-HEMA was dissolved in 95% ethanol (v/v) at 50 mg/ml and added to 6-well plate at a density of 5 mg/cm2. Cells in serum-free medium were seeded into the coated plates. At the designated time points, the suspended cells were collected and subjected to cell apoptosis assays by Caspase-3/7 Glo kit (Promega, USA), and Annexin V/PI staining (7 Sea Biotech, Shanghai, China) on flow cytometer (Beckman) according to the protocols from the respective manufacturers.
Soft agar assay
Anchorage-independent growth was assessed using the colony formation assay in soft agar on 0.35% low melting-point agar (Invitrogen, USA) overlaid on 0.6% agarose. Cell suspensions (2 × 103 cells per dish) were plated in DMEM containing 10% FBS and incubated at 37°C in a humidified 5% CO2 atmosphere. Cells were fed every 2 days with complete medium. After 10 days culture, colonies were stained with 0.05% crystal violet and counted by microscopy.
Western blotting analysis
Whole cell lysates were prepared using RIPA buffer (Sigma, USA) containing a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Roche, USA). Proteins (10-50 μg) were separated using SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were then blocked with 5% nonfat dried milk and incubated with antibodies against Rab14 (ab28639, Abcam, USA) overnight at 4°C, followed by incubation with the species-matched peroxidase-conjugated secondary antibody for 1 h at room temperature. Western blots were scanned and analyzed using the Li-COR Odyssey infrared imaging system.
In vivo lung metastasis experiment
Six-week-old male athymic nude mice were randomly divided into 2 groups (5 mice per group). Single-cell suspensions of 1 × 106 miR-NC or miR-451-overexpressing cells/0.2 ml were injected into the tail vein of the nude mice. The mice were sacrificed 30 days after injection and the lungs were isolated, fixed in formalin, sectioned, and stained with hematoxylin and eosin. The microscopic lung metastasis was counted under a light microscope. All the experiments were approved by the Medical Experimental Animal Care Commission of Shandong University.
Statistical analysis
The statistical analyses were performed using GraphPad Prism (version 6.0; GraphPad Software). The in vitro experiments were performed in triplicate wells and each experiment was performed at least two or three times. All data are presented as the means ± SDs. Statistical analysis was performed by the one-way ANOVA to express the difference within groups. Statistical significance is denoted with asterisks in the figures.
Results
Identification of dysregulated microRNAs involved in anoikis resistance of OS
Anoikis resistance is a critical character of the OS cells that are prone to distant metastasis. To identify the key microRNA(s) involved in this process, we compiled several review articles with comprehensive description of dysregulated microRNAs in OS [11-16]. And finally, a total of 9 microRNAs with tumor suppressor functions was selected, including miR-34a, miR-124, miR-133a, miR-140-5p, miR-143, miR-199a-3p, miR-224, miR-451, and miR-506. Next, Caspase-3/7 activity assay was performed to determine the implications of candidate microRNAs on cell anoikis. As shown in Figure 1A, U2OS transfected with mimics of miR-124, miR-133a, miR-140-5p, miR-199a-3p, miR-224 and miR-451 had significantly increased Caspase-3/7 activity compared with the cells transfected with miR-NC under anoikis condition for 48 h. In Saos-2 cells, miR-34a, miR-133a, miR-143, and miR-451 were able to promote cell anoikis (Figure 1B). Notably, miR-451 was mostly implicated in cell anoikis of both U2OS and Saos-2 cells.
Figure 1.
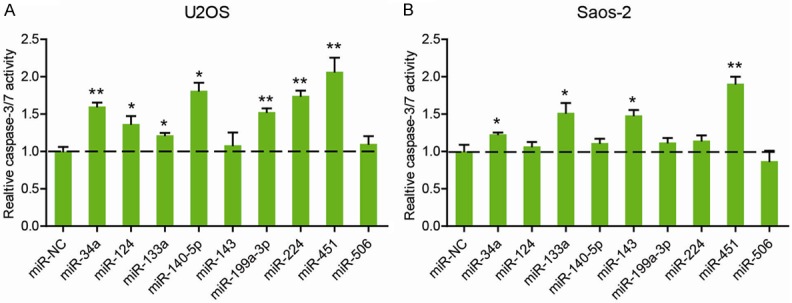
Identification of dysregulated microRNAs involved in anoikis resistance of OS. U2OS (A) and Saos-2 (B) cells were subjected to anoikis conditions and transfected with the mimics of 9 well-characterized miRs dysregulated in OS as well as their negative control for 48 h. Cell anoikis was then measured by caspase-3/7 activity. *P < 0.05; **P < 0.01.
miR-451 inhibits anoikis resistance of OS cells
Next, we focused on the role of miR-451 in the anoikis resistance of OS cells. Compared to the normal hFOB1.19 cells, U2OS, Saos-2, and MG-63 cells had relative lower expression of miR-451, whereas HOS cells had the highest expression of miR-451 in the all OS cell lines detected (Figure 2A). Consistently, transfection of miR-451 in the MG-63 cells also resulted in marked increase in cell anoikis, as demonstrated by Caspase-3/7 activity (Figure 2B), and Annexin V/PI staining (Figure 2C), respectively. To further confirm the tumor-suppressive role of miR-451 in OS, we blocked its function by transfection of miR-451 inhibitors. As illustrated in Figure 2D, miR-451 inhibitor significantly reduced the caspase-3/7 activity of HOS under anoikis condition. Similarly, Annexin V/PI staining assay showed that cell apoptosis of HOS cells were markedly decreased by miR-451 inhibitor (Figure 2E). To further determine the role of miR-451 in anoikis resistance, colony formation assay was performed. As shown in Figure 2F, transfection of miR-451 significantly reduced the anchorage-independent growth of MG63 cells. Taken together, these data above suggest that miR-451 is critically involved in the anoikis resistance of OS cells.
Figure 2.
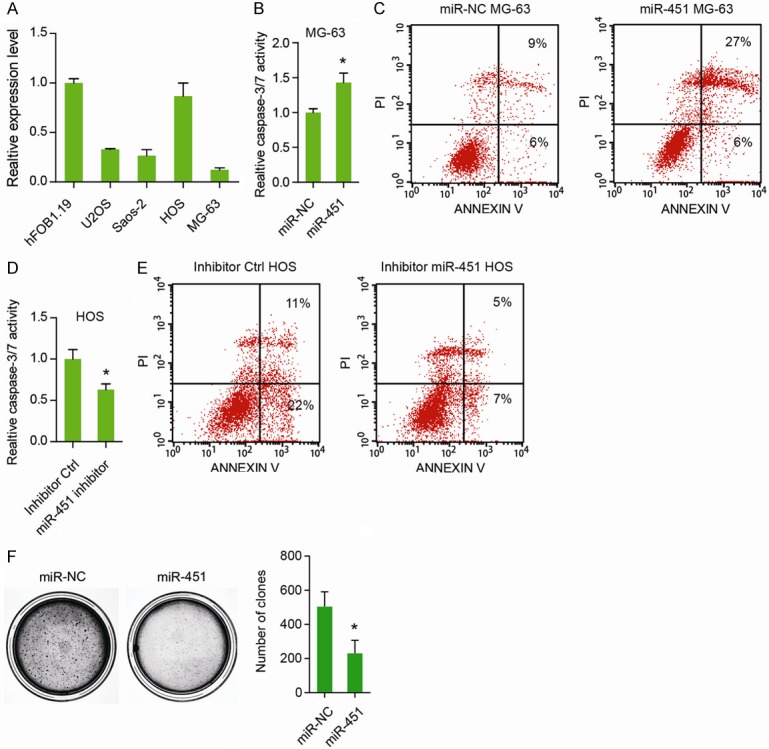
miR-451 inhibits anoikis resistance of osteosarcoma. A: The expression level of miR-451 in 4 OS cell lines and the normal hFOB1.19 cells. B: The effect of miR-451 on MG-63 cell anoikis was detected by caspase-3/7 activity. C: The effect of miR-451 on MG-63 cell anoikis as revealed by Annexin V/PI assay. D: The effect of miR-451 inhibitor on HOS cell anoikis was detected by caspase-3/7 activity. E: The effect of miR-451 inhibitor on HOS cell anoikis as revealed by Annexin V/PI assay. F: The effect of miR-451 on the anchorage-independent growth of MG-63 cells. *P < 0.05.
miR-451 directly targets ras-related protein 14 (Rab14) in OS cells
To identify the target of miR-451, we predicted the targets of miR-451 using the miRNA target prediction websites, and found miR-451 could directly bind on the 3’-UTR of Rab14 mRNA (Figure 3A). Then, we subcloned the WT or Mut Rab14 3’-UTR fragment into pGL3 dual luciferase reporter vectors. Subsequently, we found that miR-451 transfection in both U2OS and Saos-2 cells significantly inhibited the luciferase reporter activity of WT Rab14 3’-UTR, whereas mutation of Rab14 3’-UTR abrogated the repressive ability of miR-451 (Figure 3B). Moreover, qRT-PCR and western blotting were used to determine the influence of miR-451 on Rab14 expression. Expectedly, miR-451 mimics significantly decreased the mRNA and protein level of Rab14 in U2OS, Saos-2, and MG-63 cells (Figure 3C). And reversely, miR-451 mimics pronouncedly up-regulated the mRNA and protein level of Rab14 in HOS cells (Figure 3D). Collectively, these results demonstrate that miR-451 directly acts through the 3’-UTR of Rab14 in OS cells.
Figure 3.
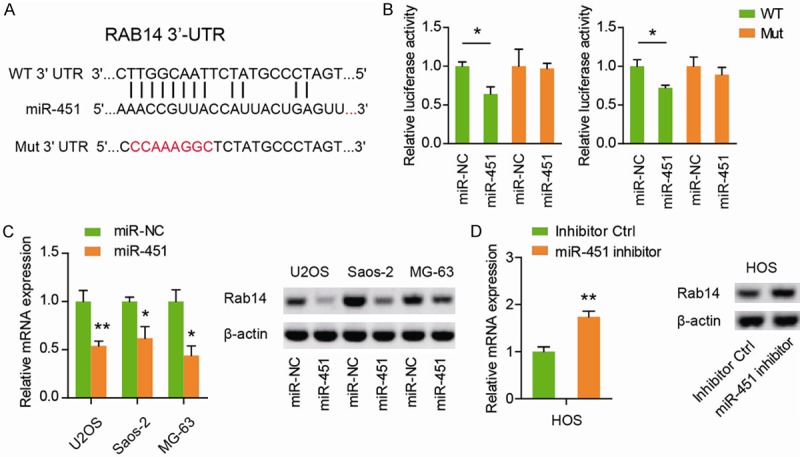
miR-451 directly targets Rab14 in OS cells. A: Putative miR-451-binding sequence in the 3’-UTR of Rab14 mRNA. Mutation was also generated on the Rab14 3’-UTR sequence in the complementary site for the seed region of miR-451. B: The wild type (WT) or mutant (Mut) reporter plasmids was co-transfected into U2OS and Saos-2 cells as well as miR-451 or negative control miR-NC. The luciferase activity was measured by a commercial kit and the result was shown as relative luciferase activity. C: The Rab14 mRNA and protein level in U2OS, Saos-2, and MG-63 cells after transfection of miR-451 was analyzed by RT-PCR assay, Western blotting, respectively. D: The Rab14 mRNA and protein level in HOS cells after transfection of miR-451 inhibitor were analyzed by RT-PCR assay, Western blotting, respectively. *P < 0.05; **P < 0.01.
Genetic silencing of Rab14 recapitulates the tumor suppressor functions of miR-451
Next, we investigated whether Rab14 was involved in miR-451-mediated effects on the anoikis of OS cells. For this purpose, we genetically silenced Rab14 by small interfering RNA (siRNA). As shown in Figure 4A, two specific siRNAs targeting Rab14 resulted in marked decrease in Rab14 protein level in both U2OS and Saos-2 cells. Compared to the si-Ctrl cells, Rab14 down-regulated cells showed increased caspase-3/7 activity (Figure 4B) and apoptosis ratio (Figure 4C). To further confirm that Rab14 targeting was involved in miR-451-induced anoikis enhancement, we then performed rescue experiments. The results showed that overexpression of Rab14 largely abrogated miR-451-mediated apoptosis enhancement in both U2OS (Figure 4D) and Saos-2 cells (Figure 4E). Furthermore, Rab14 overexpression cells had relative lower anoikis ratio compared with the control cells (Figure 4D, 4E). In conclusion, these results indicate that Rab14 might be potentially involved in miR-451-mediated tumor suppressing function on anoikis.
Figure 4.
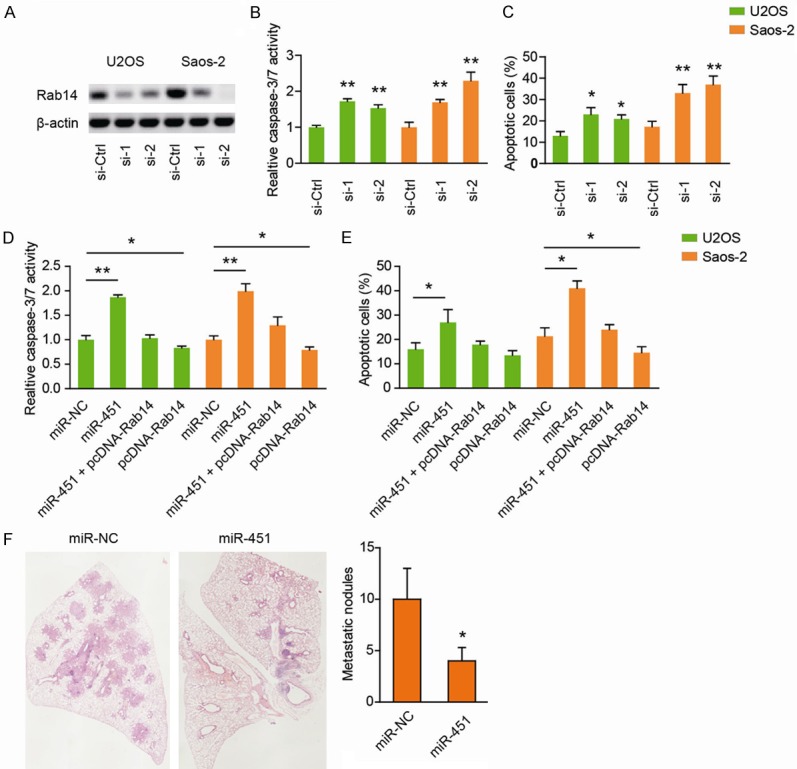
Genetic silencing of Rab14 recapitulates the tumor suppressor functions of miR-451. (A) The knockdown efficiency of Rab14 in U2OS and Saos-2 cells was tested by Western blotting assay. (B, C) The effects of Rab14 knockdown on cell anoikis of U2OS and Saos-2 cells were determined by caspase-3/7 activity (B) and Annexin V/PI assay (C), respectively. (D, E) U2OS and Saos-2 cells were cotransfected with miR-451 and pcDNA-Rab14 or empty vector. At 48 h after transfection, the cells were collected. And cell anoikis was determined by caspase-3/7 activity (D) and Annexin V/PI assay (E), respectively. (F) The numbers of visible lung metastatic nodules on the surface of the lung as revealed by HE staining. *P < 0.05; **P < 0.01.
miR-451 inhibits lung metastasis in vivo
Finally, to confirm whether the miR-451 suppresses metastasis in OS, we generated an in vivo lung metastasis model by injecting transfected cells into the tail vein of nude mice. Overexpression of miR-451 significantly reduced the ability of the cells to establish lung metastases, as reflected by the metastatic nodules (Figure 4F).
Discussion
Resistance to anoikisis a key feature of metastatic cancer cells, and protection from anoikis has been proven to facilitate both the survival and expansion of metastatic cells [17]. In this study, we showed that miR-451/Rab axis is a novel modulator of anoikis resistance in OS.Forced expression of miR-451 in OS cells increased anoikis sensitivity. Genetic silencing of Rab14 recapitulated the cardinal tumor suppressor function of miR-451. Our work adds a new understanding of anoikis resistance in OS.
Accumulating evidence suggests that miRNAs play important roles in variety of biological processes, such as cell proliferation, apoptosis, differentiation, invasion, migration and anoikis [10,11]. In this study, by screening miRNA function in OS cells under anoikis condition, miR-451 was identified as a driver miRNA contributed to anoikis sensitivity in OS cells. Previously, miR-451 was found to be down-regulated in many cancers and could affect diverse biological functions of human tumor cells including proliferation, apoptosis, metastasis, adaption to metabolic stress and sensitivity to chemo or radiotherapy [18-20]. For example, over-expression of miR-451 in gastric and colorectal cancer cells inhibited cell proliferation and increased sensitivity to radiotherapy by regulating macrophage migration-inhibitory factor production [21]. In multiple myeloma, miR-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway [22]. Particularly, the tumor suppressor functions of miR-451 in OS were also widely demonstrated [20,23-25]. However, no reportsare available for its anti-tumor effect on anoikis. Interestingly, increased miR-451 expression promoted anoikis of non-small cell lung cancer (NSCLC) cells [26]. Consistent with this observation, by loss-of-function and gain-of-function studies, we for the first time revealed that miR-451 was profoundly involved in the anoikis resistance of OS cells. Although our screening was not at a large-scale, the result, at least, to some extent demonstrated the tumor-suppressive role of miR-451 on the anoikis resistance of OS.
MiR-451 has multiple targets in human OS. Liu et al. showed that miR-451 suppresses proliferation, migration, and promotes apoptosis by targeting macrophage migration-inhibitory factor [20]. Ni et al. showed that miR-451 inhibits cell growth, migration, and angiogenesis via down-regulation of IL6R [23]. It was also shown that miR-451 targets liver receptor homolog-1 to inhibit the proliferation of OS cells [25]. Furthermore, miR-451 inhibits cell growth and invasion by targeting CXCL16 in OS [24]. In this study, we identified Rab14 as a direct functional target of miR-451. Knockdown of Rab14 fully recapitulated the inhibitory role of miR-451 on anoikis resistance. Consistently, it has been reported that miR-451 functions as a tumor suppressor in NSCLC by targeting Rab14 [27]. And miR-451 is able to increase radio sensitivity of nasopharyngeal carcinoma cells by targeting Rab14 [28]. Rab14 is a member of RAS oncogene family, and its oncogenic functions have been demonstrated in several cancers, including gastric cancer, breast cancer, and ovarian cancer [29-31]. Specifically, we uncovered a novel regulatory role of Rab14 in anoikis resistance of OS cells. However, several limitations are present in this part of work. Firstly, we cannot fully rule out other targets except for Rab14 that mediate the inhibitory role of miR-451 in OS anoikis resistance. Secondly, the downstream signaling pathway of Rab14 in OS remains further investigation.
In conclusion, we found that miR-451 is a tumor suppressor in OS and miR-451 involved OS anoikis resistance by direct targeting Rab14. Our experimental data suggest that the miR-451/Rab14 axis might be a novel mechanism underlying the anoikis resistance in OS.
Disclosure of conflict of interest
None.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Berner K, Johannesen TB, Bruland OS. Clinical epidemiology of low-grade and dedifferentiated osteosarcoma in norway during 1975 and 2009. Sarcoma. 2015;2015:917679. doi: 10.1155/2015/917679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:593–599. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Strauss SJ, Ng T, Mendoza-Naranjo A, Whelan J, Sorensen PH. Understanding micrometastatic disease and anoikis resistance in ewing family of tumors and osteosarcoma. Oncologist. 2010;15:627–635. doi: 10.1634/theoncologist.2010-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wazir U, Orakzai MM, Khanzada ZS, Jiang WG, Sharma AK, Kasem A, Mokbel K. The role of death-associated protein 3 in apoptosis, anoikis and human cancer. Cancer Cell Int. 2015;15:39. doi: 10.1186/s12935-015-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Montero CM, Wygant JN, McIntyre BW. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur J Cancer. 2006;42:1491–1500. doi: 10.1016/j.ejca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Cantiani L, Manara MC, Zucchini C, De Sanctis P, Zuntini M, Valvassori L, Serra M, Olivero M, Di Renzo MF, Colombo MP, Picci P, Scotlandi K. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and met signaling. Cancer Res. 2007;67:7675–7685. doi: 10.1158/0008-5472.CAN-06-4697. [DOI] [PubMed] [Google Scholar]
- 9.Wan X, Kim SY, Guenther LM, Mendoza A, Briggs J, Yeung C, Currier D, Zhang H, Mackall C, Li WJ, Tuan RS, Deyrup AT, Khanna C, Helman L. Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene. 2009;28:3401–3411. doi: 10.1038/onc.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esquela-Kerscher A, Slack FJ. OncomirsmicroRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 11.Kushlinskii NE, Fridman MV, Braga EA. Molecular mechanisms and microRNAs in osteosarcoma pathogenesis. Biochemistry (Mosc) 2016;81:315–328. doi: 10.1134/S0006297916040027. [DOI] [PubMed] [Google Scholar]
- 12.Palmini G, Marini F, Brandi ML. What is new in the miRNA world regarding osteosarcoma and chondrosarcoma? Molecules. 2017;22 doi: 10.3390/molecules22030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: review. Front Pediatr. 2015;3:69. doi: 10.3389/fped.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nugent M. microRNA and bone cancer. Adv Exp Med Biol. 2015;889:201–230. doi: 10.1007/978-3-319-23730-5_11. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Shrestha S, LaChaud G, Scott MA, James AW. Review of microRNA in osteosarcoma and chondrosarcoma. Med Oncol. 2015;32:613. doi: 10.1007/s12032-015-0613-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH, Wang WJ. MicroRNAs in osteosarcoma. Clin Chim Acta. 2015;444:9–17. doi: 10.1016/j.cca.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Malagobadan S, Nagoor NH. Evaluation of MicroRNAs regulating anoikis pathways and its therapeutic potential. Biomed Res Int. 2015;2015:716816. doi: 10.1155/2015/716816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12:1153–1162. doi: 10.1158/1535-7163.MCT-12-0802. [DOI] [PubMed] [Google Scholar]
- 19.Guo R, Gu J, Zhang Z, Wang Y, Gu C. MiR-451 promotes cell proliferation and metastasis in pancreatic cancer through targeting CAB39. Biomed Res Int. 2017;2017:2381482. doi: 10.1155/2017/2381482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Liu W, Liu SY, He YB, Huang RL, Deng SY, Ni GX, Yu B. MiR-451 suppresses proliferation, migration and promotes apoptosis of the human osteosarcoma by targeting macrophage migration inhibitory factor. Biomed Pharmacother. 2017;87:621–627. doi: 10.1016/j.biopha.2016.12.121. [DOI] [PubMed] [Google Scholar]
- 21.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Liu S, He J, Liu X, Qu Y, Yan W, Fan J, Li R, Xi H, Fu W, Zhang C, Yang J, Hou J. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget. 2015;6:14993–15007. doi: 10.18632/oncotarget.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu SY, Deng SY, He YB, Ni GX. miR-451 inhibits cell growth, migration and angiogenesis in human osteosarcoma via down-regulating IL 6R. Biochem Biophys Res Commun. 2017;482:987–993. doi: 10.1016/j.bbrc.2016.11.145. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Huang W, Sheng M, Liu T. MiR-451 inhibits cell growth and invasion by targeting CXCL16 and is associated with prognosis of osteosarcoma patients. Tumour Biol. 2015;36:2041–2048. doi: 10.1007/s13277-014-2811-2. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Wu S, Lv S, Wang H, Wang Y, Guo Q. Suppression of liver receptor homolog-1 by microRNA-451 represses the proliferation of osteosarcoma cells. Biochem Biophys Res Commun. 2015;461:450–455. doi: 10.1016/j.bbrc.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang XC, Tian LL, Jiang XY, Wang YY, Li DG, She Y, Chang JH, Meng AM. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett. 2011;311:203–209. doi: 10.1016/j.canlet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14) Oncogene. 2011;30:2644–2658. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, Sun Q, Liu T, Chen J, Du S, Ren C, Liao G, Yuan Y. MiR-451 increases radiosensitivity of nasopharyngeal carcinoma cells by targeting ras-related protein 14 (RAB14) Tumour Biol. 2014;35:12593–12599. doi: 10.1007/s13277-014-2581-x. [DOI] [PubMed] [Google Scholar]
- 29.Guo B, Wang W, Zhao Z, Li Q, Zhou K, Zhao L, Wang L, Yang J, Huang C. Rab14 act as oncogene and induce proliferation of gastric cancer cells via AKT signaling pathway. PLoS One. 2017;12:e0170620. doi: 10.1371/journal.pone.0170620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Wang L, Yang H, Ding D, Zhang L, Wang J, Chen Q, Zou Q, Jin Y, Liu X. Rab14 suppression mediated by MiR-320a inhibits cell proliferation, migration and invasion in breast cancer. J Cancer. 2016;7:2317–2326. doi: 10.7150/jca.15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou R, Jiang L, Yang Z, Wang S, Liu Q. Rab14 is overexpressed in ovarian cancers and promotes ovarian cancer proliferation through Wnt pathway. Tumour Biol. 2016 doi: 10.1007/s13277-016-5420-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


