Abstract
Glyoxalase 1 (Glo1) is an enzyme that plays a role to metabolize and inactivate methylglyoxal. Previous studies also have confirmed that Glo1 is closely related with tumorigenesis, metastasis, and drug-resistant, but its prognostic value in breast cancer has never been explored. In this study, we investigated the expression of Glo1 in breast cancer cell lines and tissues using real-time PCR, western blot and immunohistochemical analysis. We found Glo1 was frequently up-regulated in human breast cancer cells and tissues, and high expression of Glo1 was associated with positive lymph node, lymphovascular invasion, and TNM stage (all P<0.05). The Kaplan-Meier survival curve demonstrated that patients with high Glo1 expression had a shorter overall survival (OS) and recurrence-free survival (RFS) (Both P<0.001) than those with low Glo1 expression. Moreover, the univariate and further multivariate analysis revealed that Glo1 expression was an independent prognostic factor for both OS and RFS of breast cancer patients. Next, with CCK-8 assay, cell apoptosis analysis, colony formation assay, transwell invasion/migration assay, and wound-healing assay, we validated knock-down of Glo1 suppressed invasion and migration and promoted apoptosis of breast cancer cells. Taken together, we demonstrated the tumor-promoter Glo1 may serve as a prognostic biomarker for breast cancer.
Keywords: Breast cancer, prognosis, metastasis, proliferation, Glyoxalase 1
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and the leading cause of cancer death among females in less developed countries, with an estimated 1.7 million cases and 521,900 deaths in 2012, accounting for 25% of cancer cases and 15% of cancer deaths among females [1]. Despite advances in developing more efficient surgical techniques and novel chemotherapeutic interventions, and the 5-year relative survival rate reached to 89% [2], the metastasis and recurrence is still the main cause of cancer-related mortality.
In malignant cells, glucose metabolism and growth control are strictly linked [3]. Breast cancer cells can rely on glycolysis as a major source of energy rather than oxidative phosphorylation, even in normoxic conditions, according the Warburg effect which has been well described [4]. Aerobic glycolysis led to a remodeling of the cytoskeleton facilitating cell migration as another important physiological characteristic of tumor cells and hallmark of cancer [5]. Glyoxalase 1 (Glo1) is an enzyme that plays a role to metabolize and inactivate methylglyoxal (MG), one of the side-product of glycolysis [6,7], which had been frequently detected higher expression in breast cancer [8-10]. Previous studies also have confirmed that Glo1 is closely related with tumorigenesis [11], metastasis [12,13], and drug-resistant [14,15], but its prognostic value in breast cancer patients has never been explored.
In this study, we investigated the expression of Glo1 in breast cancer cell lines and primary invasive ductal carcinoma (IDC) tissues using real-time PCR, western blot and immunohistochemical analysis. We found high expression of Glo1 was associated with aggressive clinicopathological features and poor clinical outcome of breast cancer patients. Moreover, knock-down of Glo1 suppressed invasion and migration and promoted apoptosis of BC cells.
Materials and methods
Patients and tissue samples
Primary invasive ductal carcinomas of breast were obtained from 121 female patients at the Department of Breast and Thyroid Surgery, the Eastern hospital of First Affiliated Hospital, Sun Yat-sen University, from January 2007 to December 2012. Pathological diagnosis, as well as ER, PR and Her2 status, was verified by two different pathologists. Patients with invasive carcinomas, other than DCIS, underwent six cycles of postoperative adjuvant chemotherapy with FAC regimen (5-fluorouracil 500 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2). Subsequently, patients with ER(+) tumors underwent endocrine therapy according to NCCN guideline. No distal metastasis was identified in the patients upon diagnosis. In addition, fresh samples of normal breast tissue, benign breast tumor tissues and invasive ductal carcinoma tissues were collected from patients who undergone mastectomy or lumpectomy for benign or malignant breast diseases. All samples were snap-frozen for mRNA assessment and were collected with informed written consent from patients. The complete clinical and pathological features of these patients were collected and stored in our database by a researcher fellow. The study protocol followed the Ethical Guidelines of the 1975 Declaration of Helsinki, revised in 2000. All related procedures were performed with the approval of the Internal Review and the Ethics Boards of the First Affiliated Hospital, Sun Yat-sen University.
Immunohistochemistry
Archived paraffin-embedded tumor tissues collected from 121 consecutive patients with breast cancer treated in our hospital between 2007 and 2012 were used for tissue microarray construction and immunohistochemistry (IHC). The IHC was performed using the polymer HRP detection system (Zhongshan Goldenbridge Biotechnology, Beijing, China) as the protocol described. Glo1 expression level was scored semi-quantitatively using the IRS (immunoreactive score) = SI (staining intensity) × PP (percentage of positive cells) as described [16,17]. Briefly, SI was determined as 0, negative; 1, weak; 2, moderate and 3, strong. PP was defined as 0, <1%; 1, 1-10%; 2, 11-50%; 3, 51-80% and 4, >80% positive cells. Five visual fields from different areas of each tumor were used for the IRS evaluation. IRS≤4 was defined as low Glo1 expression and IRS>4 were defined as high Glo1 expression.
Cell lines preparation
The human breast cancer cell line MDA-MB-231, MDA-MB-468, MCF7, MCF-10A, BT-549, T-47D were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in DMEM medium (Gibco BRL) with 10% fetal bovine serum (Gibco BRL). The cell line was maintained in a humidified atmosphere containing 5% CO2 at 37°C. The culture medium was renewed every 2 to 3 days.
Analysis of Glo1 mRNA expression
RNAs were extracted from tissues and cell lines by Trizol (Invitrogen, USA) according to the manufacturer’s instructions. mRNA was reverse transcribed to cDNA using commercial kit (Advantage® RT-for-PCR Kit, Takara, China). Each sample was prepared in triplicate. The mean values were used for calculation. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as alleles to obtain a relative level of gene expression. Forward and reverse primers were as follows: Glo1: F: 5’-TTTTCACTCTACTTCTTGGCTT-3’, R: 5’-AACTCTGGGTCTCATCATCTT-3’, GAPDH: F: 5’-CGTATCGGACGCCTGGTTA-3’, R: 5’-CGCCAGTAGACTCCACGACAT-3’. Quantitative real-time PCR analysis was performed using LightCycler480 (Roche, Switzerland) Real-Time PCR System. Each well (20 ul reaction volume) contained 10 ul power SYBR Green PCR master mix (SYBR® Advantage® qPCR Premix, Takara, China).
siRNA transfection
Glo1-siRNA, which was purchased from Ribobio company in Guangzhou, China. siRNA (sequences: 5’-GATGGCTACTGGATTGAAA-3’) transfection was performed using Lipofectamine® 2000 Transfection Reagent (Invitrogen™, Garlsbad, CA, USA) according to the manufacturer’s instructions. The knockdown efficiency was assessed by qPCR and Western blot analyses.
Western blot analysis
Total proteins were extracted with RIPA lysis buffer and separated by SDS-PAGE and then transferred to the PVDF membrane (Roche Life Sciences, Switzerland). The membrane were blocked with 5% skimmed milk and incubated with the appropriate antibody. The membranes were incubate with different primary antibodies (dilution: Glo1, 1:2000, GAPDH, 1:2000, abcam) and horseradish peroxidase-conjugated anti-rabbit goat polyclonal secondary antibodies (dilution, 1:5000, ZSGB-BIO). Signals were visualized by chemiluminescence (ChemiQ3650, Bioshine, Guangzhou, China) and quantitated using Image J software.
In vitro cell behavior assays
To assess the cell viability, the Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, USA) was used according to the manufacturer’s instructions. The absorbance were measured at 450 nm on a Microplate reader (Molecular Device, SpectraMax M5e, USA) every 12 h.
For colony formation assay, cells were seeded into 6-well plates at a density of 500 cells/well and cultured for 2 weeks at 37°C. The numbers of colonies per dish were counted after staining with crystal violet (Beyotime Institute of Biotechnology). Only positive colonies (diameter >40 um) in the dishes were counted and compared.
Cell apoptosis analysis was conducted by flow cytometry (Beckman-Coulter, Fullerton, CA, USA) using Annexin V-FITC/PI Apoptosis Detection Kit (Keygen Biotechnology, Nanjing, China).
For the transwell invasion assay, 1×105 cells in serum-free medium were seeded into the upper chamber of 8-μm transwell inserts with a matrigel coated membrane (BD Biosciences, Franklin Lakes, NJ), while medium containing 10% bovine serum albumin was in the lower chamber. After several hours of incubation at 37°C, gel and cells in the upper chamber were removed carefully and cells adhering to the underside of the membrane were stained with 0.1% crystal violet (Beyotime Institute of Biotechnology, Shanghai, China) and 20% methanol. The number of cells was counted under an inverted microscope (Nikon, Chiyoda-Ku, JP).
For the transwell migration assay, 5×104 cells in serum-free medium were seeded into the upper chamber of 8-μm transwell inserts (BD Biosciences, Franklin Lakes, NJ). And medium containing 10% bovine serum albumin was in the lower chamber. After several hours of incubation at 37°C, cells in the upper chamber were removed carefully and cells adhering to the underside of the membrane were stained with 0.1% crystal violet (Beyotime Institute of Biotechnology, Shanghai, China) and 20% methanol. The number of cells was counted under an inverted microscope (Nikon, Chiyoda-Ku, JP).
For wound-healing assays, cells were seeded into 6-well plates and cultured for 48 h to obtain 90% confluent monolayer. Created a scratch by a plastic pipette tip and replaced with a fresh 10% DMEM medium. Images were captured at 0 and 48 h.
All of the above experiments were performed in triplicate. The detailed procedure was as previously described [18,19].
Statistical analysis
The correlation between Glo1 expression and clinicopathological parameters was analyzed by Spearman rank-correlation analysis. Kaplan-Meier method constructed survival curves and evaluated the difference of these groups by using the log-rank test. The Cox proportional hazard regression model was used to identify factors that were independently associated with overall survival and recurrence-free survival. Only factor which was P<0.05 in univariate analysis could be analyzed in multivariate Analysis. Continuous data in this study were presented as mean ± standard deviation (SD) from at least three independent experiments. The differences between groups were analyzed by Student’s t test. SPSS 17 software and GraphPad Prism 5 was used for performing all kinds of statistical analyses. In this study, P value <0.05 was considered significant.
Results
Glo1 is frequently up-regulated in human breast cancer cells and tissues
To investigate Glo1 expression traits in breast cancer, we comparatively analyzed the Glo1 mRNA and protein profiles in different breast cancer cell lines and 8 paired surgical specimens of IDC. Real-time PCR and western blot analysis revealed that 5 different breast cancer cell lines, including MDA-MB-231, MDA-MB-468, MCF-7, BT-549 and T-47D, exhibited higher levels of Glo1 expression compared to that of primary normal breast cell line MCF-10A at both the mRNA and protein levels (Figure 1A and 1C). Further comparative analysis demonstrated that Glo1 was differentially up-regulated in all eight detected samples paired with corresponding paracancer tissues from the same patient (Figure 1B and 1D), and this was further confirmed by the immunohistochemical staining results (Figure 1E). With these findings, our results indicate that Glo1 is frequently up-regulated in breast cancer.
Figure 1.
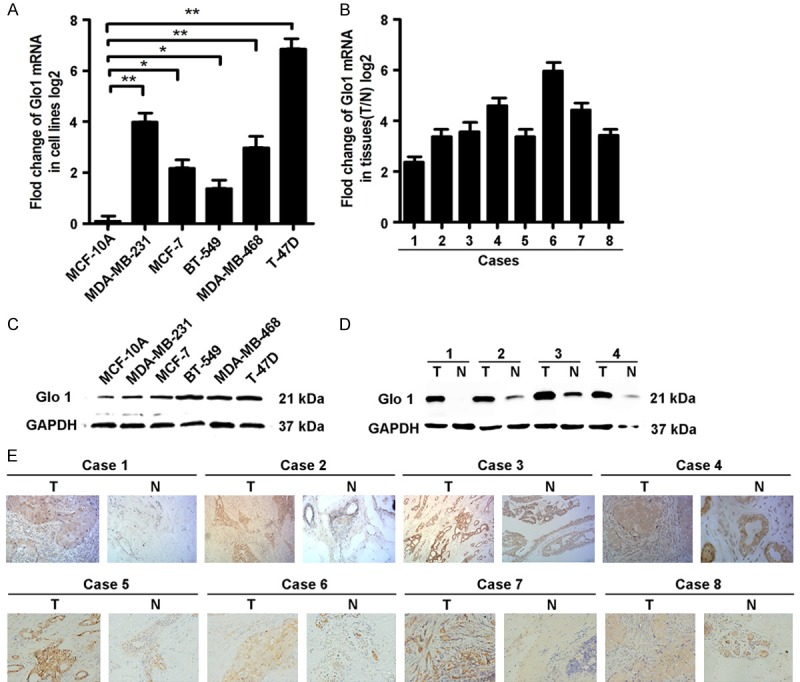
Glo1 is frequently up-regulated in human breast cancer cells and tissues. A. Glo1 mRNA levels in breast cancer cell lines was observably higher than mammary epithelial cell (MCF-10A) analyzed by Real-time PCR. B. The tumor tissue to adjacent nontumorous tissue (ANT) ratio of Glo1 expression was quantified by real-time RT-PCR. C. Glo1 protein was detected by Western blot and also showed that Glo1 protein level in breast cancer cell lines was observably higher than that in mammary epithelial cell (MCF-10A). D. Western blot analysis of Glo1 expression in each of the primary IDC tissue (T) and paired ANT (N) from four patients. E. IHC confirmed that the Glo1 protein was significantly elevated in primary IDC tissues (T) compared to that of paired ANT (N) from eight patients. Magnifications: ×100.
High expression of Glo1 in breast cancer tissues is associated with aggressive clinicopathological features and lower postoperative survival rate
To further elaborate the clinical significance of Glo1 expression in breast cancer, 121 patients’ paraffin-embedded tissue specimens were selected for microarray specimens and IHC staining. Patients were dichotomized according to low (IRS≤4) or high (IRS>4) expression of Glo1 (Figure 2A). It showed that the expression level of Glo1 was significantly associated with the following clinicopathological features: positive lymph node, lymphovascular invasion, and TNM stage (all P<0.05, Table 1). We next analyzed the relationship between Glo1 expression and the patients’ prognosis. The Kaplan-Meier survival curve demonstrated that patients with high Glo1 expression had a shorter overall survival (OS) and recurrence-free survival (RFS) (Both P<0.001, Figure 2B) than those with low Glo1 expression. Moreover, the univariate and further multivariate analysis revealed that Glo1 expression was an independent prognostic factor for both OS and RFS of breast cancer patients (Tables 2, 3). The above results demonstrated that Glo1 was closely correlated with poor survival and might be used as a novel independent prognostic biomarker for breast cancer. These data also suggest that Glo1 might be involved in the regulation of malignancy of breast cancer.
Figure 2.
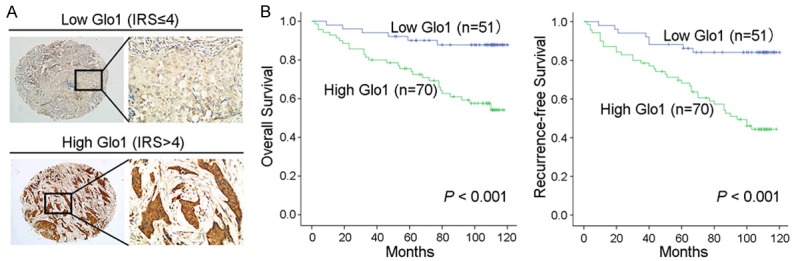
High expression of Glo1 in breast cancer tissues is associated with lower postoperative survival rate. A. Representative immunohistochemistry (IHC) images of Glo1 in breast cancer with low/high expression. Magnifications: ×100, ×400. B. The Kaplan-Meier curves showed overall survival (OS) and recurrence-free survival (RFS) of breast cancer patients with low/high Glo1 expression. P value was shown in each panel respectively.
Table 1.
The correlations of Glo1 with clinicopathological features of breast cancer patients
| Clinicopathologic Variable | n | GLO1 expression | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (year) | ||||
| ≤45 | 35 | 14 | 21 | |
| >45 | 86 | 37 | 49 | 0.760 |
| Histological grade | ||||
| I | 30 | 13 | 17 | |
| II-III | 91 | 38 | 53 | 0.880 |
| Positive lymph node | ||||
| ≤3 | 83 | 44 | 39 | |
| >3 | 38 | 7 | 31 | <0.001 |
| Lymphovascular invasion | ||||
| Absence | 73 | 40 | 33 | |
| Presence | 48 | 11 | 37 | <0.001 |
| Tumor size (cm) | ||||
| ≤2 | 30 | 14 | 16 | |
| >2 | 91 | 37 | 54 | 0.563 |
| ER | ||||
| Positive | 83 | 39 | 44 | |
| Negative | 38 | 12 | 26 | 0.111 |
| PR | ||||
| Positive | 57 | 22 | 35 | |
| Negative | 64 | 29 | 35 | 0.455 |
| Her2 | ||||
| Negative | 67 | 31 | 36 | |
| Positive | 54 | 20 | 34 | 0.307 |
| TNM stage | ||||
| I-II | 79 | 43 | 36 | |
| III | 42 | 8 | 34 | <0.001 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; Her2, Human Epidermal Growth Factor Receptor type 2; TNM, tumor node metastasis.
Table 2.
Univariate and multivariate analysis of factors associated with overall survival in breast cancer patients
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| Variable | n | RR (95% CI) | P | RR (95% CI) | P |
| Age (year) | |||||
| ≤45 | 35 | 1 | |||
| >45 | 86 | 1.416 (0.643-3.119) | 0.387 | n.a. | n.a. |
| Histological grade | |||||
| I | 30 | 1 | |||
| II-III | 91 | 2.033 (0.789-5.241) | 0.142 | n.a. | n.a. |
| Positive lymph node | |||||
| ≤3 | 83 | 1 | 1 | ||
| >3 | 38 | 2.159 (1.110-4.200) | 0.023 | 1.702 (0.213-13.588) | 0.616 |
| Lymphovascular invasion | |||||
| Absence | 73 | 1 | 1 | ||
| Presence | 48 | 3.588 (1.784-7.216) | <0.001 | 2.708 (1.290-5.681) | 0.008 |
| Tumor size (cm) | |||||
| ≤2 | 30 | 1 | |||
| >2 | 91 | 2.338 (0.907-6.031) | 0.079 | n.a. | n.a. |
| ER | |||||
| Positive | 83 | 1 | 1 | ||
| Negative | 38 | 1.955 (1.005-3.804) | 0.048 | 1.690 (0.855-3.340) | 0.131 |
| PR | |||||
| Positive | 57 | 1 | |||
| Negative | 64 | 1.159 (0.593-2.264) | 0.666 | n.a. | n.a. |
| Her2 | |||||
| Negative | 67 | 1 | 1 | ||
| Positive | 54 | 2.279 (1.158-4.485) | 0.017 | 2.196 (1.111-4.341) | 0.024 |
| TNM stage | |||||
| I-II | 79 | 1 | 1 | ||
| III | 42 | 2.123 (1.094-4.122) | 0.026 | 0.326 (0.039-2.742) | 0.302 |
| GLO1 expression | |||||
| Low | 51 | 1 | 1 | ||
| High | 70 | 4.193 (1.739-10.113) | 0.001 | 2.716 (1.063-6.939) | 0.037 |
Abbreviations: n.a.: Not application; ER, estrogen receptor; PR, progesterone receptor; Her2, Human Epidermal Growth Factor Receptor type 2; TNM, tumor node metastasis.
Table 3.
Univariate and multivariate analysis of factors associated with recurrence-free survival in breast cancer patients
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| Variable | n | RR (95% CI) | P | RR (95% CI) | P |
| Age (year) | |||||
| ≤45 | 35 | 1 | |||
| >45 | 86 | 1.033 (0.542-1.968) | 0.922 | n.a. | n.a. |
| Histological grade | |||||
| I | 30 | 1 | |||
| II-III | 91 | 1.905 (0.851-4.266) | 0.117 | n.a. | n.a. |
| Positive lymph node | |||||
| ≤3 | 83 | 1 | 1 | ||
| >3 | 38 | 2.282 (1.270-4.102) | 0.006 | 1.682 (0.222-12.775) | 0.615 |
| Lymphovascular invasion | |||||
| Absence | 73 | 1 | 1 | ||
| Presence | 48 | 3.192 (1.746-5.837) | <0.001 | 2.356 (1.245-4.456) | 0.008 |
| Tumor size (cm) | |||||
| ≤2 | 30 | 1 | |||
| >2 | 91 | 1.385 (0.686-2.799) | 0.364 | n.a. | n.a. |
| ER | |||||
| Positive | 83 | 1 | |||
| Negative | 38 | 1.568 (0.863-2.848) | 0.140 | n.a. | n.a. |
| PR | |||||
| Positive | 57 | 1 | |||
| Negative | 64 | 1.099 (0.610-1.978) | 0.754 | n.a. | n.a. |
| Her2 | |||||
| Negative | 67 | 1 | 1 | ||
| Positive | 54 | 1.942 (1.078-3.499) | 0.027 | 1.881 (1.042-3.399) | 0.036 |
| TNM stage | |||||
| I-II | 79 | 1 | 1 | ||
| III | 42 | 2.192 (1.221-3.936) | 0.009 | 0.395 (0.049-3.166) | 0.382 |
| GLO1 expression | |||||
| Low | 51 | 1 | 1 | ||
| High | 70 | 4.151 (1.931-8.923) | <0.001 | 2.917 (1.296-6.563) | 0.010 |
Abbreviations: n.a.: Not application; ER, estrogen receptor; PR, progesterone receptor; Her2, Human Epidermal Growth Factor Receptor type 2; TNM, tumor node metastasis.
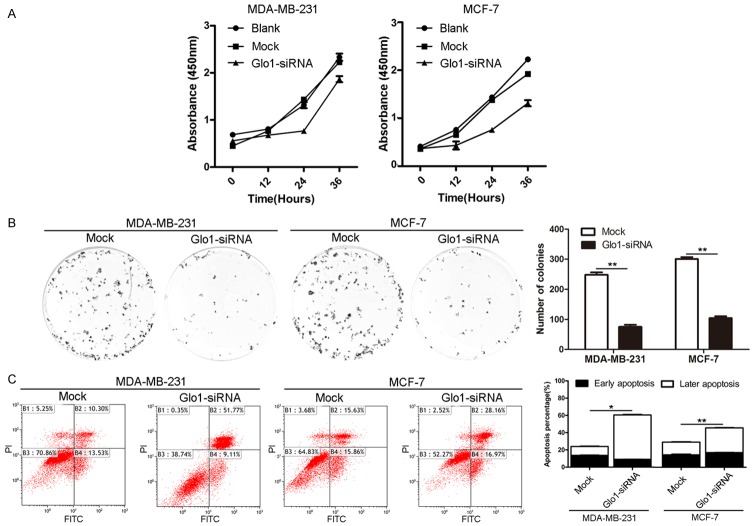
Knockdown of Glo1 suppresses cell proliferation and promotes cell apoptosis in breast cancer
In order to examine the influence of Glo1 on the biological behaviors of breast cancer, siRNA-Glo1 was used to inhibit Glo1 expression in MDA-MB-231 and MCF-7 cell lines respectively, and the knockdown efficiency of Glo1 was confirmed by real-time PCR and western blot (Figure 3). We firstly detected its role in cell proliferation. CCK-8 assay showed down-regulation of Glo1 significantly subdued the proliferative ability in MDA-MB-231 cells and MCF-7 cells, respectively (Figure 4A). Colony formation assay showed a significant decrease in the number of colonies after knocking down Glo1 in MDA-MB-231 cells and MCF-7 cells (Figure 4B). Then to explore whether knockdown of Glo1 induced obvious apoptosis, the effects of Glo1 on cell survival were assessed using flow cytometric analysis. Compared to the normal group, there was a significantly increased apoptosis rate, mainly later apoptosis, after knocking down Glo1 in MDA-MB-231 cells and MCF-7 cells, whereas the early apoptosis was not markedly affected (Figure 4C). These results demonstrated down-regulation of Glo1 inhibited cell proliferation and promoted cell apoptosis in breast cancer.
Figure 3.
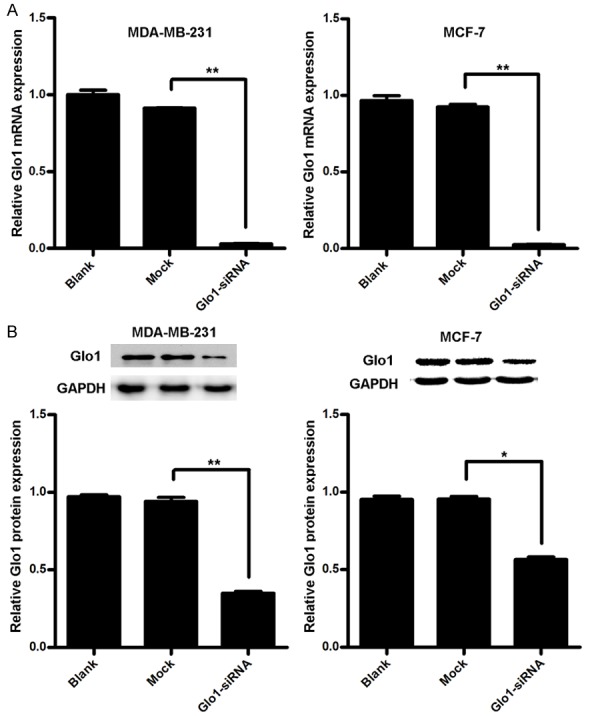
To confirm the silencing efficiency on Glo1. A. Silencing efficiency of Glo1 in mRNA level by siRNA in indicated cells were identified by qRT-PCR. **P<0.01. B. Silencing efficiency of Glo1 in protein level by siRNA in indicated cells were identified by western blot. *P<0.05, **P<0.01.
Figure 4.
Knockdown of Glo1 suppresses cell proliferation and promotes cell apoptosis in breast cancer. A. CCK-8 results showed the cell proliferation ability of Glo1-siRNA group was significantly decreased from 24 h to 72 h than Blank and Mock groups (P<0.05, respectively). B. The effect of Knockdown of Glo1 on cell proliferation was measured by colony formation assay. Representative images of colony formation are shown (left panel). Results represented the mean ± SD in triplicate using bar graph (right panel). **P<0.01. C. The effect of Knockdown of Glo1 on cell apoptosis was measured by flow cytometry analysis.
Knockdown of Glo1 suppresses breast cancer cell invasion and migration
Next we continued to explore the role of Glo1 in breast cancer cell invasion and migration. We performed transwell invasion/migration assay and found invasion and migration of both MDA-MB-231 cells and MCF-7 cells were significantly decreased after knocking down Glo1 (Figure 5A). Moreover, we adopted wound-healing assay to further evaluate its effect on migration capacity, and results showed cells with Glo1 knocked down had a markedly slower wound closure rate than Mock cells (Figure 5B). All these data indicated Knockdown of Glo1 suppressed breast cancer cell invasion and migration.
Figure 5.
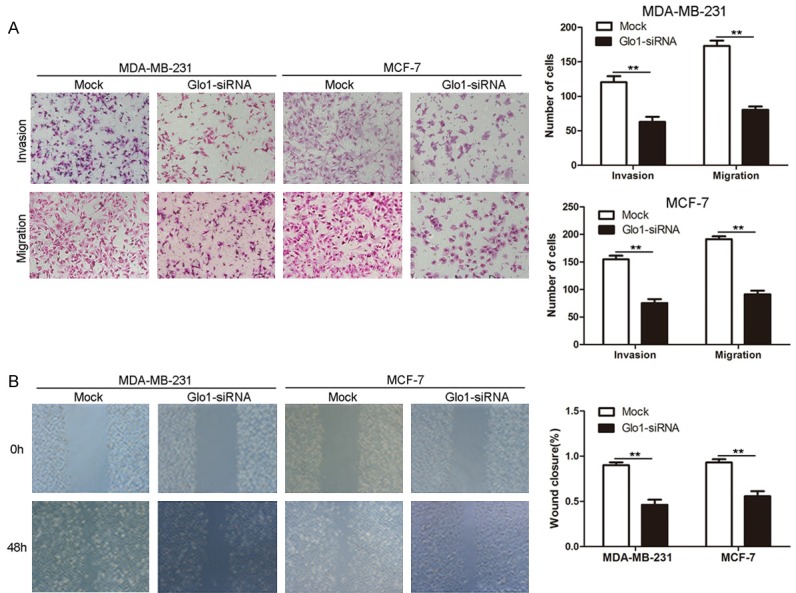
Knockdown of Glo1 suppresses breast cancer cell invasion and migration. A. The effect of Knockdown of Glo1 on cell invasion and migration was measured by transwell invasion/migration assay. Representative images are shown (left panel). Magnification: ×100. Results represented the mean ± SD in triplicate using bar graph (right panel). **P<0.01. B. The effect of Knockdown of Glo1 on cell migration was measured by wound-healing assay. Representative images are shown (left panel). Magnification: ×100. Results represented the mean ± SD in triplicate using bar graph (right panel). **P<0.01.
Taking all of these in vitro results into consideration, it suggests Glo1 play an oncogenic role in breast cancer.
Discussion
In recent years, there have been major breakthroughs in the targeted therapy of breast cancer. However, a targeted therapeutic agent inhibiting one key pathway in a tumor may not completely shut off a hallmark capability, allowing some cancer cells to survive with residual function until they or their progeny eventually adapt to the selective pressure imposed by the therapy being applied [20]. In breast cancer, around 15% of patients develop disseminated metastasis before or after diagnosis, and distant metastasis is responsible for approximately 90% of breast cancer-associated mortality [21]. Therefore, identifying novel key regulators in cancer metastasis and targeting all of these supporting pathways therapeutically become increasingly important. In this study, we identified Glo1 promoted cell proliferation, invasion and migration and suppressed cell apoptosis in breast cancer, and high expression of Glo1 was associated with aggressive clinicopathological features and poor clinical outcome of breast cancer patients. Yet there still exist some potential problems to be further discussed and explored.
The glyoxalase system is a ubiquitous detoxification pathway consisting of glyoxalase 1 (Glo1) and glyoxalase 2 (Glo2), which act in concert to convert the spontaneously formed hemithioacetal adduct between glutathione and MG into D-lactate and glutathione [22-24]. Increasing evidences have indicated Glo1 play oncogenic role in various cancers. Several studies also focused on its clinical significance. Antognelli et al [25] performed an Italian case-control study, and found GLO1 gene polymorphisms were associated with an increased risk of breast cancer. Fonseca-Sanchez et al [10] confirmed Glo1 overexpression by western blot in paired normal and tumor breast tissues in clinical stages I-III, and by immunohistochemistry on tissue microarrays (TMA) comprising a cohort of 98 breast tumors and 20 healthy specimens. They found that Glo1 was overexpressed in 79% of tumors, and its up-regulation correlated with advanced tumor grade. But interestingly, our cohort study demonstrated Glo1 was not correlated with histological grade. Maybe this difference was caused by different ethnic population. Their research sample was Mexican, whereas ours was from China. This difference also promoted us to hypothesize that the mechanism of tumorigenesis and progression of breast cancer might be different in different population. Chiavarina et al [26] reported Glo1 immunostaining performed on human triple negative and triple positive breast cancer lesions did not show any significant difference; conversely, the measurement of Glo1 activity performed on fresh samples showed a significant increase in Glo1 enzymatic activity in triple negative tumors. This finding suggests Glo1 enzymatic activity might be also used as a prognostic indicator, and this needs to be further explored. Moreover, to be strictly complied with REMARK guidelines for reporting prognostic biomarkers in cancer [27] and thus further corroborate our finding that Glo1 high expression indicated poor prognosis in breast cancer, another cohort form a different research center is necessary. In future, we will attempt to solve this shortage.
In summary, our data demonstrated that Glo1, frequently up-regulated in breast cancer, associated with aggressive tumor phenotypes, was an independent prognostic indicator for breast cancer patients. In addition, the in vitro assays validated knock-down of Glo1 suppressed invasion and migration and promoted apoptosis of breast cancer cells. Therefore, we suppose strategies designed to down-regulate Glo1 may provide a promising method to alleviate breast cancer progression.
Acknowledgements
We acknowledge Prof. Zun-fu Ke (Department of Pathology, the First Affiliated Hospital of Sun Yat-sen University) and his colleagues for the help of pathological diagnoses and guidance.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol. 2012;23:352–361. doi: 10.1016/j.semcdb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–1348. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 7.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rulli A, Carli L, Romani R, Baroni T, Giovannini E. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res Treat. 2001;66:67–72. doi: 10.1023/a:1010632919129. [DOI] [PubMed] [Google Scholar]
- 9.Santarius T, Bignell GR, Greenman CD, Widaa S, Chen L, Mahoney CL, Butler A, Edkins S, Waris S, Thornalley PJ, Futreal PA, Stratton MR. GLO1-A novel amplified gene in human cancer. Genes Chromosomes Cancer. 2010;49:711–725. doi: 10.1002/gcc.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca-Sanchez MA, Rodriguez CS, Mendoza-Hernandez G, Bautista-Pina V, Arechaga OE, Hidalgo MA, Quintanar JV, Marchat LA, Alvarez-Sanchez E, Perez PC, Lopez-Camarillo C. Breast cancer proteomics reveals a positive correlation between glyoxalase 1 expression and high tumor grade. Int J Oncol. 2012;41:670–680. doi: 10.3892/ijo.2012.1478. [DOI] [PubMed] [Google Scholar]
- 11.Thornalley PJ, Rabbani N. Glyoxalase in tumourigenesis and multidrug resistance. Semin Cell Dev Biol. 2011;22:318–325. doi: 10.1016/j.semcdb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Scheckhuber CQ, Mack SJ, Strobel I, Ricciardi F, Gispert S, Osiewacz HD. Modulation of the glyoxalase system in the aging model Podospora anserina: effects on growth and lifespan. Aging (Albany NY) 2010;2:969–980. doi: 10.18632/aging.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Zhang Y, Yang X, Lu P, Yan X, Xiao F, Zhou H, Wen C, Shi M, Lu J, Meng QH. Effects of methylglyoxal and glyoxalase I inhibition on breast cancer cells proliferation, invasion, and apoptosis through modulation of MAPKs, MMP9, and Bcl-2. Cancer Biol Ther. 2016;17:169–180. doi: 10.1080/15384047.2015.1121346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rulli A, Antognelli C, Prezzi E, Baldracchini F, Piva F, Giovannini E, Talesa V. A possible regulatory role of 17beta-estradiol and tamoxifen on glyoxalase I and glyoxalase II genes expression in MCF7 and BT20 human breast cancer cells. Breast Cancer Res Treat. 2006;96:187–196. doi: 10.1007/s10549-005-9078-7. [DOI] [PubMed] [Google Scholar]
- 15.Nass N, Brömme H, Hartig R, Korkmaz S, Sel S, Hirche F, Ward A, Simm A, Wiemann S, Lykkesfeldt AE, Roessner A, Kalinski T. Differential response to α-Oxoaldehydes in tamoxifen resistant MCF-7 breast cancer cells. PLoS One. 2014;9:e101473. doi: 10.1371/journal.pone.0101473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chui X, Egami H, Yamashita J, Kurizaki T, Ohmachi H, Yamamoto S, Ogawa M. Immunohistochemical expression of the c-kit proto-oncogene product in human malignant and nonmalignant breast tissues. Br J Cancer. 1996;73:1233–1236. doi: 10.1038/bjc.1996.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrichs K, Gluba S, Eidtmann H, Jonat W. Overexpression of p53 and prognosis in breast cancer. Cancer-Am Cancer Soc. 1993;72:3641–3647. doi: 10.1002/1097-0142(19931215)72:12<3641::aid-cncr2820721215>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Ke Z, He W, Lai Y, Guo X, Chen S, Li S, Wang Y, Wang L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget. 2014;5:9410–9424. doi: 10.18632/oncotarget.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y, Feng C, Chen X, Zhang Y, Lin M, Wang L, Ke Z. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget. 2015;6:35813. doi: 10.18632/oncotarget.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainaut P, Plymoth A. Targeting the hallmarks of cancer. Curr Opin Oncol. 2013;25:50–51. doi: 10.1097/CCO.0b013e32835b651e. [DOI] [PubMed] [Google Scholar]
- 21.Weigelt B, Peterse JL, Van’T Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 22.Antognelli C, Mezzasoma L, Fettucciari K, Talesa VN. A novel mechanism of methylglyoxal cytotoxicity in prostate cancer cells. Int J Biochem Cell Biol. 2013;45:836–844. doi: 10.1016/j.biocel.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Rabbani N, Thornalley PJ. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakadate Y, Uchida K, Shikata K, Yoshimura S, Azuma M, Hirata T, Konishi H, Kiyama H, Tachibana T. The formation of argpyrimidine, a methylglyoxal-arginine adduct, in the nucleus of neural cells. Biochem Biophys Res Commun. 2009;378:209–212. doi: 10.1016/j.bbrc.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Antognelli C, Del BC, Ludovini V, Gori S, Talesa VN, Crino L, Barberini F, Rulli A. CYP17, GSTP1, PON1 and GLO1 gene polymorphisms as risk factors for breast cancer: an Italian case-control study. BMC Cancer. 2009;9:115. doi: 10.1186/1471-2407-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiavarina B, Nokin MJ, Durieux F, Bianchi E, Turtoi A, Peulen O, Peixoto P, Irigaray P, Uchida K, Belpomme D, Delvenne P, Castronovo V, Bellahcene A. Triple negative tumors accumulate significantly less methylglyoxal specific adducts than other human breast cancer subtypes. Oncotarget. 2014;5:5472–5482. doi: 10.18632/oncotarget.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]