Abstract
Transient receptor potential vanilloid 1 (TRPV1), the receptor of capsaicin, is a nonselective cation channel that is highly permeable to Ca2+. TRPV1 is involved in the activation of immune cells and plays a role in the pathogenesis of experimental colitis. The expression of TRPV1 in colonic epithelium and its correlation with inflammatory bowel disease (IBD) are poorly understood. In this study, colonic biopsies were taken from 60 patients with active inflammatory bowel disease, including 30 patients with ulcerative colitis (UC) and 30 patients with Crohn’s disease (CD), and 30 healthy controls. Disease activity was assessed according to the Mayo score, Crohn’s disease activity index (CDAI) score, and the level of C-reactive protein (CRP). The severity of histological inflammation was graded using a scoring system that was previously described. For immunohistochemical staining, sections were incubated with a polyclonal anti-TRPV1 antibody. Next, image analysis was performed to obtain an integrated option density (IOD) value to evaluate TRPV1 immunoreactivity of five random fields per section, which was 60973±29112 for the UC group, 61942±32083 for the CD group, and 35154±21293 for the control group. Our data showed that TRPV1 expression was significantly upregulated in colonic epithelium of IBD patients compared with controls (P<0.001). In addition, no significant differences were observed in TRPV1 expression between UC and CD groups (P>0.05). Although TRPV1 immunoreactivity was highly expressed on epithelial cells and infiltrating inflammatory cells in colonic biopsies of active IBD patients, TRPV1 expression did not significantly correlate with disease severity (P>0.05). Therefore, our findings suggested a crucial role of TRPV1 in inflammatory bowel disease, and indicated that further studies are clearly warranted to determine whether TRPV1 is a potential target for therapy.
Keywords: Capsaicin, immunohistochemistry, inflammatory bowel disease, TRPV1
Introduction
Inflammatory bowel disease (IBD), comprised of ulcerative colitis (UC) and Crohn’s disease (CD), is a group of chronic idiopathic disorders that is characterized by recurrent and remitting inflammation in gastrointestinal tract. The etiology and pathogenesis of IBD are poorly understood. Previous studies have demonstrated that environmental factors, especially diets, play an important role in the pathogenesis and disease flares [1,2]. For example, epidemiologic studies have reported that spicy food with chili peppers as additives, which is widely consumed around the world, turned out to be a risk factor of IBD, and may induce or even worsen bowel symptoms [3-5]. Capsaicin, the principal pungent component in chili peppers, produces a variety of biological effects via activating its receptor transient receptor potential vanilloid 1 (TRPV1), which is also known as the vanilloid receptor 1 (VR1) [6].
TRPV1, member of transient receptor potential (TRP) family of channels, is a nonselective cation channel that can be activated by capsaicin, noxious temperature (>43°C), low pH (pH<6.0), or endocannabinoids such as anandamide [7]. TRPV1 was first cloned in 1997, and was found to be mainly expressed by primary sensory neurons originating from dorsal root ganglia and trigeminal ganglia. However, recent studies demonstrated its expression in non-neuronal cells such as keratinocytes, endothelial cells, and urothelial cells [7]. The expression of TRPV1 on immune cells such as lymphocytes, dendritic cells, and neutrophils has also been confirmed [8-10]. The activation of TRPV1 in sensory neurons elicits neurogenic inflammation, which is characterized by vasodilatation, edema formation, recruitment of inflammatory cells, and the release of neuropeptides [7,11]. Moreover, increasing evidence suggests that TRPV1 is involved in the activation and proinflammatory effects of T cells, and the maturation and migration of dendritic cells, and plays a role in regulating the production of inflammatory cytokines [8,10].
The distribution of TRPV1 in the gastrointestinal tract has been described by Ward et al., includes nerves within myenteric ganglia, interganglionic fiber tracts and muscle layers [12]. Previous studies have shown that TRPV1 plays a crucial role in the pathogenesis of colitis [8,13,14]. Intraluminal administration of the TRPV1 agonist capsaicin caused intestinal inflammation that was inhibited by treatment with its antagonist capsazepine [13]. Administration of capsazepine or genetic deletion of TRPV1 attenuates experimental colitis [8,14]. It has been reported that the immunoreactivity of TRPV1 was increased in colonic nerves fibers, and was associated with abdominal pain in patients with IBD [15,16]. Moreover, active IBD involves inflamed mucosa, which is mainly comprised of epithelium and infiltrated inflammatory cells. However, the expression of TRPV1 in colonic epithelium of these patients still needs to be elucidated. In this study, we aimed to explore the expression of TRPV1 in colonic biopsies of patients with active IBD, and investigate its correlation with disease severity.
Materials and methods
Patients and control subjects
Sixty hospitalized patients with active IBD, including 30 patients with UC and 30 patients with CD, were randomly selected from the gastroenterology department of the West China Hospital of Sichuan University (Sichuan, China) from 2014 to 2016. The diagnosis of IBD was based on clinical, radiological, endoscopic, and histological criteria. Prior to endoscopic examination, information was collected, such as disease duration, symptoms, extra-intestinal manifestations, and current medication. The disease extent was determined by radiology and colonoscopy examinations, and assessed according to the Montreal classification [17]. For UC, clinical activity was determined by a modified Mayo score, which is a sum of the following variables: frequency of bowel movements, blood in stool, quality of life, and endoscopic evaluation. Mayo scores of 0-2, 3-5, 6-10, and 11-12 were considered as remission, mild, moderate, and severe, respectively [18]. For CD, a CDAI score was calculated as per the method developed by Best et al. CDAI scores of 0-150, 151-220, 221-450, and >450 indicated quiescent, mild, moderate, and severe disease, respectively [19]. Laboratory data was collected, including the level of CRP, to evaluate the inflammatory activity.
In this study, a total of 30 healthy controls were included who underwent colonoscopy for routine health examination. In brief, a colonoscopy with standard bowel preparation was performed, and the endoscopy revealed no lesions in the gastrointestinal tract. This study was approved by the ethics committee of West China Hospital, with the principles of the Declaration of Helsinki. Informed consent was obtained from every patient.
Immunohistochemistry
From all IBD patients, endoscopic biopsies were taken from both affected and non-affected colonic mucosa using standard biopsy forceps. For histological analyses, at least three biopsies were collected, fixed in 10% formalin, and submitted to the Department of Pathology for hematoxylin-eosin staining and histological evaluation. The severity of histological inflammation was graded using a scoring system that was previously described by Virk et al. [20]. Accordingly, histological inflammation was recorded as 1 (mild: cryptitis), 2 (moderate: crypt abscesses), or 3 (severe: erosions or ulcerations). From healthy controls, a single biopsy was taken per subject for immunohistochemical study.
Tissue samples collected for immunohistochemical analysis were fixed in 4% (m/v) paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4), embedded in paraffin, and cut at 4-μm thick sections. After deparaffinization and rehydration, antigen retrieval was performed by incubating the sections in citrate buffer (0.01 mmol/L, pH 6.0) and heating with a microwave oven (720 W) for 15 min. Endogenous peroxidase was blocked with 0.3% (m/v) hydrogen peroxide for 30 min at room temperature. Next, sections were incubated overnight at 4°C with rabbit polyclonal anti-TRPV1 antibody (Bioss, Beijing, China), followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies for 30 min. Staining was completed by transferring the sections to 3,3-diaminobenzidine (DAB) solution. Sections were counterstained with haematoxylin for nuclei labeling. As a negative control, the primary antibody was omitted.
To quantify the intensity of TRPV1 expression, images were captured with a Zeiss AX10 cam HRC microscope and converted into TIFF (Tagged Image File Format) format. Five randomly selected fields (40× objective magnification) per tissue section were scanned and analyzed using Imagepro Plus (IPP) software for Windows. The IOD SUM of each image was measured after the optical density was corrected with the segmentation set at a constant level to allow for detection of positive immunostaining. For statistical analysis, the mean values of IOD readings obtained from five fields were used.
Statistical analysis
Statistical analyses were performed using SPSS Statistics version 22.0 for Windows. For quantitative variables, data were presented as the mean ± standard deviation (SD). Normality was evaluated with the Kolmogorov-Smirnov test. For categorical variables, frequencies and percentages were determined. Student’s t test or Mann-Whitney U-test was performed to determine the difference between two groups. For comparisons between more than two groups, one-way analysis of variance (ANOVA) or non-parametric Krusal-Wallis test was performed. Pearson’s or Spearman’s correlation coefficient was calculated to analyze the correlations between two variables. A P Value <0.05 was considered as statistically significant.
Results
Clinical characteristics of patients and non-IBD controls
In this study, 60 hospitalized patients with active IBD were included, whose ages ranged from 15 to 69, with a mean age of 38±15, and a male/female ratio of 1.86 (39/21). For the UC group the disease duration was 4.2±4.2 years, whereas for the CD group this was 4.4±4.8 years. Main clinical manifestations included abdominal pain, diarrhea, and blood in stool. The CD group showed an increased risk of extra-intestinal manifestations (17%) compared with the UC group (7%). Almost half of the CD patients (14/30) demonstrated perianal disease, including perianal abscess and fistula. Moreover, two cases of rectovaginal fistula were observed. In the UC group, the mean Mayo score was 8, and in the CD group, the mean CDAI was 261. Before endoscopic examination, CRP levels were collected and were 21.48±26.62 in the UC group and 33.82±29.74 in the CD group. After admission and depending on disease extent and severity, patients were placed on 5-aminosalicylates (5-ASA), corticosteroids, azathioprine, Infliximab, or methotrexate as the primary treatment. Due to intestinal perforation or uncontrollable bleeding, one UC patient and three CD patients underwent surgery. The control group included 30 subjects, with a mean age of 57±15 and a male/female ratio of 14/16. Clinical characteristics of all IBD patients and control subjects are presented in Table 1.
Table 1.
Clinical characteristics of IBD patients and non-IBD controls included
| Clinical Characteristics | UC | CD | Controls |
|---|---|---|---|
| Number | 30 | 30 | 30 |
| Gender | |||
| Male | 21 (70%) | 18 (60%) | 14 (47%) |
| Female | 9 (30%) | 12 (40%) | 16 (53%) |
| Age (years) | 43±16 | 32±12 | 57±15 |
| Disease duration (years) | 4.2±4.2 | 4.4±4.8 | - |
| Symptoms | - | ||
| Abdominal pain | 9 | 18 | |
| Diarrhea | 14 | 17 | |
| Blood in stool | 28 | 11 | |
| Extraintestinal manifestations | - | ||
| None | 27 | 25 | |
| Rheumatic | 0 | 1 | |
| Mucocutaneous | 2 | 2 | |
| Rheumatic + mucocutaneous | 0 | 2 | |
| Others | 1 | 0 | |
| Disease extent of UC | - | ||
| E1 (proctitis) | 2 (7%) | ||
| E2 (left-sided colitis) | 9 (30%) | ||
| E3 (pancolitis) | 19 (63%) | ||
| Disease extent of CD | - | ||
| L1 (ileal) | 2 (7%) | ||
| L2 (colic) | 3 (10%) | ||
| L3 (ileocolic) | 25 (83%) | ||
| Disease behavior of CD | - | ||
| B1 (non-stricturing non-penetrating) | 13 (43%) | ||
| B2 (stricturing) | 10 (33%) | ||
| B3 (penetrating) | 7 (23%) | ||
| Perianal disease | 14 | ||
| Current medication | - | ||
| 5-ASA | 13 | 5 | |
| Corticosteroids | 17 | 3 | |
| Azathioprine | 0 | 15 | |
| Infliximab | 0 | 6 | |
| Methotrexate | 0 | 1 |
TRPV1 expression in human colonic epithelium
TRPV1 immunoreactivity was observed on both normal and inflamed colonic mucosa with significant individual variation [Figure 1]. In colonic mucosa of non-IBD controls, weak and scattered immunostaining was observed, which was localized to the basolateral membrane of epithelial cells, whereas most of the interstitial cells in the lamina propria were negative. In some individuals, a gradient of staining intensity was observed, with a strong TRPV1 immunoreactivity in surface epithelium and a weaker staining in crypt epithelium. Intense TRPV1 immunoreactivity was observed in colonic mucosa of IBD patients on both epithelial cells and infiltrated inflammatory cells. The staining intensity of epithelial cells of colonic tissues was stronger in IBD patients compared to that of controls and was not restricted to the basolateral membrane. In the epithelium and lamina propria, most infiltrated inflammatory cells showed a strong TRPV1 expression, predominantly at the membrane and cytoplasm. Moreover, nuclear staining was detected in a small population of cells.
Figure 1.

A, B. The expression of TRPV1 in colonic mucosa of controls. Weak and scattered immunostaining was found, localized to the basolateral membrane of epithelial cells, while most of the interstitial cells of lamina propria were negative for TRPV1 immunostaining. C, D. The expression of TRPV1 in colonic mucosa of patients with ulcerative colitis. Both epithelial cells and infiltrated inflammatory cells showed strong TRPV1 immunostaining (a. colonic epithelial cells; b. infiltrated inflammatory cells). E, F. The expression of TRPV1 in colonic mucosa of patients with Crohn’s disease. Strong TRPV1 immunostaining was observed on epithelial cells and infiltrated inflammatory cells (a. colonic epithelial cells; b. infiltrated inflammatory cells).
Expression of TRPV1 significantly increased in IBD patients
The expression of TRPV1 in colonic epithelium was quantified with Imagepro Plus (IPP) software, and an IOD value was obtained for each sample. The mean IOD values were 60973±29112 for the UC group, 61942±32083 for the CD group, and 35154±21293 for the non-IBD control group. These results showed a significantly increased expression of TRPV1 in colonic mucosa of patients with IBD when compared with the mucosa of non-IBD controls (P<0.001). However, TRPV1 expression was not significantly different between the UC and CD group (P>0.50) [Figure 2].
Figure 2.
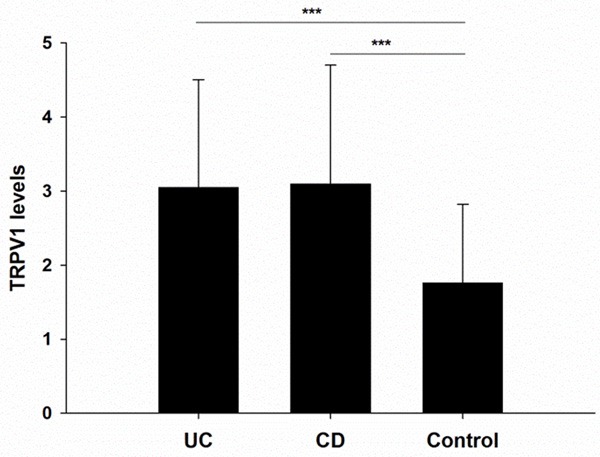
The expression of TRPV1 significantly increased in the colonic mucosa of patients with IBD compared with normal mucosa from non-IBD controls (P<0.001). No significant differences was observed between the UC and CD group (P>0.5).
The male/female ratios of UC, CD, and control groups were 21/9, 18/12, and 14/16, respectively. No significant differences in gender composition among these three groups were observed by Chi-square test (P>0.05). The difference in TRPV1 expression between males and females was evaluated, however no differences were reported (P>0.50). Given that the subjects in control group were older compared with subjects in the UC or CD group, Spearman’s correlation coefficient was used to analyze the correlation between age and TRPV1 expression. Data indicated that age was not related to the levels of TRPV1 expression in both the IBD group and control group (P>0.50). Prior to endoscopic examination, disease duration for the IBD group was recorded, which was 4.2±4.2 years for patients in the UC group, and 4.4±4.8 years for patients in the CD group. No significant correlation was observed between disease duration and the level of TRPV1 expression (P>0.50). In addition, disease extent was recorded according to the Montreal classification (Table 1). The association between disease extent and TRPV1 expression was analyzed, but no significant differences were found (P>0.05).
Correlation between TRPV1 expression and disease activity of IBD patients
To understand the association between TRPV1 expression and clinical activity, the activity was assessed using the Mayo score (for the UC group) or CDAI (for the CD group). Patients in the UC group were all active (Mayo score >2). According to the Mayo score, the number of patients with mild, moderate, and severe disease was 10, 11, and 9. No significant difference in TRPV1 expression was observed among the three groups and no significant correlation was found between Mayo score and TRPV1 levels (r=-0.316, P=0.089) [Figure 3A]. Similarly, the CDAI score, an index to evaluate clinical disease activity of CD patients, did not correlate with the TRPV1 expression levels (r=-0.274, P=0.143) [Figure 3B]. Since CRP is considered a promising biomarker to predict the disease activity of UC and CD, the correlation of CRP and TRPV1 levels was also investigated [21]. However, no significant correlation was observed between CRP levels and TRPV1 expression (r=-0.110, P=0.402) [Figure 3C].
Figure 3.
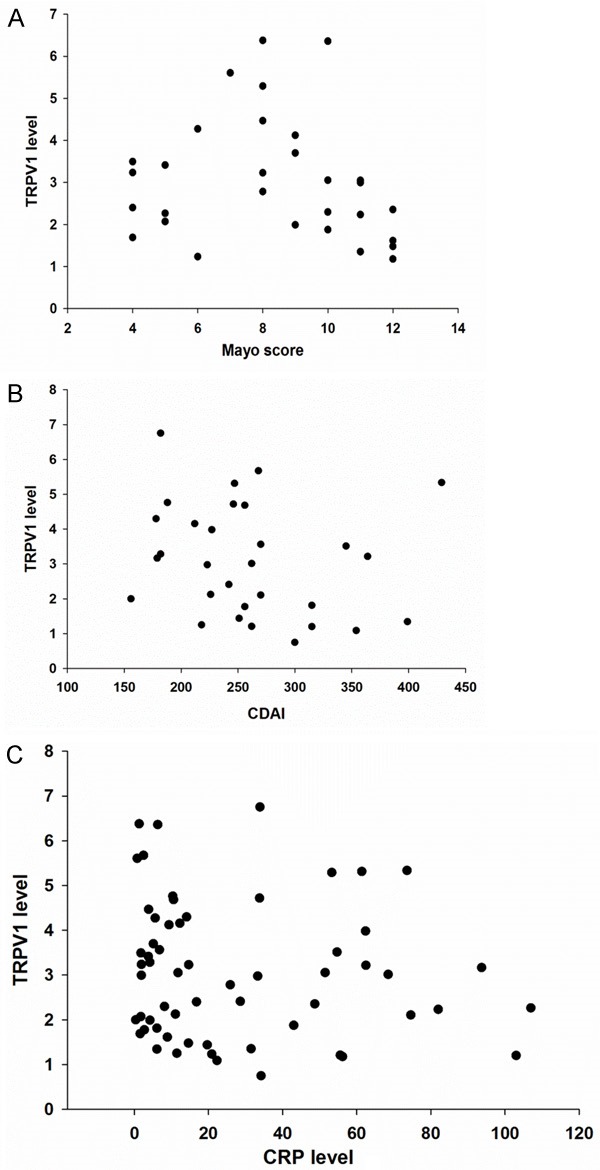
A. Scatter plot showed no significant correlation between TRPV1 expression and the Mayo score in the UC group (r=-0.316, P=0.089). B. Scatter plot showed no significant correlation between TRPV1 expression and CDAI score in the CD group (r=-0.274, P=0.143). C. Scatter plot showed no significant correlation between TRPV1 expression and CRP (r=-0.110, P=0.402).
To analyze the correlation between TRPV1 expression and the severity of inflammation in IBD patients, the severity of histological inflammation was graded as described above. Briefly, histological inflammation was recorded as 1 (mild: cryptitis), 2 (moderate: crypt abscesses), or 3 (severe: erosions or ulcerations). In the UC group, the number of patients with mild, moderate, and severe histological inflammation was 10, 11, and 9, respectively. In the CD group, the number of patients with mild, moderate, and severe histological inflammation was 11, 5, and 14, respectively. Both in the UC and the CD group, no significant correlation was observed between histological inflammation scores and TRPV1 expression (P>0.05) [Figure 4A and 4B].
Figure 4.
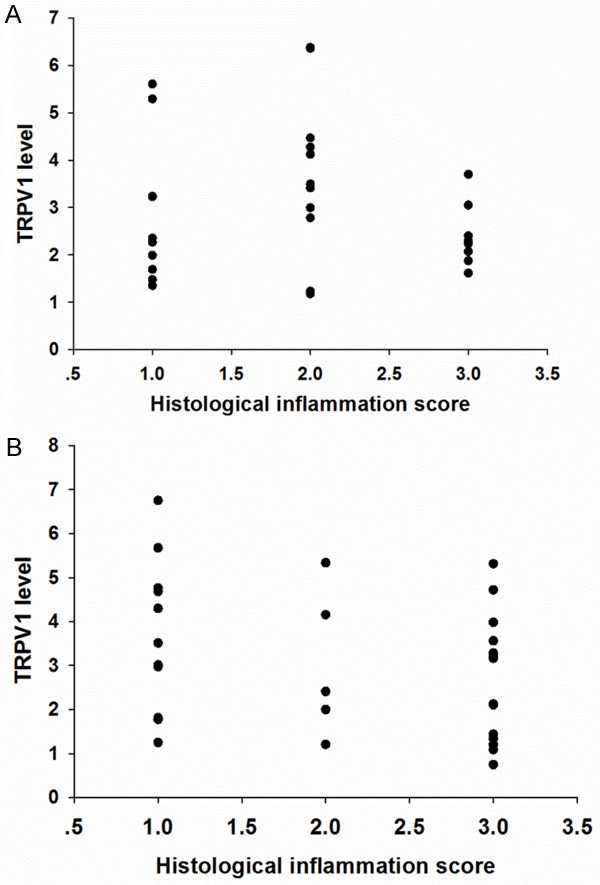
A. Scatter plot showed no significant correlation between TRPV1 expression and histological inflammation score in UC group (P>0.5). B. Scatter plot showed no significant correlation between TRPV1 expression and histological inflammation score in CD group (P>0.05).
Discussion
In this study, immunohistochemical staining revealed that TRPV1 was obviously increased in colonic epithelium of patients with active IBD. However, the expression of TRPV1 did not significantly correlate with disease duration or disease activity. Epithelial cells in inflamed colonic biopsies obtained from IBD patients showed a more intense TRPV1 immunostaining, especially at the basolateral membrane, when compared with non-IBD controls. Most inflammatory cells infiltrated in the epithelium and lamina propria expressed TRPV1, which was localized in the membrane, cytoplasm or nucleus. Since previous studies reported TRPV1 to be expressed in sensory neurons, function as a nociceptor, and play an important role in visceral hypersensitivity, its expression in colonic nerve fibers and correlation with abdominal pain has been thoroughly investigated [15,16]. It has been reported that TRPV1 expression in colonic nerve fibers was increased in patients with IBD or irritable bowel syndrome (IBS) [10,15,16,22]. This study was the first to evaluate TRPV1 expression in colonic epithelium of IBD patients and to analyze its correlation with disease activity.
The presence of TRPV1 in various gastrointestinal epithelial cells has been previously demonstrated. Activation of TRPV1 expressed on esophageal epithelial cells induces the production of chemokines, including platelet activating factor (PAF) and IL-8, thereby contributing to esophageal inflammation in gastroesophageal reflux disease (GERD) [23,24]. Besides nerve structures, immunohistochemistry and in situ hybridization confirmed the presence of TRPV1 in parietal cells of the human stomach, with a mitochondrial and sometimes nuclear distribution [25]. The mechanism is still not well understood, however, TRPV1 in stomach plays a gastroprotective role via regulating acid secretion [25]. Moreover, the expression of TRPV1 in the human colonic crypt cell line T84 cells has been reported. In T84 cells, TRPV1 was localized in the basolateral membrane and vesicular type compartments in the cytosol [26]. The present study further confirmed the presence of TRPV1 in human colonic epithelial cells and revealed a significant upregulation in patients with IBD, however, its role remains to be elucidated.
Although the results are promising, this study also has some limitations. For example, we failed to clearly identify which infiltrated inflammatory cell types express TRPV1. Recent studies have demonstrated that TRPV1 expressed by immune cells plays an immunomodulatory role. For example, TRPV1 was expressed by CD4+ T cells, co-localized with the T cell receptor (TCR) complex, contributing to TCR-induced T cell activation and production of proinflammatory cytokines [8]. Oral administration of capsaicin, a well-known agonist of TRPV1, enhanced production of the Th1 cytokines IFN-γ and IL-2 in intestinal Peyer’s patch cells [27]. TRPV1 mRNA expression on neutrophils isolated from human peripheral blood was confirmed using RT-PCR. However, despite the fact that TRPV1 is highly permeable to Ca2+, only high concentrations of capsaicin could stimulate Ca2+ entry in neutrophils [28]. In a previous study, Basu et al. revealed that TRPV1 was also expressed by dendritic cells (DCs), leading to maturation and migration of DCs after activation by capsaicin [9]. Further evaluation using RT-PCR and Western blot analysis did not confirm the expression of TRPV1 in macrophages. Moreover, an anti-inflammatory effect of capsaicin in macrophages was observed via inhibiting lipopolysaccharide (LPS)-induced NF-κB activation and prostaglandin E2 (PGE2) production in a TRPV1-independent way [29]. However, in another study, it was demonstrated that TRPV1 immunoreactivity was present on macrophages in colonic biopsies collected from IBD patients [30]. In the same study, it was also found that TRPV1 is expressed on interstitial plasma cells and some neuroendocrine cells. In contrast to our results, a significant decrease in TRPV1 was observed in active IBD patients compared to non-inflamed controls [30]. In addition, the expression of transient receptor potential ankyrin-1 (TRPA1), another member of the TRPs family that generally co-localizes with TRPV1, was upregulated in active IBD patients [30]. TRPA1 plays a protective role in T cell-mediated colitis by inhibiting TRPV1 activity, and genetic depletion of TRPA1 results in exacerbation of colitis [31]. The increased infiltration of TRPA1+TRPV1+ T cells in the colon of patients with IBD indicates involvement of TRPA1 and TRPV1 in the pathogenesis of IBD [31].
Data regarding the role of TRPV1 in colitis was primarily collected from animal models and seem conflicting. In IL-10-/- mice, knockdown or pharmacological inhibition of TRPV1 attenuates colonic inflammation and T cell-derived cytokines [8]. Capsazepine, an antagonist of TRPV1, reduces the severity of dextran sulfate sodium (DSS)-induced colitis through oral, topical, or systemic administration [32,33]. However, TRPV1-deficient mice developed more severe colonic inflammation in dinitrobenzene sulfonic acid (DNBS)-induced colitis, indicating a protective role of TRPV1 in colitis [34,35]. Activation of TRPV1 leads to the release of pro-inflammatory neuropeptides, which mainly includes substance P (SP) and calcitonin gene-related peptide (CGRP) [11]. SP interacts with its neurokinin-1 receptor (NK-1R) to activate NF-κB, p38 MAPK, and JAK/STAT pathways. In addition, SP stimulates cytokine production, and plays an important role in intestinal inflammation [36,37]. Administration with the NK-1 receptor antagonist reduced the severity of T cell-induced colitis in severe combined immunodeficiency (SCID) mice and DSS-induced colitis in rats [38,39].
Considering the crucial role of TRPV1 in the production of chemokines from epithelial cells, activation of immune cells, and the pathogenesis of experimental colitis, the upregulation of TRPV1 in colonic mucosa may contribute to the onset and recurrence of IBD. Although neurotoxic doses of capsaicin induce the depletion of nociceptive afferent nerves and reduce the severity of intestinal inflammation, capsaicin derived from food components may activate TRPV1 that is expressed in the intestinal epithelium, thereby stimulating the migration and activation of immune cells [33,39]. This may explain why spicy foods are detrimental factors for IBD. Using semiquantitative approaches, this study failed to demonstrate any significant correlation between TRPV1 expression in colonic epithelium and disease activity. Further studies are warranted to validate our results and the involvement of TRPV1 in the pathogenesis of IBD, as well as to investigate the effects of spicy foods and oral capsaicin administration on intestinal inflammation.
In conclusion, TRPV1 is extensively expressed by epithelial cells and infiltrated inflammatory cells in colonic epithelium and lamina propria of patients with active IBD. The expression of TRPV1 is significantly upregulated in IBD patients compared to controls, however no significant correlation was found between the level of TRPV1 expression and disease activity. Thus, TRPV1 is a potential target for IBD therapy.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 81570502) and Research Fund for the Doctoral Program of Higher Education of China (20130181120041).
Disclosure of conflict of interest
None.
References
- 1.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 2.Martin TD, Chan SS, Hart AR. Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci. 2015;60:1396–1405. doi: 10.1007/s10620-014-3437-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang YF, Ou-Yang Q, Xia B, Liu LN, Gu F, Zhou KF, Mei Q, Shi RH, Ran ZH, Wang XD, Hu PJ, Wu KC, Liu XG, Miao YL, Han Y, Wu XP, He GB, Zhong J, Liu GJ. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J Gastroenterol. 2013;19:1827–1833. doi: 10.3748/wjg.v19.i11.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai A, Guo Y, Shen Y, Xie Y, Lu N. Genderrelated and city- and countryside-related differences in patients with ulcerative colitis in a Chinese population. Intern Med. 2008;47:2103–2107. doi: 10.2169/internalmedicine.47.1580. [DOI] [PubMed] [Google Scholar]
- 5.Eppinga H, Peppelenbosch MP. Worsening of bowel symptoms through diet in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:E6–E7. doi: 10.1097/MIB.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 6.Deng Y, Huang X, Wu H, Zhao M, Lu Q, Israeli E, Dahan S, Blank M, Shoenfeld Y. Some like it hot: the emerging role of spicy food (capsaicin) in autoimmune diseases. Autoimmun Rev. 2016;15:451–456. doi: 10.1016/j.autrev.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181–195. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Bertin S, Aoki-Nonaka Y, de Jong PR, Nohara LL, Xu H, Stanwood SR, Srikanth S, Lee J, To K, Abramson L, Yu T, Han T, Touma R, Li X, Gonzalez-Navajas JM, Herdman S, Corr M, Fu G, Dong H, Gwack Y, Franco A, Jefferies WA, Raz E. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells. Nat Immunol. 2014;15:1055–1063. doi: 10.1038/ni.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Luckhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, Reeh PW. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J Gastroenterol. 2012;47:256–265. doi: 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- 12.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 13.McVey DC, Vigna SR. The capsaicin VR1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides. 2001;22:1439–1446. doi: 10.1016/s0196-9781(01)00463-6. [DOI] [PubMed] [Google Scholar]
- 14.Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- 16.Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- 17.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI) Gastroenterology. 1979;77:843–846. [PubMed] [Google Scholar]
- 20.Virk R, Shinagare S, Lauwers GY, Yajnik V, Stone JH, Deshpande V. Tissue IgG4-positive plasma cells in inflammatory bowel disease: a study of 88 treatment-naive biopsies of inflammatory bowel disease. Mod Pathol. 2014;27:454–459. doi: 10.1038/modpathol.2013.121. [DOI] [PubMed] [Google Scholar]
- 21.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harnett KM, Rieder F, Behar J, Biancani P. Viewpoints on acid-induced inflammatory mediators in esophageal mucosa. J Neurogastroenterol Motil. 2010;16:374–388. doi: 10.5056/jnm.2010.16.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto E, Naito Y, Handa O, Okada H, Mizushima K, Hirai Y, Nakabe N, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Yoshida N, Yoshikawa T. Oxidative stress-induced posttranslational modification of TRPV1 expressed in esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G230–G238. doi: 10.1152/ajpgi.00436.2009. [DOI] [PubMed] [Google Scholar]
- 25.Faussone-Pellegrini MS, Taddei A, Bizzoco E, Lazzeri M, Vannucchi MG, Bechi P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem Cell Biol. 2005;124:61–68. doi: 10.1007/s00418-005-0025-9. [DOI] [PubMed] [Google Scholar]
- 26.Bouyer PG, Tang X, Weber CR, Shen L, Turner JR, Matthews JB. Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1. Am J Physiol Gastrointest Liver Physiol. 2013;304:G142–G156. doi: 10.1152/ajpgi.00483.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi M, Hasegawa I, Yahagi N, Ishigaki Y, Takano F, Ohta T. Carotenoids modulate cytokine production in Peyer’s patch cells ex vivo. J Agric Food Chem. 2010;58:8566–8572. doi: 10.1021/jf101295y. [DOI] [PubMed] [Google Scholar]
- 28.Wang JP, Tseng CS, Sun SP, Chen YS, Tsai CR, Hsu MF. Capsaicin stimulates the nonstore-operated Ca2+ entry but inhibits the store-operated Ca2+ entry in neutrophils. Toxicol Appl Pharmacol. 2005;209:134–144. doi: 10.1016/j.taap.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003;140:1077–1087. doi: 10.1038/sj.bjp.0705533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kun J, Szitter I, Kemeny A, Perkecz A, Kereskai L, Pohoczky K, Vincze A, Godi S, Szabo I, Szolcsanyi J, Pinter E, Helyes Z. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One. 2014;9:e108164. doi: 10.1371/journal.pone.0108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertin S, Aoki-Nonaka Y, Lee J, de Jong PR, Kim P, Han T, Yu T, To K, Takahashi N, Boland BS, Chang JT, Ho SB, Herdman S, Corr M, Franco A, Sharma S, Dong H, Akopian AN, Raz E. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut. 2017;66:1584–1596. doi: 10.1136/gutjnl-2015-310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 33.Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med (Berl) 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Yamamoto T, Kuramoto H, Kadowaki M. TRPV1 expressing extrinsic primary sensory neurons play a protective role in mouse oxazolone-induced colitis. Auton Neurosci. 2012;166:72–76. doi: 10.1016/j.autneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol. 2006;176:5050–5059. doi: 10.4049/jimmunol.176.8.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J. 2002;368:665–672. doi: 10.1042/BJ20020950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stucchi AF, Shofer S, Leeman S, Materne O, Beer E, McClung J, Shebani K, Moore F, O’Brien M, Becker JM. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- 39.Gad M, Pedersen AE, Kristensen NN, Fernandez CF, Claesson MH. Blockage of the neurokinin 1 receptor and capsaicin-induced ablation of the enteric afferent nerves protect SCID mice against T-cell-induced chronic colitis. Inflamm Bowel Dis. 2009;15:1174–1182. doi: 10.1002/ibd.20902. [DOI] [PubMed] [Google Scholar]


