Abstract
Bladder cancer is the second most common malignant tumor of the urinary tract worldwide and is associated with significant morbidity and mortality. EZH2, the enzymatic subunit of Polycomb repressive complex 2 (PRC2), is frequently overexpressed in multiple tumor types including Bladder cancer and plays multiple roles in tumor cell proliferation and apoptosis. Previous study showed that miR-26a has different roles in different tumors and the expression of EZH2 is identified as a potential target of miR-26a which miR-26a has been found to decrease in bladder cancer. But the mechanism between EZH2 and miR-26a is not completely clear in bladder cancer. Western blot and Real-time PCR were involved to detect both expression of mRNA and protein of EZH2. And we used mimics-miR26a to elaborate the relationship between EZH2 and miR-26a in cell proliferation and apoptosis process through lots of specific assays. The results showed that EZH2 express mainly in bladder tumor tissues than para-carcinoma tissues. Meanwhile, miR26a can down-regulate the expression of EZH2 through suppressing EZH2 activity. Both miR26a and downregulated EZH2 can induce bladder cancer cell apoptosis and increase cell at G1 stage as well as suppress cell proliferation. The further assays reveal that miR-26a can suppress cell proliferation and enhance cell apoptosis through EZH2. In this study, we found that EZH2 was overexpressed in bladder tumor tissue and miR-26a could downregulate the expression of EZH2 to inhibit proliferation and enhance apoptosis in bladder cancer.
Keywords: miR-26a, EZH2, proliferation, apoptosis, bladder cancer
Introduction
Bladder cancer (BC) is the fifth most common cancer and is one of the leading causes of cancer-related death in the word [1]. BC can be categorized into 2 groups: muscle invasive BC and nonmuscle invasive BC. Almost 75% of BC patients are diagnosed with nonmuscle invasive bladder cancer which are managed by local therapies, but often still have a high risk of recurrence and a variable risk of progression [2]. In recent years, although many targeted-therapies and immunotherapy such as PD-1/PD-L1 inhibitors have excelled in clinical trials, there is still no efficient therapeutic reagent for bladder cancer [3]. Hence, elucidation of novel molecular of BC and developing a more efficient therapeutic target that prevent recurrence or progression are urgently needed.
EZH2, Enhancer of Zeste Homologue 2, is the catalytic subunit of polycomb repressive complex2 (PRC2) and catalyzes the trimethylation of K27 of H3 (H3K27me3) [4]. PRC2 is mainly composed by four different proteins in mammals (EED, SUZ12, EZH2 and RBBP7/4) [5]. The EZH2 contains different domains: H1 and H2 domains conformed as binding regions for PHF1 and SUZ12, respectively, cysteine rich domain and SET domain [6]. The EZH2 catalytic activity mainly depends on its SET domain besides EED and SUZ12 of the PRC2 complex [7]. As demonstrated by various studies before, EZH2 not only acts as epigenetic silencer, but also it can act as a gene activator which represents an oncogenic signal in several cancers including prostate, liver, colon, lung and BC. Meanwhile, EZH2 expression can be directly regulated post-transcriptionally through the interaction with miRNA, such as miR-101, miR-26a, miR-217 [8]. MicroRNAs (miRNAs) are a class of endogenously small non-coding RNAs present extensively in eukaryotes [9]. The function of miRNAs is based on the imperfect complement binding between the seed region of miRNA and 3’-UTR of target mRNA to alter the expression of target genes at transcriptional and post-transcriptional levels [10]. Previous studies have shown that miRNAs act as tumor-suppressive or oncogenic genes in different tumor types [11]. Wang et al. found that the expression of miR-26 is down-regulated in groups T1 and T2 (differentiated grade 1-3) bladder cancer and can be a significant marker in BC [12]. MiR-26 contains miR-26a and miR-26b subtypes [13]. Recent studies reveal that EZH2 can be up-regulated with miR-26a-dependent regulation in rhabdomyosarcoma and neurogliocytoma [14]. Sander et al. also found that overexpression of miR-26a in Burkitt lymphoma cell lines can down-regulate EZH2 through an axis of MYC-miR-26a-EZH2-target to affect cell cycle progression [15]. But whether the same mechanism that miR-26a can also affect the expression of EZH2 in bladder cancer is still unknown. In our study, we found that EZH2 overexpressed in bladder tumor tissues compare with paracarcinoma tissues. MiR-26a can directly down-regulates EZH2 promoter activity to suppress the expression of EZH2 in BC and suppress cell proliferation and enhance cell apoptosis through EZH2.
Methods and materials
Cell culture, transfection and bladder tissue samples
Human bladder cancer cell line (EJ cell) was purchased from ATCC and cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C with 5% CO2. Lipofectamine 2000 was used for transfect cells according to the manufacturer’s protocol. DNA plasmids, MiRNA mimics or EZH2 siRNA were mixed with Opti-MEM medium and lipofectamine 2000 reagents Before transfection. After adding it to the cells, the medium was replaced 6 h later. The BC tissues and adjacent normal bladder tissues were obtained from Affiliated Hospital of Guilin Medical University. All patients were aware that their tissue sample would be used for scientific research before study and signed a document informed consent for the sample to be used. Our study was approved by the institutional research ethics committee of Affiliated Hospital of Guilin Medical University.
Expression vectors, mimic-miR-26a and antibodies
cDNAs encoding homo sapien EZH2 gene were synthesized and inserted into pcDNA3.0 (Invitrogen) vector. The EZH2 primer sequences: sense primer-5’-GGGGTAGGACGAAGAATAATCATGG-3’; antisense primer-5’ ATAAGAATGAGGTAGCAGATGTCAAGGG-3’. Mimic-mi26a and siRNA was purchased in GenePharma Inc. The antibody of EZH2 protein used in western blot and immunohistochemistry experiments is Rabbit-anti-EZH2 (abcam).
Quantitative real time PCR (qRT-PCR)
Total RNA was extracted using Trizol (TAKARA) and cDNA synthesis using Superscript II reverse transcriptase (DBI) was primed with random hexamer primers following the manufacturer’s protocols [16]. Real-time PCR for mRNA detection were performed using Stratagene Mx3000P Real time PCR (Agilent). The sequences of the primers used were as follows: EZH2 mRNA (sense): 5’-AATCAGAGTACATGCGACTGAGA-3’, EZH2 mRNA (antisense): 5’-GCTGTATCCTTCGCTGTTTCC-3’, GAPDH mRNA (sense): 5’-TGTTCGTCATGGGTGTGAAC-3’, GAPDH mRNA (antisense): 5’-ATGGCATGGACTGTGGTCAT-3’.
Western blotting
After transfected with the designated plasmids or siRNA, cells were harvested into universal protein extraction lysis buffer (Beyotime) containing protease inhibitor cocktail (Roche). We next measured protein concentrations and equal amounts of protein were prepared for SDS-PAGE, transferred to PVDF (Millipore) and detected with primary antibody of EZH2 and GAPDH (KangCheng) and horseradish peroxidase-conjugated secondary antibodies. Specific proteins were visualized using an enhanced chemiluminescence (ECL) western blot detection system [17].
Luciferase reporter assay
In the 3’UTR of the Luciferase-containing TK-pGL3 reporter construct (Promega), we included the 3’-UTR of human EZH2 or a 3’-UTR version with mutated miR-26a binding site. For the reporter assays, EJ cells were cultured in 24-well plates and transfected with TK-pGL3-EZH2 wt or mutated plasmid mixed with negative control-mimics or siR26a-mimics using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured 24 hours after transfection with the Dual-luciferase reporter assay system (promega).
Cell cycle and apoptosis analysis by flow cytometry
The DJ cells were transfected with specific plasmid, siRNA or miR-26a-mimics for 72 hours before analysis. For cell cycle analysis, cells were harvested and washed with ice-cold PBS before being fixed with 70% ice-cold ehanol. Then, cells were collected by centrifugation and resuspended in PBS containing RNase and Propidium Iodide (PI) and incubated at 37°C for 30 minutes. Finally, the cell cycle was analyzed by FACs scan flow cytometer and the relative ratios of G1, S and G2 phases were analyzed by FlowJo 2.7 software. For apoptosis analysis, cells were suspended in PBS at a density of 106 cells/mL and incubation with reagent containing Annexin V-FITC and PI for 30 minutes at room temperature and analyzed by flow cytometer [18].
CCK-8 assays
The cell proliferation ability was analyzed using CCK-8 assay. After cells were transfected with specific plasmids, siRNA or mimics for 48 h. The collected cells were counted at final 4 × 103 cells placed in 96-well plates. Every 24 h, 10 μL CCK-8 were added in each well and then incubated at 37°C for 30 min. The absorbance was measured at 450 nm to assess the number of viable cells. All experiments were repeated at least three times [19].
Colony formation assay
Colony formation assay was used to determine the cell proliferation ability. After EJ cells were transfected with specific vector, siRNA or siR26a-mimics for 48 h and collected and placed in six-well plates with final 3 × 103 cells/well for 14 days with complete medium. The colonies were fixed with 10% formaldehyde and stained with 0.5% crystal violet. The colonies were detected under microscope. All experiments were repeated at least three times.
Statistical analysis
Experiments were repeated three times. Statistical analyses were performed using SPSS version 17.0. Differences between experimental groups were determined using Student’ t test. Values of P < 0.05 were considered as significant and indicated by asterisks in the figures.
Results and discussion
The expression of EZH2 in bladder cancer tissue and para-carcinoma tissue
Recent studies show that EZH2 is overexpressed in multiple tumor types, and plays different roles besides epigenetic silencer and gene activator [20]. In order to detect the function of EZH2 in BC, we collected many bladder tumor tissues and para-carcinoma tissues to detect protein expression of EZH2 in BC. We found that EZH2 protein strongly expressed in Bladder tumor tissues and almost no signal detected in para-carcinoma tissues (Figure 1A-D) via immunohistochemistry (IHC). Western blot assays also showed higher expression levels of EZH2 in human BC compare matched adjacent tissue (Figure 1E). Together, the results showed that EZH2 express mainly in bladder tumor tissues than para-carcinoma tissues.
Figure 1.
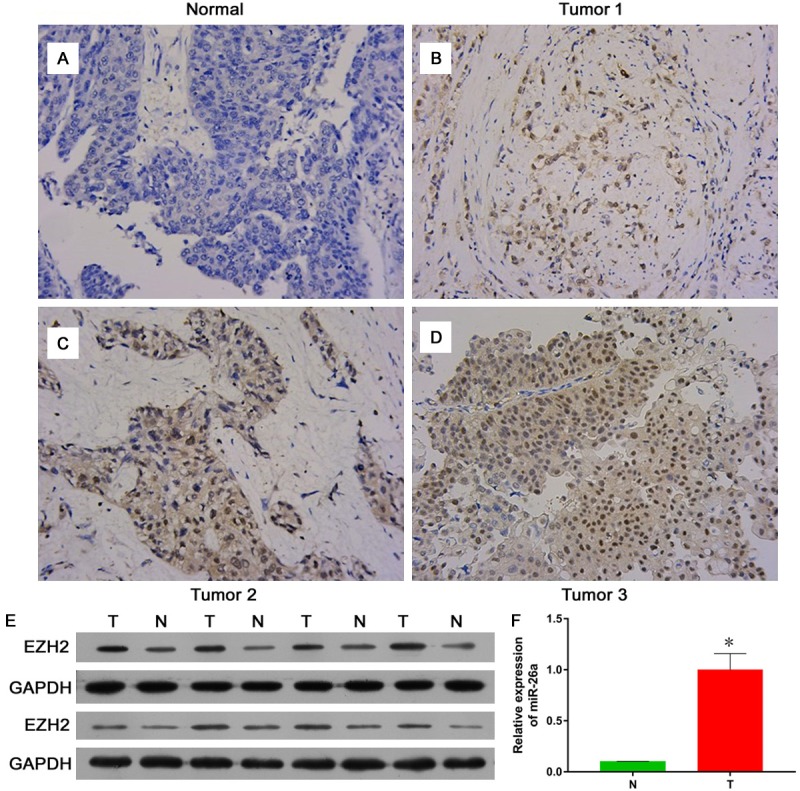
The expression of EZH2 in bladder cancer tissue and para-carcinoma tissue. In immunohistochemistry (IHC) stain, EZH2 protein strongly expressed in bladder tumor tissues and almost no signal detected in para-carcinoma tissues (A-D). Western blot assays showed higher expression levels of EZH2 in human BC compare matched adjacent tissue (E). PCR data showed miR-26a level in bladder cancer tissue was increased significantly compared to that in para-carcinoma tissue (F).
MiR-26a suppresses bladder cancer cell proliferation and promotes apoptosis
Previous studies clearly elaborated that miR-26a may participate in various biological processes through binding 3’UTR of target mRNA to repress protein expression in different tumors [9]. In our study, we found that the expression level of miR-26a in bladder tumor tissues were lower compare with para-carcinoma tissues (Figure 2A). Meanwhile, we also transfected with miR-26a-mimics in EJ cell to detected function of miR-26a at cell proliferation, apoptosis and cell cycle in vitro assay. Using CCK8 assay and colony formation assay, we found that mimics-miR26a can restrain cell proliferation and cell colony formation (Figure 2B and 2F). Meanwhile, we also detected that mimics-miR26a can promote EJ cell apoptosis and overexpression of miR-26a in vitro decrease in cells of S stage as well as induced an increase in cells of G1 stage (Figure 2E), indicating that miR-26a induced a G1 arrest. These results suggest that miR26a induce bladder cancer cell apoptosis and increase cell at G1 stage as well as suppress cell proliferation.
Figure 2.
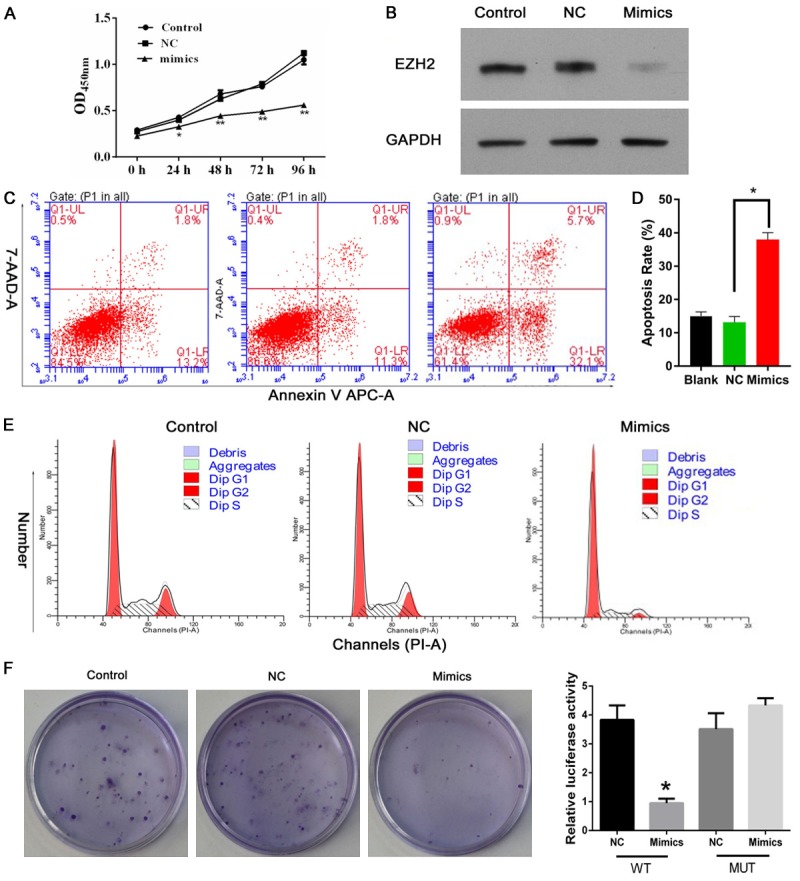
MiR-26a suppresses bladder cancer cell proliferation and promotes apoptosis. As results, the expression level of miR-26a-EZH2 in bladder tumor tissues were lower compare with para-carcinoma tissues with transfection of mimics-miR26a (A, B). Meanwhile, transfected miR-26a-mimics in EJ cell was detected function of miR-26a at cell proliferation, apoptosis and cell cycle in vitro assay. Using CCK8 assay and colony formation assay, miR26a inhibited cell proliferation and cell colony formation (C, D and F). Meanwhile, miR26a promoted EJ cell apoptosis and overexpression of miR-26a in vitro decrease in cells of S stage as well as induced an increase in cells of G1 stage (E).
MiR26a down-regulates expression of EZH2
Various studies showed that EZH2 is potential downstream target genes of miR-26 and down-regulated through MYC-miR-26a-EZH2 pathway in Burkitt lymphoma cell lines [5]. However, the molecular mechanisms of miR-26a and the target genes EZH2 in bladder cancer remain unclear. Our study detected the relationship between miR26a and EZH2 and found that miR-26a can down-regulated the protein expression of EZH2 with transfected mimics-miR26a in vitro, To determine whether EZH2 might be directly regulated by miR-26a, the full-length 3’UTR of human EZH2 containing the wild or mutant miR-26a responsive element were cloned into the 3’UTR region of the TK-pGL3 vector. The relative luciferase activity was significantly decreased with transfection of mimics-miR26a (Figure 3B) in wild EZH2 binding sequence compared with mutant EZH2 binding sequence. Collectively, these finding suggest that miR26a can down-regulate the expression of EZH2 through suppressing EZH2 promoter activity.
Figure 3.
MiR26a down-regulates expression of EZH2. MiR-26a down-regulated the mRNA and protein expression of EZH2 with transfected mimics-miR26a in vitro (A, B). To determine whether EZH2 might be directly regulated by miR-26a, the full-length 3’UTR of human EZH2 containing the wild or mutant miR-26a responsive element were cloned into the 3’UTR region of the TK-pGL3 vector. The relative luciferase activity was significantly decreased with transfection of mimics-miR26a in wild EZH2 binding sequence compared with mutant EZH2 binding sequence (C-E).
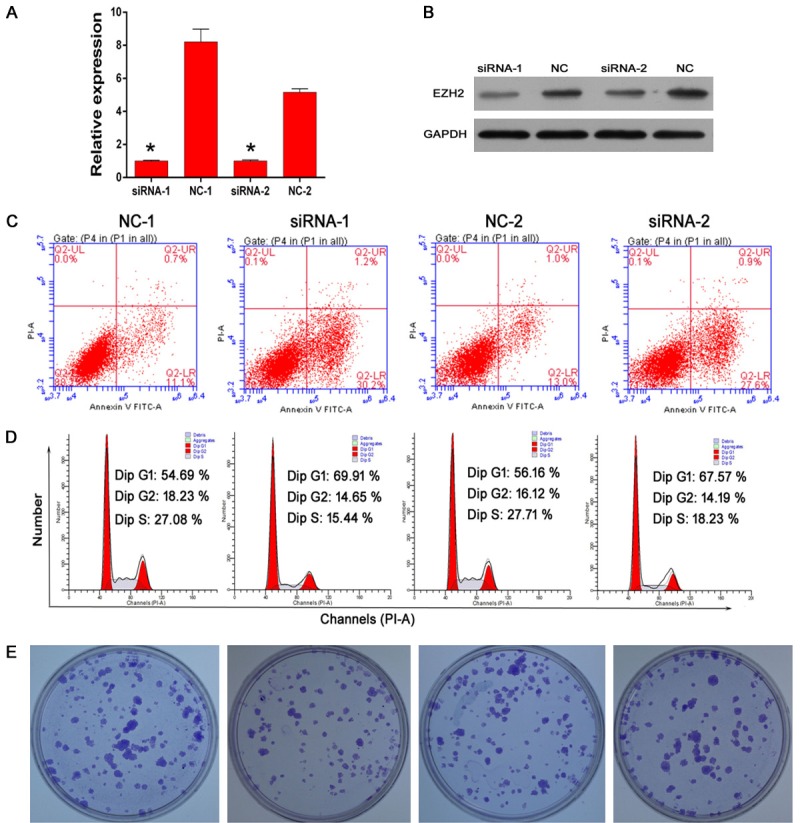
Down-regulation of EZH2 promote bladder cancer cell apoptosis and induce cell cycle arrest as well as proliferation suppressed
The implication of EZH2 in tumorigenesis has been extensively documented in various types of tumors such as prostate and breast cancer [21]. We surmise whether EZH2 regulates tumor cell proliferation and apoptosis in bladder cancer. Small interfering RNA (siRNA) for human EZH2 was transfected into EJ cells. A real-time quantitative PCR (RT-qPCR) (Figure 3C) and Western blot (WB) (Figure 3D) analysis revealed that both EZH2 mRNA and protein expression were decreased following EZH2-siRNA transfected compared with negative control-siRNA transfected. Using the flow cytometry to observe the cell apoptosis and cell cycle in EJ cells, we found that downregulated EZH2 enhanced cell apoptosis (Figure 3E) and decreased cells in S stage as well as induced an increase in cells of G1 stage by transfected with effective siRNA (Figure 3F). Meanwhile, our studies also detected that siRNA-EZH2 can suppress EJ cell colony formation in vitro (Figure 3G). Together, these results indicate that downregulated EZH2 can increase cell apoptosis and arrest cell in G1 stage as well as decrease cell proliferation.
EZH2 medicates MiR-26a-regulated tumor cell proliferation and apoptosis
Above results revealed that miR-26a can directly down-regulate expression of EZH2. Surprisingly, we also found that both mi-26a and EZH2 played the similar roles in cell apoptosis, cell cycle arrested and cell proliferation. Together analysis of these results, we were interested in and surmised whether miR26a regulated tumor cell proliferation and apoptosis via EZH2. The RT-PCR and WB assays were involved and detected that transfected EZH2 plasmids can recover EZH2 regulation by miR-26a in mRNA level and protein expression (Figure 4A, 4B). We next detected the relationship between miR-26a and EZH2 in tumor cell proliferation by performing cell counting kit-8 assays and colony formation assays. Results showed that mimics-miR26a markedly suppress cell proliferation and colony formation, which can be recovered by overexpression of EZH2 (Figure 4C and 4F). We found that overexpression of mimic-miR-26a enhanced cell apoptosis (Figure 4E) and G1 cell stage arrest (Figure 4D) through using flow cytometry analysis, which can be recovered by overexpression of EZH2. These results suggested that miR-26a can suppress cell proliferation and enhance cell apoptosis through EZH2.
Figure 4.
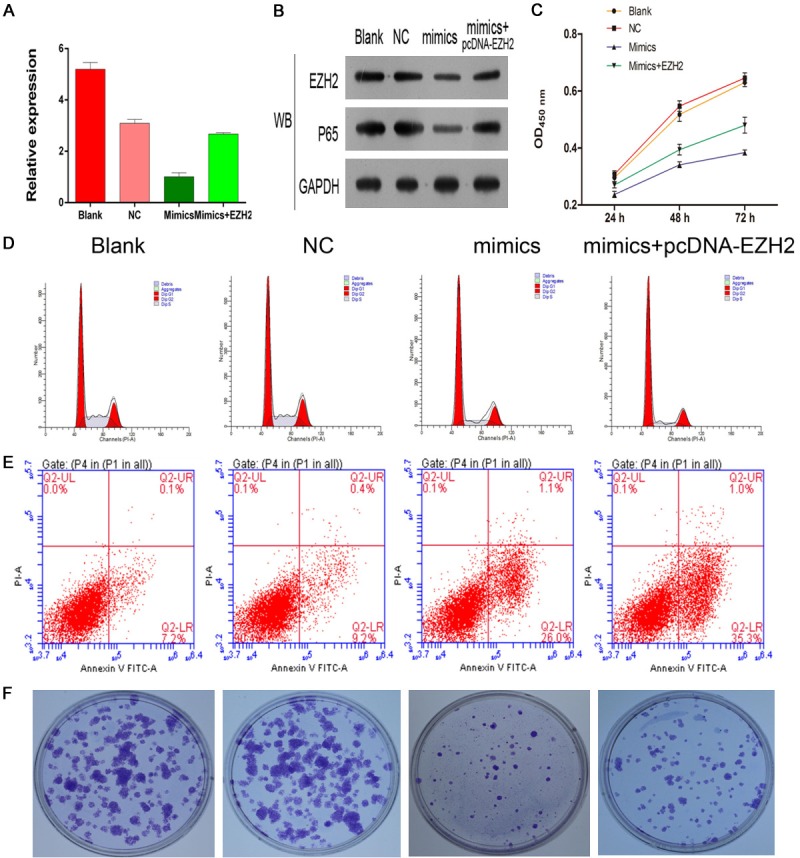
EZH2 medicates MiR-26a-regulated tumor cell proliferation and apoptosis. The RT-PCR and WB assays were involved and detected that transfected EZH2 plasmids induced EZH2 regulation by miR-26a in mRNA level and protein expression (A, B). Results from kit-8 assays and colony formation assays showed that mimics-miR26a markedly suppress cell proliferation and colony formation, which was recovered by overexpression of EZH2 (C-F).
Acknowledgements
This project is supported by the Innovation Project of Guangxi Graduate Education (YCSW2017210), the Natural Science Foundation of Guangxi Province (NO. 2016GXNSFAA380305) and Guilin Scientific Research and Technology Development Project (NO. 2016012706-15).
Disclosure of conflict of interest
None.
References
- 1.Nandagopal L, Sonpavde G. Circulating biomarkers in bladder cancer. Bladder Cancer. 2016;2:369–379. doi: 10.3233/BLC-160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammed AA, El-Tanni H, El-Khatib HM, Mirza AA, Mirza AA, Alturaifi TH. Urinary Bladder cancer: biomarkers and target therapy, new era for more attention. Oncol Rev. 2016;10:320. doi: 10.4081/oncol.2016.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Choi W, Ferguson Rd JE, Metcalfe MJ, Kamat AM. New discoveries in the molecular landscape of bladder cancer. F1000Research. 2016;5:2875. doi: 10.12688/f1000research.10031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arisan S, Buyuktuncer ED, Palavan-Unsal N, Caskurlu T, Cakir OO, Ergenekon E. Increased expression of EZH2,a polycomb group protein, in bladder carcinoma. Urol Int. 2005;75:252–257. doi: 10.1159/000087804. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Liu D, Tao D, Xiang W, Xiao X, Wang M, Wang L, Luo G, Li Y, Zeng F, Jiang G. BRD4 regulates EZH2 transcription through upregulation of C-MYC and represents a novel therapeutic target in bladder cancer. Mol Cancer Ther. 2016;15:1029–1042. doi: 10.1158/1535-7163.MCT-15-0750. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Fernandez M, Rubio C, Segovia C, Lopez-Calderon FF, Duenas M, Paramio JM. EZH2 in bladder cancer, a promising therapeutic target. Int J Mol Sci. 2015;16:27107–27132. doi: 10.3390/ijms161126000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz S, Kempkensteffen C, Christoph F, Hoffmann M, Krause H, Schrader M, Schostak M, Miller K, Weikert S. Expression of the polycomb group protein EZH2 and its relation to outcome in patients with urothelial carcinoma of the bladder. J Cancer Res Clin Oncol. 2008;134:331–336. doi: 10.1007/s00432-007-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weikert S, Christoph F, Kollermann J, Muller M, Schrader M, Miller K, Krause H. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med. 2005;16:349–353. [PubMed] [Google Scholar]
- 9.Braicu C, Cojocneanu-Petric R, Chira S, Truta A, Floares A, Petrut B, Achimas-Cadariu P, Berindan-Neagoe I. Clinical and pathological implications of miRNA in bladder cancer. Int J Nanomedicine. 2015;10:791–800. doi: 10.2147/IJN.S72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Liu QG. The role of miR-26 in tumors and normal tissues (Review) Oncol Lett. 2011;2:1019–1023. doi: 10.3892/ol.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratert N, Meyer HA, Jung M, Lioudmer P, Mollenkopf HJ, Wagner I, Miller K, Kilic E, Erbersdobler A, Weikert S, Jung K. miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J Mol Diagn. 2013;15:695–705. doi: 10.1016/j.jmoldx.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Tan S, Ding K, Li R, Zhang W, Li G, Kong X, Qian P, Lobie PE, Zhu T. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Res. 2014;16:R40. doi: 10.1186/bcr3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeitels LR, Acharya A, Shi G, Chivukula D, Chivukula RR, Anandam JL, Abdelnaby AA, Balch GC, Mansour JC, Yopp AC, Richardson JA, Mendell JT. Tumor suppression by miR-26 overrides potential oncogenic activity in intestinal tumorigenesis. Genes Dev. 2014;28:2585–2590. doi: 10.1101/gad.250951.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Tang K, Xiao H, Zeng J, Guan W, Guo X, Xu H, Ye Z. A panel of eight-miRNA signature as a potential biomarker for predicting survival in bladder cancer. J Exp Clin Cancer Res. 2015;34:53. doi: 10.1186/s13046-015-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecker N, Stephan C, Mollenkopf HJ, Jung K, Preissner R, Meyer HA. A new algorithm for integrated analysis of miRNA-mRNA interactions based on individual classification reveals insights into bladder cancer. PLoS One. 2013;8:e64543. doi: 10.1371/journal.pone.0064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Song J, Wu W, Wu X, Su M. Puerarin exerts the protective effect against chemical induced dysmetabolism in rats. Gene. 2016;595:168–174. doi: 10.1016/j.gene.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Liang L, Wu X, Ma X, Su M. Valproate acid (VPA)-induced dysmetabolic function in clinical and animal studies. Clin Chim Acta. 2017;468:1–4. doi: 10.1016/j.cca.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Guo C, Wu X, Huang Z, Chen J. FGF21 functions as a sensitive biomarker of APAPtreated patients and mice. Oncotarget. 2017;8:44440–44446. doi: 10.18632/oncotarget.17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C, Pan Q, Su M, Li R. Clinical immunophenotype of nasopharyngeal neuroendocrine carcinoma with metastatic liver cancer. Clin Chim Acta. 2017;471:283–285. doi: 10.1016/j.cca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YB, Niu HT, Chang JW, Dong GL, Ma XB. EZH2 silencing by RNA interference inhibits proliferation in bladder cancer cell lines. Eur J Cancer Care. 2011;20:106–112. doi: 10.1111/j.1365-2354.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SR, Roh YG, Kim SK, Lee JS, Seol SY, Lee HH, Kim WT, Kim WJ, Heo J, Cha HJ, Kang TH, Chung JW, Chu IS, Leem SH. Activation of EZH2 and SUZ12 regulated by E2F1 predicts the disease progression and aggressive characteristics of bladder cancer. Clin Cancer Res. 2015;21:5391–5403. doi: 10.1158/1078-0432.CCR-14-2680. [DOI] [PubMed] [Google Scholar]


