Abstract
The aim of this study was to assess the therapeutic effect of melatonin in phosgene induced acute lung injury (ALI) and to explore the related mechanisms. A rat model of phosgene induced ALI was established and the severity of the ALI was evaluated by wet/dry (W:D) ratio of lung weight, bronchoalveolar lavage (BAL) fluid cell counts and inflammatory cytokines. The rats were administrated with elatonin (MT), ulinastatin (UTI), p38 inhibitor and NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) alone or in combination. We found that MT in combination with UTI significantly improved the severity of ALI and the activation of Wnt/β-catenin signaling was involved in beneficial effect of MT. MT may be used as a therapeutic adjuvant for phosgene induced ALI.
Keywords: Melatonin, ulinastatin, phosgene, acute lung injury, Wnt/β-catenin pathway
Introduction
Phosgene, a well-known irritating and choking gas, has been used as a chemical warfare agent since the First World War. Currently, phosgene is widely used in the production of isocyanates, dyes, plastics, and pharmaceuticals. Therefore, accidental, environmental, and occupational exposure to the phosgene could not be avoided [1]. It is well-defined that exposure to phosgene causes acute lung injury (ALI) [2], which can occasionally produce symptoms similar to fatal acute respiratory distress syndrome [3]. Currently, there is no specific or effective treatment for phosgene-induced ALI.
Urinary trypsin inhibitor (UTI) exists in human urine and blood and is a multivalent Kunitz-type serine protease inhibitor [4]. UTI has been widely used in clinical practice as a drug for patients with disseminated intravascular coagulation (DIC), shock, and pancreatitis [5]. UTI exerts its effect mainly through the inhibition of proteases including trypsin, α-chymotrypsin, plasmin, cathepsin G, and leukocyte elastase. In addition, UTI has been reported to have anti-inflammatory properties in vitro. UTI inhibits the production of proinflammatory molecules such as thromboxane B2 (TXB2), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and the production of prostaglandin H2 synthase (PHS-2), which is induced in some inflammatory conditions and contributes to the inflammatory process [6]. Therefore, UTI ameliorates several inflammatory injuries such as ischemia-reperfusion injury [7], septic shock [8], and glomerulonephritis [9]. Moreover, we recently showed that UTI could reduce pathogenesis of phosgene-induced ALI through decreasing neutrophil accumulation and inflammatory cytokine production [10].
Melatonin (MT) is a hormone secreted by the pineal gland at the base of the brain and its levels are monitored by environmental light [11]. MT levels peak at night to lower body temperature and cause drowsiness [12]. MT is a regulator of circadian rhythm in different organ systems [13,14]. In addition, MT plays a protective role in inflammatory environment [15,16]. However, the role of MT in the treatment of phosgene induced ALI remains unclear. Recent study indicated that melatonin could regulate Wnt/β-catenin pathway [17]. Therefore, in this study we aimed to explore therapeutic effect of MT in phosgene induced ALI and investigate potential molecular mechanisms related to Wnt/β-catenin pathway.
Materials and methods
Animals
Adult male SD rats (weight 200 ± 20 g, 8 weeks old) were purchased from the Experimental Animal Center of the Second Military University (Shanghai, China). The experiment protocol was approved by the Ethic Committee of Animal Care in Jinshan Hospital, Fudan University. The rats were maintained in cages at 24-26°C under a 12 hr light/dark cycle with free access to food and water. The rats were randomly divided into 7 groups as follows: Control group (normal air), phosgene group (control), MT group (10 mg/kg MT and phosgene), UTI (100 U/g UTI and phosgene), MT+UTI (10 mg/kg MT, 100 U/g UTI and phosgene), p38 inhibitor (25 μg/100 g SB203580 and phosgene), NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) (120 mg/kg PDTC and phosgene). All the drugs were applied 1 h after phosgene exposure by intraperitoneal injection. Six hours after exposure to phosgene, all rats were anesthetized (i.p.) with 2% pentobarbital and sacrificed to evaluate the severity of ALI.
Exposure to phosgene
Phosgene gas was produced by dissolving triphosgene (Shanghai Guangtuo Chemical Co., Ltd, Shanghai, China) in cyclohexane (Sinopharm Chemical Reagent Co., Ltd, Shanghai China) with N, N-dimethyl formamide (Sinopharm Chemical Reagent Co., Ltd) and released at a constant rate. The animals were placed into an exposure cabinet and allowed to breath spontaneously, followed by phosgene gas inhalation at a dose of 5.0 g/m3 for 5 min. Then the animals were placed into separate cabinets with fresh air where they were not allowed to consume any food.
Bronchoalveolar lavage (BAL)
BAL was performed with the whole lung. Two mL aliquots of 37°C, sterile, pyrogen-free, 0.9% saline were flushed through the tracheotomy tube and this process was repeated for 5 times. A total of about 10 mL were recovered and pooled. The total number of cells was counted using a standard hemocytometer. The BAL fluid (BALF) was then centrifuged at 1000×g for 5 min. The supernatant was collected and stored at -80°C. Protein quantification was determined by using BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Wet-to-dry lung weight ratio
The lower right lobe of the lung was weighed. After data recording, the lung was dried in an incubator for 48 h at 80°C and weighed again. The weights of wet and dried lungs were normalized to body weight and the ratio of W/D was calculated.
ELISA
The concentrations of interleukin 10 (IL-10) and Tumor necrosis factor-α (TNF-α) in BALF were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA).
Western blot analysis
The frozen lung tissues were lysed in RIPA buffer (Beyotime, China), followed by high speed centrifugation and BCA quantification. Cellular protein was separated by electrophoresis on sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel and then transferred onto Poly vinylidene fluoride (PVDF) membranes. After blocking, the membranes were incubated with the antibodies to Wnt1, Wnt3a, β-catenin, p38, NF-κB and GAPDH (all from Cell Signaling, Danvers, MA, USA), followed by incubation with HPR conjugated secondary antibodies. The protein bands were detected with SuperSignal Ultra Chemiluminescent Substrate (Pierce, Rockford, IL, USA) on X-ray films (Koda, Rochester, NY, US).
Statistical analysis
All statistical analyses were performed using SPSS13.0 software (SPSS Inc., Chicago, IL, USA). The results were presented as mean ± standard deviation (SD). One way ANOVA was used to examine the differences among multiple groups. P<0.05 was considered as statistically significance.
Results
Establishment of phosgene induced ALI
As shown in Figure 1A, W:D lung-weight ratio of rats was significantly increased after exposure to phosgene compared to control group (4.11 ± 0.44 vs. 7.38 ± 0.58, P<0.01). The protein level in BALF was significantly increased after phosgene exposure compared to control group (0.623 ± 0.02 vs. 1.832 ± 0.02 mg/mL, P<0.01) (Figure 1B). The BAL fluid cell count was significantly higher after phosgene exposure than that in control group (1.74 ± 0.2 vs. 5.3 ± 0.38×106 cells, P<0.01) (Figure 1C).
Figure 1.
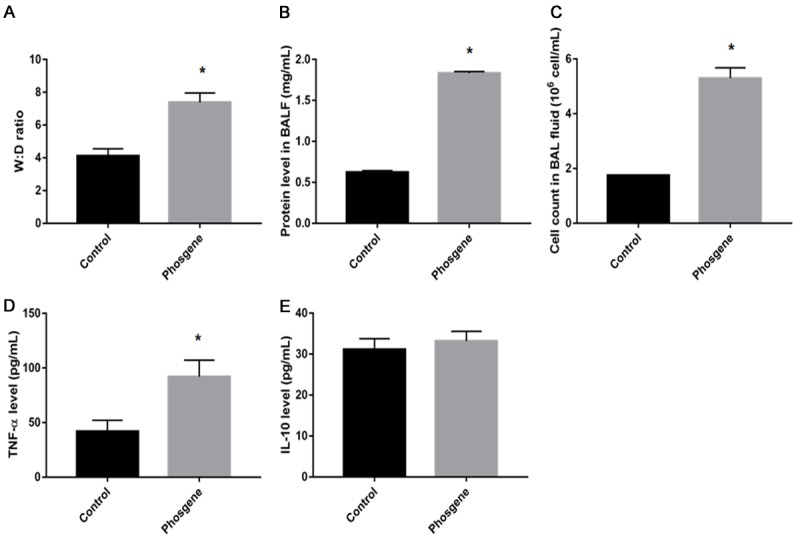
Establishment of phosgene induced acute lung injury in rats. A. The Wet/Dry lung weight ratio; B. The protein level in BAL fluid; C. The BAL fluid cell counts; D. The level of TNF-α; E. The level of IL-10 after phosgene exposure. Data were presented as mean ± SD (n=3). *P<0.05 vs. Control group.
The concentration of TNF-α in BALF was significantly increased in phosgene-exposed group (Figure 1D) (92.01 ± 15.14 pg/mL vs. 41.89 ± 10.19 pg/mL in control group, P<0.001). There was no difference in IL-10 concentration between phosgene group and control group (31.12 ± 2.66 pg/mL vs. 33.23 ± 2.34 pg/mL in control group, P<0.05) (Figure 1E).
MT and UTI attenuated the severity of ALI
We examined the severity of ALI at 6 h post phosgene exposure. W:D ratio of the lung was lower in MT (5.00 ± 0.39, P<0.05), UTI (4.84 ± 0.33, P<0.05), MT+UTI (4.54 ± 0.29, P<0.01), p38 inhibitor (5.46 ± 0.48, P<0.05), PDTC group (5.31 ± 0.41, P<0.05) than in phosgene group (7.38 ± 0.58, P<0.05) (Figure 2A). The protein levels in BALF were lower in MT (0.824 ± 0.014, P<0.05), UTI (0.733 ± 0.011, P<0.05), MT+UTI (0.677 ± 0.014, P<0.01), p38 inhibitor (1.190 ± 0.029, P<0.05), PDTC group (1.007 ± 0.019, P<0.05) than in phosgene group (1.832 ± 0.015, P<0.05) (Figure 2B). As shown in the Figure 2C, the BAL fluid cell count (×106 cells) was significantly decreased in MT (3.00 ± 0.25, P<0.05), UTI (2.65 ± 0.23, P<0.05), MT+UTI (2.29 ± 0.21, P<0.01), p38 inhibitor (3.77 ± 0.28, P<0.05), PDTC group (3.53 ± 0.27, P<0.05) than in phosgene group (5.30 ± 0.38, P<0.05). The concentration of TNF-α was lower in MT (65.64 ± 12.25, P<0.05), UTI (63.14 ± 11.23, P<0.05), MT+UTI (57.86 ± 10.21, P<0.01), p38 inhibitor (70.93 ± 15.28, P<0.05), PDTC group (68.90 ± 13.25, P<0.05) than in phosgene group (92.01 ± 15.14, P<0.05) (Figure 2D). These data suggest that MT and UTI could alleviate the severity of ALI.
Figure 2.
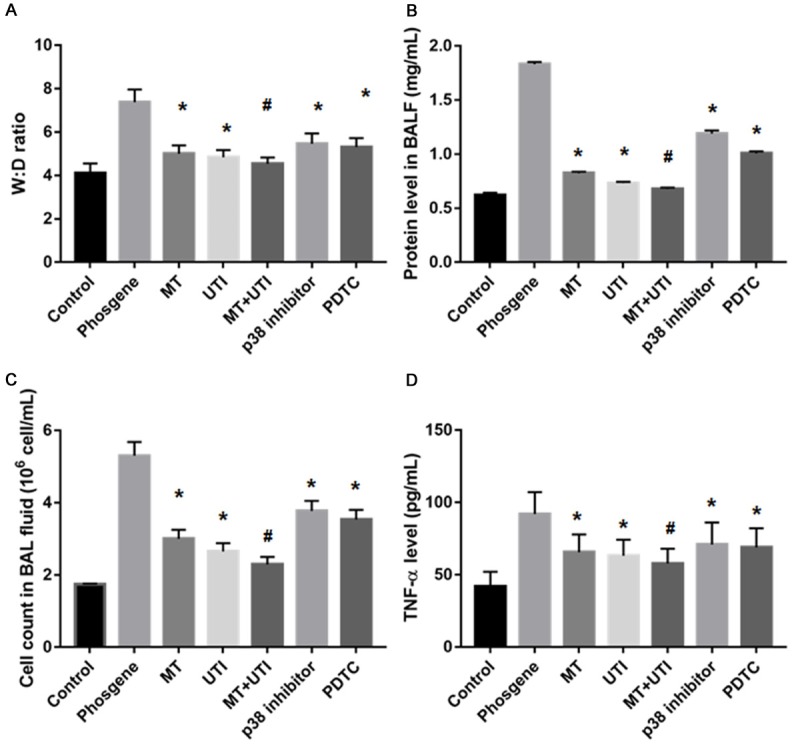
Treatment of phosgene induced acute lung injury by MT, UTI, p38 inhibitor and PDTC. A. The Wet/Dry lung weight ratio; B. The protein level in BAL fluid; C. The BAL fluid cell counts; D. The level of TNF-α. Data were presented as mean ± SD (n=3). *P<0.05 vs. Phosgene group. #P<0.01 vs. Phosgene group.
Altered expression of the proteins in Wnt/β-catenin pathway
Then we collected the lung tissues and performed Western blot analysis. As shown in Figure 3, the expression of WNT1 significantly increased in MT and UTI group (P<0.05) while decreased significantly in PDTC group (P<0.01), compared to phosgene group. The expression of WNT3a significantly increased in p38 inhibitor and PDTC group (P<0.05) compared to phosgene group (Figure 3C). The expression of β-catenin significantly increased in MT, UTI, MT+UTI, p38 inhibitor, PDTC group compared to phosgene group (Figure 3D).
Figure 3.
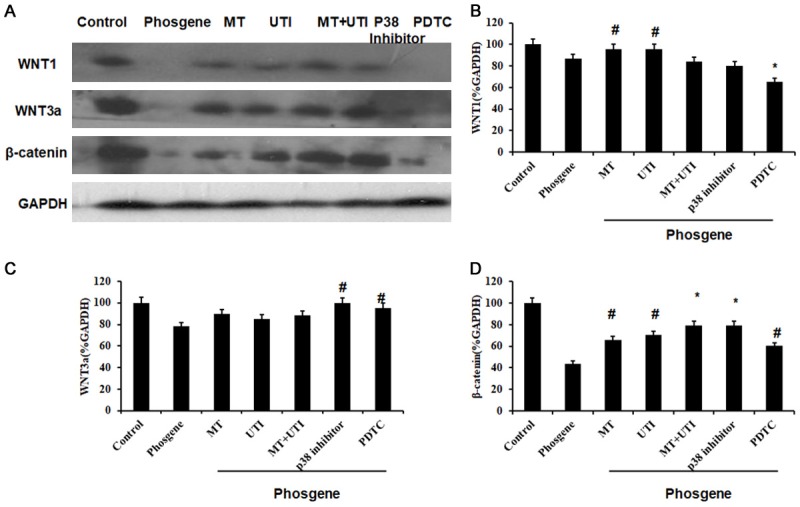
The expression of proteins in Wnt/β-catenin pathway after different treatments. (A) Western blot analysis of WNT1, WNT3a and β-catenin. Relative expression quantification of WNT1 (B), WNT3a (C) and β-catenin (D). GAPDH was loading control. Data were presented as mean ± SD (n=3). #P<0.05 vs. Phosgene group. *P<0.01 vs. Phosgene group.
Altered levels of p-p38 and NF-κB
We also examined the levels of p-p38 and NF-κB and found that p-p38 level significantly decreased in MT (P<0.05), MT+UTI (P<0.05), p38 inhibitor (P<0.05) and PDTC group (P<0.01) compared to phosgene group. In addition, NF-κB level significantly decrease in MT (P<0.05), MT+UTI (P<0.05), p38 inhibitor (P<0.05) and PDTC group (P<0.01) compared to phosgene group (Figure 4).
Figure 4.
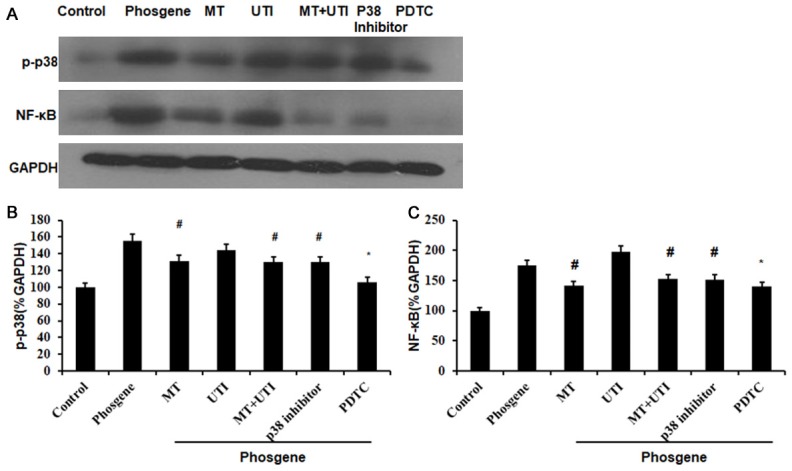
The levels of p-p38 and NF-κB after different treatments. (A) Western blot analysis of p-p38 and NF-κB. Relative expression quantification of p-p38 (B) and NF-κB (C). GAPDH was loading control. Data were presented as mean ± SD (n=3). #P<0.05 vs. Phosgene group. *P<0.01 vs. Phosgene group.
Discussion
Animal studies have shown that the exposure to phosgene resulted in acute pathological changes specifically in the broncho alveolar region which were characterized by alveolar and interstitial edema, fibrin accumulation, and hemorrhage [18,19]. In present study, we established a rat model of phosgene induced ALI. Using this model we demonstrated that MT and UTI could significantly reduce the severity of phosgene induced ALI. The combination of MT and UTI was better than using MT or UTI alone.
W:D lung weight ratio has been used as an important index of the severity of lung edema [20]. The increase of W:D lung-weight ratio is mainly caused by an increasing extravasation of plasma constituents into the alveolus when the capillary permeability increases. In this study, W:D lung-weight ratio and BAL fluid cell count increased after phosgene exposure and were significantly higher than those in control group. Chen et al. [21] demonstrated the greatest increase in W:D lung weight ratio and BAL fluid cell count at 6 h, consistent with our results. It can be concluded that phosgene exposure could induce ALI, and the most severe ALI is presented at 6 h post-exposure.
Next we used five different treatments to treat the rats with phosgene induced ALI and examined the severity of ALI. We found that MT and UTI exerted protective effect on ALI. Shao et al. [22] proved that p38 inhibitor SB203580 could significantly decrease the W:D lung weight ratio and neutrophil cell counts by reducing the activated expression of p38. We also found significant inhibitory effect on ALI by p38 inhibitor. In addition, we used NF-κB inhibitor PDTC to treat the rats and found that it reduced the severity of ALI. According to previous studies [23,24], the inhibition of NF-κB could be considered as a main mechanism in phosgene induced ALI. However, no direct evidence has shown the results on application of NF-κB inhibitor in the treatment of phosgene-induced ALI.
Furthermore, we found that MT could significantly reduce the severity of phosgene induced ALI and the concentration of inflammatory cytokines. In addition, we found that MT could significantly increase the expression of β-catenin. We also combined MT with UTI and found the maximal effect on alleviating the severity of ALI. The combination effect of inflammatory cytokine regulation and Wnt pathway inhibition may account for the results. Interestingly, GSK-3β could regulate NF-κB signaling pathway through post-transcriptional regulation of NF-κB complex [25].
In conclusion, our data suggest that MT might be used as a therapeutic adjuvant for phosgene-induced ALI. The combination of MT and UTI can serve as a better choice in phosgene ALI treatment. However, the application of MT and UTI in the clinical practice should be further tested.
Acknowledgements
The authors thank the Laboratory Center of Jinshan Hospital for excellent technology support. This work was supported by grant for National Youth Nature Science Foundation of China (No. 81101412) and Phase II Outstanding Young Medical Personnel Training Fund of Jinshan District Health Systems, Shanghai, China (No. JWKJ-RCYQ-201207).
Disclosure of conflict of interest
None.
References
- 1.Pauluhn J, Hai CX. Attempts to counteract phosgene-induced acute lung injury by instant high-dose aerosol exposure to hexamethylenetetramine, cysteine or glutathione. Inhal Toxicol. 2011;23:58–64. doi: 10.3109/08958378.2010.541951. [DOI] [PubMed] [Google Scholar]
- 2.Sciuto AM. Assessment of early acute lung injury in rodents exposed to phosgene. Arch Toxicol. 1998;72:283–288. doi: 10.1007/s002040050503. [DOI] [PubMed] [Google Scholar]
- 3.Sciuto AM, Stotts RR, Hurt HH. Efficacy of ibuprofen and pentoxifylline in the treatment of phosgene-induced acute lung injury. J Appl Toxicol. 1996;16:381–384. doi: 10.1002/(SICI)1099-1263(199609)16:5<381::AID-JAT355>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H, Miyazaki J. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–1160. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Yoshino S, Sato H, Yoshikawa T. Protective role of urinary trypsin inhibitor in acute lung injury induced by lipopolysaccharide. Exp Biol Med (Maywood) 2005;230:281–287. doi: 10.1177/153537020523000408. [DOI] [PubMed] [Google Scholar]
- 6.Inoue K, Takano H. Urinary trypsin inhibitor as a therapeutic option for endotoxin-related inflammatory disorders. Expert Opin Investig Drugs. 2010;19:513–520. doi: 10.1517/13543781003649533. [DOI] [PubMed] [Google Scholar]
- 7.Chen HM, Huang HS, Ruan L, He YB, Li XJ. Ulinastatin attenuates cerebral ischemia-reperfusion injury in rats. Int J Clin Exp Med. 2014;7:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YJ, Li M, Meng M, Feng M, Qin CY. The effect of ulinastatin on the small intestine injury and mast cell degranulation in a rat model of sepsis induced by CLP. Exp Toxicol Pathol. 2009;61:481–490. doi: 10.1016/j.etp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Feng M, Shu Y, Yang Y, Zheng X, Li R, Wang Y, Dai Y, Qiu W, Lu Z, Hu X. Ulinastatin attenuates experimental autoimmune encephalomyelitis by enhancing anti-inflammatory responses. Neurochem Int. 2014;64:64–72. doi: 10.1016/j.neuint.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Shen J, Gan Z, Zhao J, Zhang L, Xu G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol Ind Health. 2012;30:785–793. doi: 10.1177/0748233712463776. [DOI] [PubMed] [Google Scholar]
- 11.Piesiewicz A, Kedzierska U, Podobas E, Adamska I, Zuzewicz K, Majewski PM. Seasondependent postembryonic maturation of the diurnal rhythm of melatonin biosynthesis in the chicken pineal gland. Chronobiol Int. 2012;29:1227–1238. doi: 10.3109/07420528.2012.719964. [DOI] [PubMed] [Google Scholar]
- 12.Barrell GK, Montgomery GW. Absence of circadian patterns of secretion of melatonin or cortisol in Weddell seals under continuous natural daylight. J Endocrinol. 1989;122:445–449. doi: 10.1677/joe.0.1220445. [DOI] [PubMed] [Google Scholar]
- 13.Crowley SJ, Eastman CI. Melatonin in the afternoons of a gradually advancing sleep schedule enhances the circadian rhythm phase advance. Psychopharmacology (Berl) 2013;225:825–837. doi: 10.1007/s00213-012-2869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendez N, Abarzua-Catalan L, Vilches N, Galdames HA, Spichiger C, Richter HG, Valenzuela GJ, Seron-Ferre M, Torres-Farfan C. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS One. 2012;7:e42713. doi: 10.1371/journal.pone.0042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmadiasl N, Banaei S, Alihemmati A, Baradaran B, Azimian E. The anti-inflammatory effect of erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Adv Pharm Bull. 2014;4:49–54. doi: 10.5681/apb.2014.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian YF, Lin CH, Hsu SF, Lin MT. Melatonin improves outcomes of heatstroke in mice by reducing brain inflammation and oxidative damage and multiple organ dysfunction. Mediators Inflamm. 2013;2013:349280. doi: 10.1155/2013/349280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD, Yu X, Kumbar SG. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharm Des. 2013;19:3420–3428. doi: 10.2174/1381612811319190008. [DOI] [PubMed] [Google Scholar]
- 18.Duniho SM, Martin J, Forster JS, Cascio MB, Moran TS, Carpin LB, Sciuto AM. Acute changes in lung histopathology and bronchoalveolar lavage parameters in mice exposed to the choking agent gas phosgene. Toxicol Pathol. 2002;30:339–349. doi: 10.1080/01926230252929918. [DOI] [PubMed] [Google Scholar]
- 19.Pauluhn J, Carson A, Costa DL, Gordon T, Kodavanti U, Last JA, Matthay MA, Pinkerton KE, Sciuto AM. Workshop summary: phosgene-induced pulmonary toxicity revisited: appraisal of early and late markers of pulmonary injury from animal models with emphasis on human significance. Inhal Toxicol. 2007;19:789–810. doi: 10.1080/08958370701479133. [DOI] [PubMed] [Google Scholar]
- 20.Sciuto AM. Posttreatment with ETYA protects against phosgene-induced lung injury by amplifying the glutathione to lipid peroxidation ratio. Inhal Toxicol. 2000;12:347–356. doi: 10.1080/089583700196194. [DOI] [PubMed] [Google Scholar]
- 21.Chen HL, Hai CX, Liang X, Zhang XD, Liu R, Qin XJ. Correlation between sPLA2-IIA and phosgene-induced rat acute lung injury. Inhal Toxicol. 2009;21:374–380. doi: 10.1080/08958370802449712. [DOI] [PubMed] [Google Scholar]
- 22.Shao YR, Shen J, Li W, Yuan Z, He DK. Effects of phosphorylated mitogen-activated protein kinases on phosgene inhalation-induced lung injury in rats and its relationship with matrix metalloproteinase. Zhonghua Shao Shang Za Zhi. 2013;29:261–266. [PubMed] [Google Scholar]
- 23.He DK, Shao YR, Zhang L, Shen J, Zhong ZY, Wang J, Xu G. Adenovirus-delivered angiopoietin-1 suppresses NF-κB and p38 MAPK and attenuates inflammatory responses in phosgene-induced acute lung injury. Inhal Toxicol. 2014;26:185–192. doi: 10.3109/08958378.2013.872213. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Ye XL, Liu R, Chen HL, Liang X, Li WL, Zhang XD, Qin XJ, Bai H, Zhang W, Wang X, Hai CX. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp Toxicol Pathol. 2013;65:311–318. doi: 10.1016/j.etp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]


