Abstract
Interactions between angiotensin-converting enzyme-2 (ACE2) gene polymorphisms and high salt intake increase the risk of hypertension (HTN); however, this association is not well-established in the Chinese Wa population. In this study, we investigated the prevalence and associated factors of HTN in the Chinese Wa ethnic minority in Yunnan Province, China. In addition, we assessed the associations of single nucleotide polymorphisms (SNPs) in ACE2 with blood pressure and environmental factors. Among a total of 838 Wa individuals, the overall prevalence, awareness, treatment and control rates of HTN were 31.03%, 32.81%, 10.77%, and 0.70%, respectively. In addition, 260 hypertensive patients and 290 normotensive individuals were randomly selected for investigations of salt intake and ACE2 SNPs. The levels of e24-h salt intake in female hypertensive patients were significantly higher that those in normotensive individuals. The ACE2 rs2285666 T allele or TT genotype and rs714205 G allele or GG genotype were identified as risk factors for the development of HTN in female Wa individuals. The CGTG haplotype was a risk factor in hypertensive patients. Moreover, high salt intake increased the occurrence of hypertension among ACE2 rs2285666 TT and rs714205 GG individuals. In this study, we not only identified an association between ACE2 gene polymorphism and HTN in the Chinese Wa population, but also a possible link interaction between ACE2 polymorphism type and high salt intake in increasing the risk of HTN in this population.
Keywords: Hypertension, genetic polymorphisms, angiotensin-converting enzyme-2, salt sensitivity, sodium
Introduction
Hypertension (HTN) is a major contributor to cardiovascular disease and death, and is becoming increasingly widespread in China. The precise etiology of HTN remains poorly understood; however, evidence suggests that both genetic and environmental factors contribute to susceptibility to HTN [1,2]. To date, hundreds of hypertension related gene, (Such as the rennin angiotensin aldosterone system, RAAS). The angiotensin-converting enzyme-2 (ACE2) gene has been identified by various groups as a candidate locus for HTN suseptibility [3,4].
With 26 ethnic groups in total, Yunnan has the highest number of ethnic groups among all the Chinese Provinces. All 26 ethnic groups other than the Han are minorities, of which 15 are native. As a typical southern Chinese population, the Wa ethnic minority (>347,000) is a native group living in the southwest of Yunnan Province. At an elevation of 1,300 meters, this remote geographical location renders the Wa minority group unique as an isolated population that is genetically homogeneous due to centuries of a relatively low rate of migration and intermarriage. Consequently, the Wa represent an ideal group for population genetic polymorphism studies.
ACE2 plays a key role as the central negative regulator of the renin-angiotensin system (RAS). Activation of ACE2 has been shown to attenuate the devastating effects of Ang II in the cardiovascular system by reducing Ang II degradation and increasing Ang-(1-7) generation leading to Mas receptor activation [5]. However the few studies that have examined this association have yielded mixed results. In this study, we investigated the prevalence and associated factors of HTN in the Chinese Wa population in Yunnan Province, and assessed the association between single nucleotide polymorphisms (SNPs) in the ACE2 gene with blood pressure and environmental factors in this unique ethnic group.
Materials and methods
Study subjects
Participants were recruited through random sampling of individuals in Si Pai Shan village, which is located in Gengma Autonomous County and inhabited predominantly by the Wa ethnic group. A total of 838 individuals of Wa ethnicity were screened using a questionnaire designed to assess basic information and cardiovascular risk factors. Baseline investigations regarding demographics, educational level, medical history, intake of salt with any sort of food, diet (vegetarian/non-vegetarian), tobacco/alcohol habits and medication were recorded. Height, weight, waist, and hip circumference were measured. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Blood pressure was measured on the right arm using a sphygmomanometer with the subject in a sitting position after resting in the seated position for 10 min.
A total of 260 hypertensive patients and 290 normotensive individuals (controls) were enrolled for investigation based on the inclusion/exclusion criteria. Diagnostic criteria of hypertension were accordance with World Health Organization/International Society of Hypertension (WHO/ISH) hypertension guidelines (systolic blood pressure (SBP) ≥140 mmHg, and/or diastolic blood pressure (DBP) ≥90 mmHg) or current anti-hypertensive medication. The exclusion criteria were as follows: individuals with secondary hypertension (hypertension due to secondary causes such as renovascular disease, renal failure, pheochromocytoma, aldosteronism or other causes of secondary hypertension) and white coat hypertension. A control group with no history of HTN was recruited from the same geographic area and was matched for age and sex with the HTN patients. Written informed consent was obtained from all patients and controls.
This study was approved by the Institutional Review Boards of Yan’an Affiliated Hospital of Kunming Medical University (China).
Blood samples
At least 2 ml of venous blood was collected aseptically from each individual after overnight fasting and stored in EDTA vials. Blood was transferred to the laboratory under -20°C conditions. From 1 ml of blood, plasma was separated within 3 h by centrifugation at 3,000 rpm for 3 min and stored at -20°C prior to biochemical analysis.
Data collection and laboratory measurements
Total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides (TG), fasting blood glucose (FBG), serum sodium (Na), serum potassium (K) and the indicators of kidney function status (urea and creatinine) were analyzed using an automatic analyzer (Cobas Integra 400, Roche Diagnostics, Germany) with commercially available reagent kits supplied by the same company. Spot voiding urinary samples were also taken to measure the urinary concentration of sodium, potassium, creatinine and microalbuminuria. All measurements were determined using standard laboratory procedures at SRL, Inc. (Tokyo, Japan). The estimated daily salt intake was calculated according to a previously published published formula [6] as follows: PRCr (mg/day) = -2.04 × age + 14.89 × weight (kg) + 16.14 × height (cm)-2244.45; SUNa = Na concentration in the spot voiding urine (mEq/L); SUCr = creatinine concentration in the spot voiding urine (mg/dL); XNa = SUNa/SUCr × PRCr; 24HuNaV = Estimated population mean levels of 24-h urinary sodium excretion (mEq/day); 24HuNaV (mEq/day) = 21.98 × XNa0.392; Estimated daily salt intake (g/day) = 24 HuNaV (mEq/day) × 0.0585.
SNP selection and genotyping
We selected 20 ACE2 SNPs (rs1514283, rs1514282, rs2074192, rs233575, rs714205, rs4240157, rs4646176, rs4646174, rs879922, rs4646156, rs4646155, rs4646188, rs4646140, rs2158083, rs2285666, rs2106809, rs1978124, rs6632677, rs2301692, and rs2306193) for investigation. All of these SNPs had minor allele frequencies (MAFs) of >5% in the Asian Population HapMap and had previously been reported to be associated with hypertension. Genomic DNA was extracted from peripheral blood samples using the Axyprep Blood Genomic DNA Miniprep Kit (Axygen, USA), and the DU530 UV/VIS spectrophotometer (Beckman Instruments, Fullerton, CA, USA) was used to determine DNA concentration according to the manufacturer’s protocol. MassARRAY Assay Design 3.0 Software (Sequenom, San Diego, CA, USA) was used to design Multiplex SNP MassEXTEND assays. Genotyping was performed using the Sequenom MassARRAY RS1000 following a standard protocol recommended by the manufacturer [7], and data were analyzed using Sequenom TYPER 4.0 Software (Sequenom, San Diego, CA, USA) [8]. For all tested samples, a 5% random sample was reciprocally tested, and the reproducibility was 100%.
Statistical analysis
The ACE2 gene maps to the X chromosome; therefore, male and female patient groups were analyzed separately. Hardy-Weinberg equilibrium was tested in the female population. Unpaired t-tests, chi-squared tests or Fisher’s exact test were used to compare the characteristics of the two groups and to compare the characteristics according to different genotypes. Alleles in females were compared by one-way ANOVA. Odds ratios (OR) were calculated with 95% confidence intervals (95% CI). Multivariate logistic regression models were used to estimate the associations between SNPs and hypertension after adjustment for age, BMI, FBG, TG, TC, LDL-C, e24-h salt intake, protein-creatinine ratio and waist-to-hip ratio. In addition, haplotype constructions and Mendel linkage disequilibrium were analyzed using the online computer platform SHEsis and Haploview software (http://analysis.bio-x.cn/myAnalysis. php). Haplotypes with frequencies >3% in the combined cases and controls were examined. All statistical analysis was performed using SPSS 17.0 software (SPSS INC., IL, USA). P<0.05 was considered to indicate statistical significance.
Results
Prevalence of hypertension
In total, 838 individuals of Wa ethnicity (421 females and 417 males) were screened by our team. The mean age of the group was 48.62±12.86 years, with SBP readings of (134.65±12.43) mmHg and significantly lower DBP readings of (82.68±9.94) mmHg. The overall prevalence of hypertension among the Wa ethnic group aged ≥18 in Yunnan Province was 31.03% (260/838). The rate of awareness of hypertension was 32.81% (275/260), while only 10.77% people received treatment. However, among the individuals who received medication, blood pressure control was achieved in only 0.70%. Approximately 50.35% of the group were illiterate, with those whose highest level of education was primary school, junior high school, senior high school accounting for 34.25%, 11.69%, and 3.70%, respectively. Almost 45.94% of the subjects were smokers, and 35.92% reported drinking alcohol (Table 1). Because of the predominance of women in the group and some significant differences between men and women found across several socioeconomic and demographic variables, the following analyses were sex-stratified.
Table 1.
Demographic and clinical characteristics of the study sample (n = 838)
| Variable | Summary statistics |
|---|---|
| Age (years) | 48.62±12.86 |
| Prevalence of hypertension (%) | 31.03% |
| Awareness of hypertension (%) | 275 (32.81%) |
| Treatment rates of hypertension (%) | 90 (10.77%) |
| Control rates of hypertension (%) | 6 (0.70%) |
| Smoking (current) | 385 (45.94%) |
| Drinking (≥1 glass/day) | 301 (35.92%) |
| Education level: No school | 422 (50.35%) |
| Primary school | 287 (34.25%) |
| Junior high school | 98 (11.69%) |
| Senior high school | 31 (3.70%) |
| BMI (kg/m2) | 23.48±3.15 |
| SBP (mmHg) | 134.65±12.43 |
| DBP (mmHg) | 82.68±9.94 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Baseline characteristics of the study population
A total 260 hypertensive patients and 290 normotensive individuals (controls) were enrolled for salt intake investigations and SNP analysis. The clinical characteristics of the patients and controls are listed in Table 2. For both sexes, there were no significant differences between hypertensive patients and normotensive controls in terms of TC, LDL-C, serum K, urinary Na, uNa/K, microalbuminuria, protein-creatinine ratio, and waist-to-hip ratio (P>0.05). The levels of e24-h salt intake, SBP and DBP, and age were significantly higher in patients than in the control group (P<0.05). Relative to normotensive controls, hypertensive patients had higher UA and TG levels for females and higher fasting glucose level for males.
Table 2.
The baseline characteristics of all study participants
| Characteristics | Females | Males | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HBP | NBP | P | HBP | NBP | P | |
|
|
|
|||||
| (n = 152) | (n = 168) | (n = 108) | (n = 122) | |||
| Age (years) | 53.47±11.61* | 44.41±12.20 | 0.000 | 50.98±14.93 | 43.21±12.86 | 0.03 |
| BMI (kg/m2) | 25.32±4.03* | 22.50±3.53 | 0.000 | 23.54±4.74 | 23.19±2.79 | 0.633 |
| SBP (mmHg) | 153.93±14.28* | 116.30±10.96 | 0.000 | 149.59±16.12* | 120.69±9.19 | 0.000 |
| DBP (mmHg) | 89.04±9.95* | 71.98±8.83 | 0.000 | 92.46±15.07* | 77.25±6.14 | 0.000 |
| Serum Cr (umol/L) | 57.58±12.25 | 59.62±21.35 | 0.472 | 72.45±15.77* | 78.42±15.81 | 0.048 |
| UA (umol/L) | 291.26±70.03* | 247.93±51.91 | 0.000 | 375.06±90.69 | 349.69±72.75 | 0.104 |
| FBG (mmol/L) | 5.14±1.11 | 4.83±1.12 | 0.082 | 5.33±1.51* | 4.75±0.45 | 0.009 |
| TC (mmol/L) | 5.10±1.20 | 5.03±0.94 | 0.702 | 5.37±1.18 | 5.14±0.89 | 0.251 |
| TG (mmol/L) | 2.34±1.49* | 1.61±0.84 | 0.000 | 2.47±2.43 | 1.82±1.36 | 0.09 |
| LDL-C (mmol/L) | 3.08±0.82 | 3.09±0.80 | 0.954 | 3.17±0.79 | 3.24±0.78 | 0.663 |
| Serum Na (mmol/L) | 137.81±2.02* | 136.95±2.21 | 0.012 | 137.92±2.33 | 137.76±1.71 | 0.691 |
| Serum K (mmol/L) | 4.88±0.57 | 4.91±0.59 | 0.790 | 5.17±0.61 | 4.97±0.08 | 0.075 |
| Urinary sodium (mmol/L) | 141.93±53.18 | 158.90±51.96 | 0.129 | 154.66±51.68 | 136.98±54.76 | 0.159 |
| Urinary potassium (mmol/L) | 34.62±12.17* | 44.67±16.51 | 0.002 | 44.11±21.70 | 40.14±15.18 | 0.344 |
| e24-h Salt intake, g per day | 18.97±5.67* | 15.47±3.65 | 0.000 | 17.14±3.17* | 14.34±4.03 | 0.030 |
| urine Na/K | 4.49±2.00 | 4.09±2.21 | 0.368 | 4.26±2.63 | 3.77±1.71 | 0.322 |
| Microalbuminuria mg/L | 36.07±47.25 | 33.00±58.34 | 0.810 | 67.55±89.34 | 40.40±57.95 | 0.156 |
| Urine creatinine (umol/L) | 8986.42±755.77 | 10700.93±784.53 | 0.068 | 10597±3604.91* | 12717.00±3687.56 | 0.018 |
| Urinary albumin/creatinine ratio (mg/mmol) | 36.87±8.51 | 36.91±6.91 | 0.242 | 63.94±98.86 | 30.76±54.46 | 0.108 |
| Waist-to-hip ratio | 0.89±0.07 | 0.87±0.181 | 0.312 | 0.92±0.31 | 0.86±0.05 | 0.186 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; Cr, creatinine; UA: Uric acid; FBG: fasting blood glucose; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein cholesterol; e24-h salt intake: estimated daily 24 h salt intake.
P values were computed by unpaired t-test or Mann-Whitney U-test for quantitative variables.
SNP analysis
We detected five SNPs (rs2074192, rs2106809, rs2285666, rs6632677, rs714205) of the ACE2 gene in this Chinese Wa population. The genotype distributions and allele frequencies of the five examined polymorphisms are presented in Table 3. The gene encoding ACE2 is assigned on the X chromosome; therefore, Hardy-Weinberg equilibrium testing was possible in the female population and the observed frequencies were in accordance with the expected frequencies for each polymorphism (P>0.05). As shown in Table 3, analyses of single SNPs indicated no differences in genotypes/allele frequencies of ACE2 rs2106809, rs2074192, and rs6632677 between hypertensive patients and controls, in either men or women. Although the frequency of the ACE2 rs2285666 T allele or TT genotype was significantly higher in female hypertensive patients than in female controls (Table 3), the difference was still statistically significant (P<0.05); however, this difference was not found in men. Analysis of the at-risk ACE2 rs2285666 revealed a significant association between the the T allele and TT genotype with hypertension (OR = 1.521, 95% CI: 1.113-2.078, P = 0.008; OR = 1.623, 95% CI: 1.021-2.741, P = 0.049). Logistic regression analysis revealed that the ACE2 rs2285666 T allele and TT genotype were hypertensive risk factors in females (OR = 1.48, 95% CI: 1.05-2.241, P = 0.013; OR = 1.534, 95% CI: 1.001-2.54, P = 0.031). Although the frequency of the ACE2 rs714205 G allele and GG genotype were significantly higher in female hypertensive patients than in female controls, the difference was still statistically significant (P<0.05); however, this difference was not found in men. Logistic regression analysis revealed that the ACE2 rs714205 G allele and GG genotype were hypertensive risk factors in females (OR = 1.499, 95% CI: 1.088-2.231, P = 0.005; OR = 1.342, 95% CI: 1.001-2.59, P = 0.048).
Table 3.
Genotype distributions and allele frequencies of five ACE2 gene polymorphisms in hypertensive patients and normotensive controls according to sex
| SNPs | Gender | Genotype/allele | Frequency | P * | OR; 95% CI; P# | Logistic regression analysis OR; 95% CI; P ★ | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Hyp | Con. | ||||||
| rs2074192 | Male | CT | 0 | 0 | |||
| TT | 52 | 54 | |||||
| CC | 56 | 68 | |||||
| C | 112 | 136 | 0.404 | 0.855; (0.592-1.235); 0.404 | 0.765; (0.651-1.34); 0.32 | ||
| T | 104 | 108 | 1.169; (0.810-1.689); 0.404 | 1.56; (0.754-1.701); 0.563 | |||
| Female | CT | 80 | 74 | 0.289 | 1.411; (0.908-2.193); 0.125 | 1.233; (0.867-2.33); 0.41 | |
| TT | 28 | 34 | 0.890; (0.510-1.553); 0.681 | 0.762; (0.467-1.493); 0.79 | |||
| CC | 44 | 60 | 0.733; (0.458-1.175); 0.197 | 0.601; (0.456-1.089); 0.34 | |||
| C | 88 | 194 | 0.492 | 1.150; (0.772-1.714); 0.492 | 0.97; (0.742-1.654); 0.32 | ||
| T | 56 | 142 | 0.492 | 0.869; (0.583-1.296); 0.492 | 0.67; (0.521-1.378); 0.51 | ||
| rs2106809 | Male | GG | 56 | 60 | - | ||
| GA | 0 | 0 | |||||
| AA | 52 | 62 | |||||
| A | 104 | 124 | 0.56 | 0.89; (0.62-1.29); 0.56 | 0.82; (0.56-1.31); 0.42 | ||
| G | 112 | 120 | 1.11; (0.77-1.60); 0.56 | 0.99; (0.68-1.56); 0.46 | |||
| Female | GG | 42 | 56 | 0.59 | 0.79; (0.49-1.28); 0.34 | 0.75; (0.51-1.34); 0.48 | |
| GA | 78 | 80 | 1.226; (0.79-1.91); 0.37 | 1.02; (0.76-1.34); 0.40 | |||
| AA | 28 | 32 | 0.992; (0.56-1.74); 0.97 | 0.85; (0.64-1.43); 0.52 | |||
| A | 134 | 144 | 0.49 | 1.12; (0.882-1.53); 0.49 | 0.98; (0.75-1.12); 0.34 | ||
| G | 160 | 192 | 0.87; (0.653-1.227); 0.49 | 0.71; (0.75-1.37); 0.32 | |||
| rs2285666 | Male | TT | 60 | 64 | |||
| TC | 0 | 0 | |||||
| CC | 48 | 58 | |||||
| T | 120 | 128 | 0.506 | 1.133; (0.784-1.636); 0.506 | 0.943; (0.68-1.56); 0.45 | ||
| C | 96 | 116 | 0.506 | 0.883; (0.611-1.275); 0.506 | 0.765; (0.587-1.215);0.456 | ||
| Female | TT | 42 | 32 | 0.028 | 1.623; (1.021-2.741); 0.049 | 1.534; (1.001-2.54); 0.031 | |
| TC | 78 | 80 | 1.159; (0.747-1.799); 0.509 | 1.062; (0.756-1.65); 0.601 | |||
| CC | 32 | 56 | 0.533; (0.322-0.884); 0.014 | 0.523; (0.28-0.79); 0.022 | |||
| T | 162 | 144 | 0.008 | 1.521; (1.113-2.078); 0.008 | 1.48; (1.05-2.241); 0.013 | ||
| C | 142 | 192 | 0.657; (0.481-0.898); 0.008 | 0.56; (0.399-0.79); 0.007 | |||
| rs6632677 | Male | GG | 106 | 118 | 0.687 | ||
| CG | 0 | 0 | |||||
| CC | 2 | 4 | |||||
| C | 4 | 8 | 0.338 | 0.557; (0.165-1.875); 0.338 | 0.543;(0.243-1.771); 0.433 | ||
| G | 212 | 236 | 0.338 | 1.797; (0.533-6.052); 0.338 | 1.652; (0.481-5.478); 0.432 | ||
| Females | GG | 142 | 158 | 0.320 | 0.899; (0.363-2.222); 0.817 | 0.844; (0.352-2.38); 0.727 | |
| CG | 10 | 8 | 1.408; (0.541-3.667); 0.481 | 1.34; (0.78-2.74); 0.39 | |||
| CC | 0 | 2 | 1.012; (0.995-1.029); 0.177 | 1.08; (0.877-1.432); 0.24 | |||
| C | 10 | 12 | 0.038 | 0.918; (0.391-2.157); 0.038 | 0.765; (0.45-1.891); 0.04 | ||
| G | 294 | 324 | 1.089; (0.464-2.558); 0.038 | 0.98; (0.77-2.34); 0.052 | |||
| rs714205 | Males | GC | 0 | 0 | |||
| GG | 60 | 68 | |||||
| CC | 48 | 54 | |||||
| C | 96 | 108 | 0.969 | 1.007; (0.697-1.456); 0.969 | 0.94; (0.543-1.526); 0.85 | ||
| G | 120 | 136 | 0.969 | 0.993; (0.687-1.435); 0.969 | 0.901; (0.877-1.62); 0.67 | ||
| Females | GC | 76 | 76 | 0.016 | 1.211; (0.780-1.879); 0.394 | 1.09; (0.69-1.92); 0.453 | |
| GG | 46 | 36 | 1.591; (1.020-2.638); 0.041 | 1.342; (1.001-2.59); 0.048 | |||
| CC | 30 | 56 | 0.492; (0.295-0.821); 0.006 | 0.43; (0.31-0.93); 0.008 | |||
| C | 136 | 188 | 0.005 | 0.637; (0.466-0.871); 0.005 | 0.599; (0.412-0.85); 0.007 | ||
| G | 168 | 148 | 1.569; (1.148-2.144); 0.005 | 1.499; (1.088-2.231); 0.005 | |||
Note: Females Hardy-Weinberg. P>0.05.
P was calculated by χ2 test contingency table with the corresponding degree of freedom.
No adjustment for age, BMI, glucose, TG, TC, LDL-C, e24-h salt intake, protein-creatinine ratio, waist-to-hip ratio.
Adjustment for age, BMI, glucose, TG, TC, LDL-C, e24-h salt intake, protein-creatinine ratio, waist-to-hip ratio.
Haplotype and linkage disequilibrium analysis
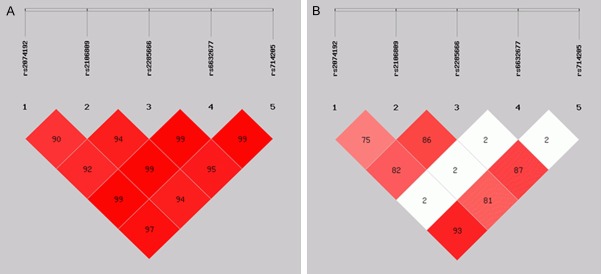
Linkage disequilibrium of the five ACE2 SNPs and haplotype frequencies were analyzed using the SHEsis software. The linkage disequilibrium map was constructed according to the distribution of the rs2074192, rs2106809, rs2285666, rs6632677, and rs714205 sites. We found that rs2074192, rs2106809 and rs2285666 as well as rs714205 were linked (D’>0.9 and r2>0.7) (Table 4). All haplotypes with a frequency <0.03 were excluded from our analysis. According to the analysis, eight haplotypes of four SNPs were identified. One of the haplotypes, C G T G, was associated with significantly increased risk in hypertensive patients (P = 0.043, OR = 1.283; 95% CI: 1.046-1.387) (Figure 1).
Table 4.
Haplotype analysis of rs2074192, rs2106809, rs2285666, and rs714205 single nucleotide polymorphism sites
| Haplotype | HBP (frequency) | NBP (frequency) | P | OR, 95% CI |
|---|---|---|---|---|
| C G T G | 234.00 (0.457) | 231.73 (0.400) | 0.043 | 1.283 [1.046-1.387] |
| T A C C | 254.00 (0.496) | 295.79 (0.510) | 0.528 | 0.923 [0.721-1.183] |
| C A C C | 0.00 (0.000) | 6.00 (0.010) | / | / |
| C A C G | 10.00 (0.020) | 12.05 (0.021) | / | / |
| C A T G | 0.00 (0.000) | 16.16 (0.028) | / | / |
| T A T C | 4.00 (0.008) | 4.05 (0.007) | / | |
| T G C C | 0.00 (0.000) | 14.21 (0.025) | / | |
| T G T G | 10.00 (0.020) | 0.00 (0.000) | / |
Figure 1.
Linkage disequilibrium map of five ACE2 SNPs (rs2074192, rs2106809, rs2285666, rs6632677, and rs714205). (A: D’ values and B: r2 values of the five SNPs).
Statistical analysis of the interaction between ACE2 variants and high salt intake
The influence of the interaction between ACE2 variants and high salt intake [9] (e24-h salt intake ≥11 g) on the risk of hypertension in females was evaluated by logistic regression (Table 5). (P<0.000), and the OR value of patients with both a history of high salt intake and the rs714205 GG genotype was 3.055 (P<0.000). In contrast, the OR values of individuals with a history of high salt intake or the rs714205 GG genotype alone were 3.972 and 1.270, respectively. These findings suggested that high salt intake increased the hypertensive OR for individuals with the rs2285666 TT and rs714205 GG genotypes.
Table 5.
Statistical analysis of the interaction between ACE2 variants and hypertension in females
| HBP | NBP | χ 2 | p | OR; 95% CI | |
|---|---|---|---|---|---|
| rs2285666 TT+/high salt intake+ | 28 | 9 | 13.319 | 0.000 | 4.012 [1.816-8.762] |
| rs2285666 TT+/high salt intake- | 14 | 23 | 1.566 | 0.225 | 0.64 [0.316-1.293] |
| rs2285666 TT-/high salt intake+ | 64 | 26 | 27.993 | 0.000 | 3.972 [2.343-6.733] |
| rs2285666 TT-/high salt intake- | 46 | 110 | 39.604 | 0.000 | 0.229 [0.143-0.336] |
| rs714205 GG+/high salt intake+ | 31 | 13 | 10.779 | 0.001 | 3.055 [1.532-6.089] |
| rs714205 GG+/high salt intake- | 15 | 23 | 1.114 | 0.305 | 0.690 [0.346-1.378] |
| rs714205 GG-/high salt intake+ | 60 | 57 | 1.058 | 0.353 | 1.270 [0.805-2.003] |
| rs714205 GG-/high salt intake- | 46 | 75 | 7.017 | 0.011 | 0.538 [0.339-0.853] |
Discussion
In this study, we found an overall prevalence of 31.03% for hypertension among the Wa ethnic group aged ≥18 years in Yunnan Province, which was higher than that reported (29.6%) in a national survey of Chinese adults aged ≥18 years in 2014 [10]. However, the rate of awareness, treatment and control of hypertension were 32.81%, 10.77% and 0.70%, respectively, in the Wa ethnic group.
A hypertension survey conducted between 2001 and 2003 in a European population aged between 26 and 65 years indicated that the prevalence of hypertension was 24.4% [11]. Wa people have a unique ethnic origin, environment, and customs. Most live in Gengma, which has a mild climate and fertile land. The Wa people are mainly engaged in agriculture, which accounts for the low incidence of overweight and obesity among this population. However, the prevalence of hypertension in the Wa group was found to be higher than that reported in other areas of China. This could be accounted for by a number of factors. Wa people report a preference for preserved food, the high salt intake of which may contribute to the progression of hypertension. In addition, Wa people have high rates of smoking and drinking, which influence sympathetic stimulation in addition to the effects mediated by chronic impairment of endothelial function and increased arterial stiffness.
ACE2, encodes an 805 amino acid protein that is the first known human homolog of ACE. ACE2 is highly expressed in normal kidney, heart, and retina [12-14] and is an important regulator of the RAS through its ability to degrade angiotensin II. Evidence indicates that the ACE2-mediated diversion of the vasoconstrictor angiotensin II toward the vasodilator angiotensin-(1-7) peptides plays a protective role in the pathogenesis of hypertension. To date, few studies regarding associations of ACE2 variants and hypertension have been reported [15]. Although the ACE2 variants identified in this study were intronic rather than exonic polymorphisms, introns are important for DNA stability, suggesting that intronic mutations are as important as those in exons.
In the current study, we found that ACE2 variants (rs2285666 and rs714205) were associated with hypertension in the female Wa ethnic population. The ACE2 rs2285666 T allele/TT genotype and rs714205 G allele/GG genotype were hypertensive risk factors in females. However, the relationships between ACE2 variants and hypertension have yielded mixed results. In three Chinese studies, significant associations of women with the rs2285666 SNP and either hypertension or increased SBP or DBP were reported. In two of these studies, high blood pressure was associated with the A allele, although an association with the G allele was reported in another study. A large family-based study indicated that genetic variants in the APLNR and ACE2 genes are significantly associated with blood pressure responses to dietary sodium intervention. In another study, ACE2 SNPs (rs2285666, rs714205, and rs4646176) were reported to be significantly associated with SBP, DBP, or MAP responses to low-sodium intervention [16]. However, Fan et al. investigated orthostatic blood pressure responses in 3,630 Chinese Han subjects and found no association with the rs2285666 SNP. Moreover Fan et al. did not find an association of the rs228566 or rs879922 SNPs with hypertension, but found that the rs2106809 T allele conferred a higher risk of hypertension. Differences in the study design, including sample selection and phenotypes, may explain the inconsistencies in the results of these studies. In addition, sex and sex hormones may affect components of the RAS [17]. Moreover, functional studies of this polymorphism are required.
ACE2 is one of the components of RAS that is located on the X chromosome. Therefore, the escape of X inactivation reported for many genes may contribute to the sex-related differences in the ACE2 gene in this Chinese cohort of patients with hypertension [6,18]. In the present study, we focused on ACE2 variants in the Yunnan Wa ethnic population. None of the subjects had a history of migration history or marriage with other regional populations within three generations. A total of 20 ACE2 SNPs were detected. Two SNPs (rs2285666 and rs714205) were found to be significantly associated with hypertension in females. The ACE2 rs2285666 T allele, TT genotype and the rs714205 G allele, GG genotype implicated as risk factors for hypertension in Chinese Wa females. Linkage disequilibrium analysis revealed linkage of rs2074192, rs2106809 and rs2285666 as well as rs714205. Moreover, haplotype analysis showed an that the CGTG haplotype of ACE2 was associated with increased risk of hypertension.
Interestingly, the hypertensive patients among the Wa ethnic population in Yunnan Province had high levels of e24-h salt intake. Thus, we hypothesized that salt intake has an independent effect on hypertension. In this study, the estimated daily salt intake was estimated using the method developed by Tanaka et al. and Kawasaki, which involves estimation of 24-h urinary Na and K excretion from spot voiding urinary samples collected in medical check-ups, with no restriction on collection time [6,19]. This method can be used to estimate the population average of Na intake is employed in epidemiological studies and health education. In our study, the levels of e24-h salt intake were (18.97±5.67) g in females hypertensive patients and (17.14±3.17) g in males. These values exceed the population nutrient intake goal of 5 g of salt per day recommended by the WHO and the Food and Agriculture Organization of the United Nations. Numerous epidemiologic, evolutionary, and clinical studies have confirmed that salt intake is an important factor in elevating blood pressure in humans [20]. Alderman et al. and Cohen et al. both suggested that excessive salt reduction increased the incidence of cardiovascular diseases [21,22]. Alderman proposed that the relationship between salt intake and the risk of cardiovascular diseases is J-shaped and that salt intake at 5 to 6 g per day is associated with the lowest risk of cardiovascular diseases [23].
Blood pressure is a complex phenotype that is influenced by multiple genetic and environmental factors and their interactions. In our study, we selected candidate ACE2 genes and analyzed the interaction between ACE2 variants and dietary sodium intake. The odds ratio values of hypertensive patients with both a history of high salt intake and either the rs2285666 TT or rs714205 GG genotype were higher than those for individuals with a hsitory of high salt intake alone. These results indicate that high salt intake increases the hypertensive OR for individuals with the rs2285666 TT or rs714205 GG genotype. Evidence of abnormalities in sodium balance as a cause of hypertension and the underlying mechanisms have been obtained in numerous studies conducted in genetically hypertensive rat strains [24]. The changes in the components of the intrarenal RAS in response to alterations in salt intake have also been evaluated in several studies [23,25]. In addition, excess salt intake has functional and pathological effects on the vasculature that are independent of blood pressure.
Some limitations of the present study should be noted. First, a larger sample size is necessary for a more accurate analysis. Second, functional investigations of the association between the CGTG haplotype and hypertension are also required. Last but not least, the exact biological mechanism and other possible factors underlying the association of the ACE2 gene with hypertensive individuals remain to be clarified.
In conclusion, the present study demonstrates a correlation between the high prevalence of hypertension among the Wa ethnic group in Yunnan Province with both high salt intake and ACE2 polymorphisms. ACE2 variants (rs2285666 and rs714205) are associated with hypertension in the female Wa ethnic population. Furthermore, the interactions between high salt intake and ACE2 SNPs are implicated in increased risk of hypertension.
Acknowledgements
This work was supported by the “Special and Joint Program” of Yunnan Provincial Science and Technology Department and Kunming Medical University [grant numbers 2012FB098, 2013FZ286] and the Fund from the Bureau of Medicine, Kunming, People’s Republic of China.
Disclosure of conflict of interest
None.
References
- 1.Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, Hixson JE, Henkel RD, Sharp RM, Comuzzie AG, Vande-Berg JL, Stern MP, MacCluer JW. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 1996;94:2159–2170. doi: 10.1161/01.cir.94.9.2159. [DOI] [PubMed] [Google Scholar]
- 2.Choh AC, Czerwinski SA, Lee M, Demerath EW, Wilson AF, Towne B, Siervogel RM. Quantitative genetic analysis of blood pressure response during the cold pressor test. Am J Hypertens. 2005;18:1211–1217. doi: 10.1016/j.amjhyper.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Yang M, Zhao J, Xing L, Shi L. The association between angiotensin-converting enzyme 2 polymorphisms and essential hypertension risk: a meta-analysis involving 14,122 patients. J Renin Angiotensin Aldosterone Syst. 2015;16:1240–1244. doi: 10.1177/1470320314549221. [DOI] [PubMed] [Google Scholar]
- 4.Chen LJ, Xu R, Yu HM, Chang Q, Zhong JC. The ACE2/Apelin signaling, microRNAs, and hypertension. Int J Hypertens. 2015;2015:896861. doi: 10.1155/2015/896861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell LM, Harrap SB, Velkoska E, Patel SK. The ACE2 gene: its potential as a functional candidate for cardiovascular disease. Clin Sci (Lond) 2013;124:65–76. doi: 10.1042/CS20120269. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009 doi: 10.1002/0471142905.hg0212s60. Chapter 2: Unit 2.12. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, Wang M, Feng W, Zander T, MacConaill L, Lee JC, Nicoletti R, Hatton C, Goyette M, Girard L, Majmudar K, Ziaugra L, Wong KK, Gabriel S, Beroukhim R, Peyton M, Barretina J, Dutt A, Emery C, Greulich H, Shah K, Sasaki H, Gazdar A, Minna J, Armstrong SA, Mellinghoff IK, Hodi FS, Dranoff G, Mischel PS, Cloughesy TF, Nelson SF, Liau LM, Mertz K, Rubin MA, Moch H, Loda M, Catalona W, Fletcher J, Signoretti S, Kaye F, Anderson KC, Demetri GD, Dummer R, Wagner S, Herlyn M, Sellers WR, Meyerson M, Garraway LA. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 9.Cavka A, Jukic I, Ali M, Goslawski M, Bian JT, Wang E, Drenjancevic I, Phillips SA. Shortterm high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J Hypertens. 2016;34:676–684. doi: 10.1097/HJH.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Zhang L, Wang F, Liu L, Wang H. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens. 2014;27:1355–1361. doi: 10.1093/ajh/hpu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costanzo S, Di Castelnuovo A, Zito F, Krogh V, Siani A, Arnout J, Cappuccio FP, Miller MA, van Dongen M, de Lorgeril M, de Gaetano G, Donati MB, Iacoviello L. Prevalence, awareness, treatment and control of hypertension in healthy unrelated male-female pairs of European regions: the dietary habit profile in European communities with different risk of myocardial infarction--the impact of migration as a model of gene-environment interaction project. J Hypertens. 2008;26:2303–2311. doi: 10.1097/HJH.0b013e328311ce04. [DOI] [PubMed] [Google Scholar]
- 12.Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227. doi: 10.3389/fphys.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, Grant MB, Lewin AS, Hauswirth WW, Raizada MK, Li Q. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malard L, Kakinami L, O’Loughlin J, Roy-Gagnon MH, Labbe A, Pilote L, Hamet P, Tremblay J, Paradis G. The association between the Angiotensin-Converting Enzyme-2 gene and blood pressure in a cohort study of adolescents. BMC Med Genet. 2013;14:117. doi: 10.1186/1471-2350-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Hixson JE, Rao DC, Gu D, Jaquish CE, Rice T, Shimmin LC, Chen J, Cao J, Kelly TN, Hamm LL, He J. Genetic variants in the apelin system and blood pressure responses to dietary sodium interventions: a family-based association study. J Hypertens. 2010;28:756–763. doi: 10.1097/HJH.0b013e3283370d32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 18.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 20.Ha SK. Dietary salt intake and hypertension. Electrolyte Blood Press. 2014;12:7–18. doi: 10.5049/EBP.2014.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25:1144–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 22.Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow-up study. Am J Med. 2006;119:275, e277–214. doi: 10.1016/j.amjmed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Alderman MH. Presidential address: 21st Scientific Meeting of the International Society of Hypertension: dietary sodium and cardiovascular disease: the ‘J’-shaped relation. J Hypertens. 2007;25:903–907. doi: 10.1097/HJH.0b013e3280c14394. [DOI] [PubMed] [Google Scholar]
- 24.Greene AS, Yu ZY, Roman RJ, Cowley AW Jr. Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol. 1990;258:H508–514. doi: 10.1152/ajpheart.1990.258.2.H508. [DOI] [PubMed] [Google Scholar]
- 25.Shao W, Seth DM, Prieto MC, Kobori H, Navar LG. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am J Physiol Renal Physiol. 2013;304:F505–514. doi: 10.1152/ajprenal.00587.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]