Abstract
There are drastic changes that occur in the impaired regions after spinal cord injury (SCI), however, improvement of the detrimental pathological process after injury is limited in the mammalian adult, which is due a large part to the failure of local axons to grow. Non-muscle myosin II (NMII) has been proved having essential role in the regulation of cytoskeletal structure and genetic silencing NMII markedly accelerates axon growth in vitro. Our purpose is to explore the association between phosphorylated NMII expression and axonal regeneration after SCI in rats and determine whether gene silencing NMII can improve the locomotor function in rats with SCI. The results showed that phosphorylated NMII level was up regulated after SCI and may even play important role in inhibiting neuronal survival and axonal regeneration. After silencing NMII, the viability of neurons, proliferation of axons, synaptic connection and locomotor functional recovery were promoted significantly after SCI. Our study provides an effective way by direct regulation of neuron viability, the proliferation of axons and synaptic connection for treating SCI, which may be a novel method for repairing SCI. However, the specific signaling pathway mechanisms about the recovery require further study.
Keywords: Spinal cord injury, non-muscle myosin II, axon regeneration, neuronal survival, functional recovery
Introduction
Spinal cord injury is a neurological disorder with high disability rate, which affects thousands of individuals and brings enormous socioeconomic burden to individuals and our society [1-3]. Current treatments for SCI are as follows: high dose methylprednisolone, surgical interventions, and rehabilitative care. Nonetheless, how to successfully cure SCI is still a major conundrum.
After SCI, neurons fail to regenerate axons in the lesion site due to the limited capacity of lesioned CNS axons, the inhibitory microenvironment full of inhibitory factors, and lacking of the growth-promoting factors after injury [4-7]. Promoting axon growth after SCI seems to be an attractive strategy. Targeting inhibitory factors intrinsic to injured neurons, such as PTEN, PDE4, KLF4, SOCS3 and so on have been confirmed to promote axon growth and functional recovery in various pre-clinical models [8-11]. However, the NMII related to contusive spinal cord injury (CSCI) still need further explorations.
Previous studies demonstrated that lesions of central nervous system activates the Rho/Rock signaling pathway which inhibits axon growth and sprouting. NMII, a two-headed conventional myosin has been proved to be of vital role in regulating cytoskeletal structure and place in downstream of Rho-Rock signaling [9,10]. Vitro experiment proven that genetic silencing of NMII markedly regulates the cell morphology, function and migration [11-15].
The present study is to explore the association between NMII and axonal regeneration in rats with SCI. And whether genetic silencing of NMII could promote axon regeneration and functional recovery in vivo.
Materials and methods
Animals
Forty female adult Wistar rats aged 8 weeks and weighing 200 ± 30 g, were provided by Beijing HFK Bioscience Co., Ltd., China (license No. SCXK (Jing) 2009-0004). They were housed separately in plastic cages with a 12 h light/dark cycle and free access to food and water.
Contusive SCI
Contusive spinal cord injury was induced with the Impact Model II device. Rats were subjected to laminectomy at the T9-11 level after intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 mg). T10 level of the spinal cord at was exposed from the back side and suffered a 10 g rod impact (2.5 mm in diameter) from a height of 25 mm. Subsequently, rats were spasmed several times, and this was followed by flaccid paralysis, indicating a successful model.
After injury, penicillin was injected and the skin and muscles were sutured layer by layer. Rats were raised in a plastic cage at 22-25°C, with regular ventilation. The bedding in the rearing cages was regularly changed to keep it dry. Physiological saline was administered by subcutaneous intraperitoneal injection at a volume of 5 mL, once or twice a day to prevent fluid and electrolyte disorders. Manual evacuation of bladder were conducted twice per day to prevent intestinal obstruction and pressure ulcers until recovery of normal bladder function.
The procedures involving animals were conducted in conformity to the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China.
Construction of NMIIA lentiviral vector
Search for Coding sequence (Coding The Sequence, CDS) of NMII genes (Gene ID: 4627) on National Center for Biotechnology Information (NCBI). Three target sequence of NMIIA were designed, there were NMIIA-1 (CCTCAAGGAGCGTTACTAC), NMIIA-2 (GGGACTTGTCCCAAGTCTG) and NMIIA-3 (GGCCAAACCTGCCGAATAA) by Whitehead Institute (http://jura.wi.mit.edu/siRNAext) and siRNA Wizard v3.1 (http://sirnawizard.com/). According to the sequence of siRNA, the stem loop sequence of shRNA was designed (Table 1). Then the NMIIA lentiviral vector was constructed to inhibit NMIIA. After this, 1 μg of pEGFP-N1-NMIIA plasmid were transfected into HEK293T cells, so do the pSIREN-RetroQ-RNAi (shRNA 1-3) plasmids, respectively. After 48 h culture, the supernatant was harvested and filtered through a 0.2-μm SFCA syringe filter. Then the remains were concentrated by ultracentrifugation under 11000 rpm for 4 h at 4°C. The pellets were resuspended with 150 µl PBS. Finally, the lentivirus was frozen at -80°C. The whole structure of the three-plasmid system was showed in Figure 1.
Table 1.
shRNA oligonucleotide sequences
| Name | Top strand | Bottom strand |
|---|---|---|
| shRNA 1 | 5’-GATCCCCTCAAGGAGCGTTACTACTTCAAGAGAGTAGTAACGCTCCTTGAGGTTTTTTG-3’ | 5’-ATTCAAAAAACCTCAAGGAGCGTTACTACTCTCTTGAAGTAGTAACGCTCCTTGAGGG-3’ |
| shRNA 2 | 5’-GATCCGGGACTTGTCCCAAGTCTGTTCAAGAGACAGACTTGGGACAAGTCCCTTTTTTG-3’ | 5’-ATTCAAAAAAGGGACTTGTCCCAAGTCTGTCTCTTGAACAGACTTGGGACAAGTCCCG-3’ |
| shRNA 3 | 5’-GATCCGGCCAAACCTGCCGAATAATTCAAGAGATTATTCGGCAGGTTTGGCCTTTTTTG-3’ | 5’-AATTCAAAAAAGGCCAAACCTGCCGAATAATCTCTTGAATTATTCGGCAGGTTTGGCCG-3’ |
The underlined sequences are siRNA and italic sequences are hairpin loops.
Figure 1.
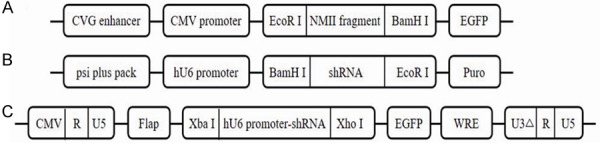
Plasmid construction. A: Structure of pEGFP-N1-NMII plasmid. B: Structure of pSIREN-RetroQ-RNAi plasmids. C: Structure of FG12-RNAi plasmids. All recombinant plasmids were validated by gene sequencing before further experiments.
Injection of slow vector virus into injured site
The site of injury was exposed as described above. Then, slow vector virus was injected into the injured site within 48 hours before the SCI. Briefly, a volume of 1 μl was injected 1.5 mm rostral and caudal to the injury site at a depth of 1.5 mm. A time lapse of 5 min was allowed after injection before removing the needle to prevent leakage of the vehicle/virus. Following injection, the muscle and skin above the exposed spinal cord was sutured.
Western blot
The concrete procedures were according to previous study with minor modification [16]. Briefly, tissue near damage area (0.5 mm) were collected and homogenized in SDS sample buffer. After separating protein samples and transferring, the membranes was incubated overnight at 4°C with primary antibodies specific for NMII (mouse monoclonal, abcam, USA), Neu (mouse monoclonal, Santa Cruz, USA), Syn (mouse monoclonal, Santa Cruz, USA), respectively. Membranes were washed three times and incubated with secondary antibody (Santa Cruz, USA), and then the bands were visualized and quantified following the manufacturer’s instructions.
Immunohistochemistry for neuron and glial scar in the spinal cord injury site
Rats were sacrificed at 8 weeks post injury and the injured spinal cords (T10, 3 cm length) were fixed in 4% (v/v) paraformaldehyde solution. Sections cut at a thickness of 5 um were prepared from paraffinembedded tissues. Dried slices were immersed in dimethylbenzene I and II for 30 minutes for deparaffinization, and washed with 1×PBS solution twice. Endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide for 5-10 minutes.
Sections were incubated overnight with NF200 and GFAP primary antibody (ectoderm, polyclonal; Boster, Wuhan, China; 1:50) at 4°C. Sections were washed two times with 1×PBS and incubated with secondary antibody (ectoderm, polyclonal; Boster) for 20 minutes at 37°C. Strept avidin-biotin complex (immunoassay kit, Boster) was added and incubated with sections at 37°C for 20 minutes, followed by four PBS washes. 3,3’-Diaminobenzidine reagent was applied for 5 minutes and the sections were then washed with mini-Q water. Sections were counterstained with hematoxylin for 2 minutes and disunited in 70% alcoholic solution mixed with 1% hydrochloric acid. The reaction was terminated using ammonia wash water. Sections were immersed in dimethylbenzene for transparency, and neutral gum was applied to seal the slices.
Functional assessment of rats with spinal cord injury
The BBB score was used to evaluate the functional motion of hind limbs. The scale ranged from a score of 0 to 21. Score 0 indicates no obvious motion of hind limb, and a gradually increasing score indicates the improved function of the hind limb; a score of 21 represents normal function in the hind limb.
Statistical analysis
All statistical analyses were performed using the statistical software SPSS13.0. Data were described as the mean ± SD. If sets of data (groups ≥3) were fitted normal distribution and constant variances, one-way ANOVA with an LSD-t (equal variance assumed) or Bonferroni was performed. If sets of data (groups =2) were compared, T test or Wilcoxon were used. The P value of less than 0.05 was considered as statistically significant.
Results
Up-regulation of phosphorylated NMII after spinal cord injury
Vitro experiment proven that genetic silencing of NMII markedly accelerates axon growth. However, NMII expression change after SCI in vivo is still not clear. We observed the total NMII level and phosphorylated NMII level after SCI in different time point. The results showed that the total NMII expression has not changed before and after SCI, but the phosphorylated NMII level was up regulated after SCI. And in the third day after SCI, phosphorylated NMII expression level reached peak (Figure 2A-C). The difference between the phosphorylated NMII groups was statistically significant by One-way ANOVA (F=4913.203, P<0.05), without no obvious difference in total NMII group (F=4913.203, P>0.05). The results suggested that although there was no significant change of total NMII expression, NMII was phosphorylated after SCI and may play a vital role in the pathological mechanism after spinal cord injury.
Figure 2.
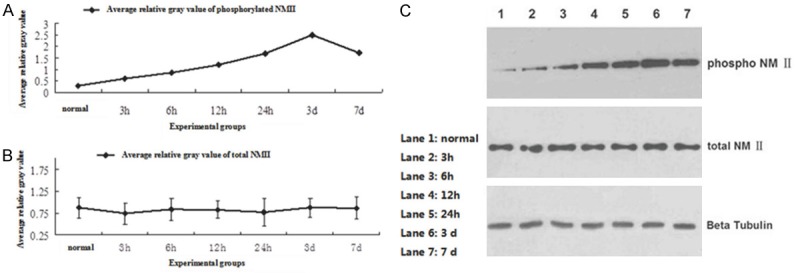
A: The phosphorylated NMII level was up regulated after SCI, and reached the peak in the third day; B: The total NMII expression was not change significantly before and after SCI; C: The western-blot result of total NMII level and phosphorylated NMII level before and after SCI in different time point (normal, 3 h, 6 h, 12 h, 24 h, 3 d, 7 d).
The relationship between phosphorylated NMII level and survival neurons after SCI
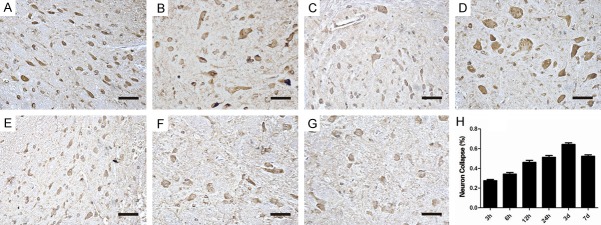
Phosphorylated NMII just expressed at low level in normal spinal cord tissue, and high expression was observed in spinal cord injured tissue. Previous studies demonstrated that NMII inhibition changes its morphology and function by reorganization of cytoskeletal structures in Embryonic DRG neurons [17]. We next investigated whether the phosphorylated NMII level had relevant to the number of survival neurons and its arrangement in vivo after spinal cord injury. The immunohistochemistry results showed that the neuron nucleus was obvious and the cells were arranged in order in normal group. However, in the injured group, cell arrangement was disorder, the cell nucleus couldn’t be seen and the morphology of neurons were stained heterogeneous. In the injured tissue after 24 h, almost most of completed neurons were collapsed (Figure 3A-G). The percentage of the neuron collapse (%) was shown in Figure 3. Neuron collapse (%) was up regulated after SCI. And in 3 d after SCI, the ratio of collapsed neuron reached peak, more than 60% (Figure 3H). Together, following elevated expression of phosphorylated NMII, the neuron collapse increased after spinal cord injury.
Figure 3.
The immunohistochemistry results of the neuron morphology and arrangement. A: Normal group: the morphology of normal neuron is complete; B-G: Injured groups (3 h, 6 h, 12 h, 24 h, 3 d, 7 d): the cell arrangement is disorder, the cell nucleus cannot be seen and the morphology of neurons are stained heterogeneous even cannot be observed. (Scale bar =2.5 um); H: Quantified neuron collapse (%) from immunohistochemistry results. Growth cone collapse (%) was up regulated after SCI (3 h, 6 h, 12 h, 24 h, 3 d, 7 d). And in 3 d after SCI, the ratio of collapsed neuron reached peak, more than 60%.
NMII inhibition promotes viability of neuron cell after spinal cord injury
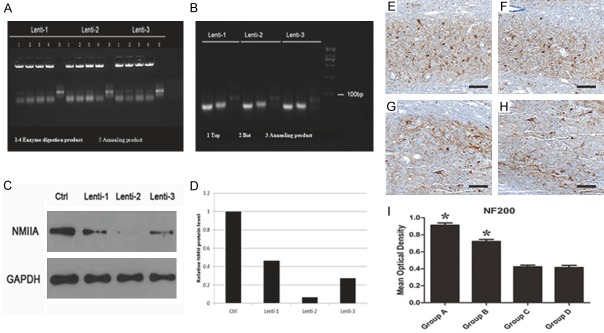
Three siRNA sequences were designed for the target gene NMII (Figure 4A, 4B). The silencing efficiency of three siRNA was 63%, 95% and 79%, respectively (Figure 4C, 4D). We choose the siRNA sequences with highest (siRNA 2) and lowest (siRNA 1) silencing efficiency as experiment groups. Two siRNA sequences were packaged with slow virus carrier which infected into spinal cord injured rats. Then the NF200 which labeled neuron was determined to access the viability of neuron cells. The results showed that the number of NF200+ cell was relatively more in NMII inhibition group, especially in Group A (Group A: siRNA 2 group; Group B: siRNA 1 group; Group C: control group; Group D: empty vector group) (Figure 3E-H). After spinal cord injury, the amount of NF200+ cells in the group A and B was significantly higher than the other two groups (Figure 3I, P<0.05). This result indicated NMII inhibition can promote neuron survival ability.
Figure 4.
(A) The results of objective fragment synthesis and identification; (B) The results of plasmid identification; (C, D) Blots of NMII proteins, comparing the efficiency of different siRNAs for silencing NMII gene, the silencing efficiency of three siRNA was 63%, 95% and 79%, respectively; (E, F) The NF200 which labeled neuron was observed, and the neuron expression level was highest in Group A (E: Group A; F: Group B; G: Group C; H: Group D) (scale bar =100 um); (I) Quantified mean optical density for NF200 (lower panel). *P<0.05.
Silencing NMII can promote the proliferation of axons and synaptic connection after SCI
Although inhibiting NMII can promote the viability of neuron cells, however, transmission of electrical signals depends on axonal regeneration and synaptic connections. Hence, proliferation of axons and synaptic connection after inhibiting NMII were observed through the specific label Neu and SYN. The results indicated the level of Neu significantly increased after silencing NMII (Figure 5A, 5C, P<0.05), however, the expression of SYN significant increased only in Group A (Figure 5B, 5D, P<0.05). Overall, the amount of axons and synaptic connections were significant increased after silencing NMII in spinal cord injured rats. But more therapeutic effect may be seen in Group A with highest (siRNA 2) silencing efficiency.
Figure 5.
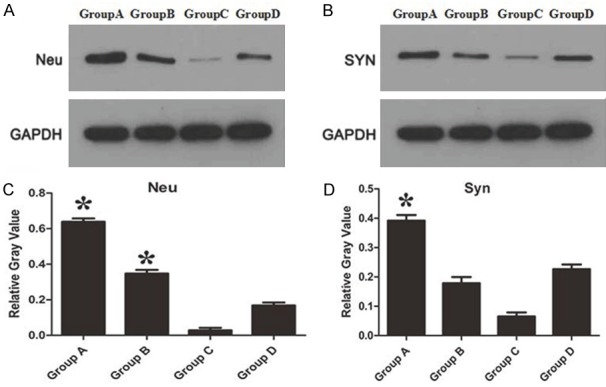
A: Comparing the expression of Neu protein which labeled axons; B: Comparing the expression of SYN protein which labeled synaptic connections; C, D: The relative gray value of Neu and SYN protein expression, respectively. *P<0.05.
Silencing NMII cannot inhibit the formation of glial scar after spinal cord injury
The GFAP which labeled glial cells was observed after inhibiting NMII in spinal cord injured rats. The results showed though GFAP+ area may seem to be rare in NMII inhibition group (Group A: siRNA 2 group; Group B: siRNA 1 group; Group C: control group; Group D: empty vector group), no significant difference of GFAP expression between four groups was seen (Figure 6A-D). Mean optical density also verified this consequence (Figure 6E, P>0.05). So the glial scar formation cannot be inhibited by silencing NMII. This also indirectly shows that the formation of glial scar is not regulated by the RhoA/Rock pathway.
Figure 6.
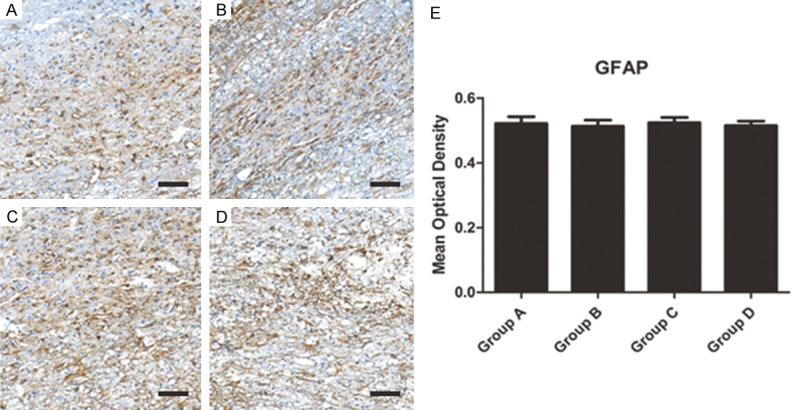
(A-D) The GFAP which labeled glial cells were observed and no significantly different of glial scar formation was observed in different groups (A: Group A; B: Group B; C: Group C; D: Group D) (scale bar =100 um). (E) Quantified mean optical density for GAFP.
Silencing NMII promote the functional recovery of locomotion after SCI
Finally, we determined whether inhibiting NMII expression can promote the functional recovery after SCI. We evaluated the functional recovery of locomotion after spinal cord injury with BBB scale. All rats showed posterior limb paralysis after SCI and the mean BBB score was 0-2 points at day 3. A gradual recovery of hind limb locomotion happened along with the following 8 weeks. The functional recovery presented a significant difference in the silencing NMII groups, which was better than control groups (Figure 7). In Group A, a significant difference compared with control group or empty vector group began to be seen from 1 week, but from 3 week in Group B (Figure 7, P<0.05). This result prompted that inhibiting NMII expression can promote locomotor function recovery after SCI and higher silencing efficiency is much more favorable.
Figure 7.
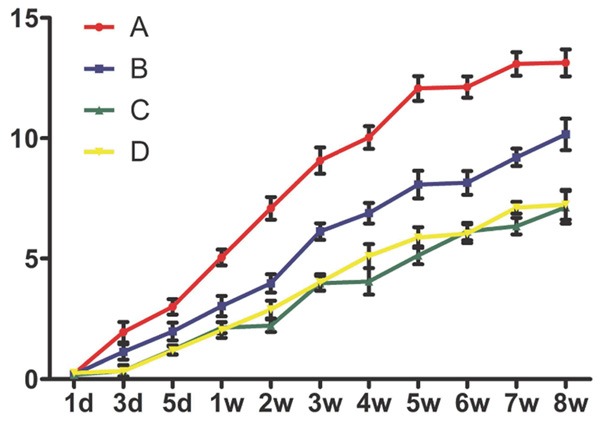
BBB scale was performed for evaluating the recovery of locomotor function after SCI. Silencing NMII promotes functional neurobehavioral recovery after SCI, Group A obtained a significantly higher BBB score in comparison to control group or empty vector group (P<0.05) at 1, 2, 3, 4, 5, 6, 7, 8 week; at 3, 4, 5, 6, 7, 8 week in Group B (P<0.05). *P<0.05.
Discussion
Eun-Mi Hur et al. [17] confirmed axon regeneration was accelerated under inhibitory microenvironment by NMII knock-out in vitro. Moreover, NMII knock-out could increase the expression of self-renewal regulators Oct3/4 Nanog, which can promote the viability of cells [18]. However, whether blockade of NMII activity can improve neurological deficits and promote functional recovery after SCI in vivo is not clear.
Current study indicated total NMII expression has not changed significantly before and after SCI in rats, but the phosphorylated NMII level was up regulated after SCI significantly. What’s more, following expression of phosphorylated NMII, the neuron collapse increased after SCI. These results proved that the high expression of phosphorylated NMII was positively related to the neuron collapse after SCI. However, the reason why neuron collapse followed phosphorylated NMII is still uncertain. Then, to investigate the role of NMII in vivo, siRNA technique was performed to silence NMII gene in spinal cord injured rats. And the results indicated NMII inhibition can promote neuron survival ability, which may relate to self-renewal regulators Oct3/4 Nanog. However, the glial scar formation cannot be inhibited by silencing NMII. We surmised that the glial scar formation may be independent of the NMII related pathway. As we all know, the best strategy to repair spinal cord injury is to accelerate long-distance axon regeneration. Then we observed the amount of axons and synaptic connections were significant increased after silencing NMII in spinal cord injured rats, especially with higher silencing efficiency. This also laid a foundation for the functional recovery of rats. What’s more, the recovery of hind limb locomotion presented a significant improvement in the silencing NMII rats, which prompted that inhibiting NMII expression can promote locomotor function recovery after SCI.
NMII plays vital roles in multiple basic biological functions, such as mitosis, migration and polarization [19,20]. Previous study proven that NMII served as a necessary component in Rho-Rock signaling [21,22], so the effect of promoting axon growth by NMII inhibition might be regulated by Rho-ROCK pathway. What’s more, the actin cytoskeleton and NMII have been confirmed to be indispensable in mediating TNF signaling, which related to regulating cell apoptosis [23-25]. Hence, NMII inhibition can promote neuron survival ability, axons regeneration and synaptic connections formation.
Conclusion
Our study confirmed that phosphorylated NMII play an important role in inhibiting neuronal survival and axonal regeneration. We provide an effective way by regulation of neuron viability and axonal regeneration for treating SCI. However, the specific signaling pathway mechanisms require further study.
Acknowledgements
We thank the support of the following funding: State Key Program of National Natural Science Foundation of China (81330042); Special Program for Sino-Russian Joint Research Sponsored by the Ministry of Science and Technology, China (2014DFR31210); International Cooperation Program of National Natural Science Foundation of China (81620108018); Key Program Sponsored by the Tianjin Science and Technology Committee, China (14ZCZDSY00044, 13RCGFSY19000); National Natural Science Foundation of China (81472070); National Science Foundation for Young Scientists of China (81501889).
Disclosure of conflict of interest
None.
References
- 1.Huber E, Curt A, Freund P. Tracking trauma-induced structural and functional changes above the level of spinal cord injury. Curr Opin Neurol. 2015;28:365–72. doi: 10.1097/WCO.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin BH, Havton LA. Basic advances and new avenues in therapy of spinal cord injury. Annu Rev Med. 2004;55:255–282. doi: 10.1146/annurev.med.55.091902.104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Mao G, Chen L, Liu A. Progress and challenges with clinical cell therapy in neurorestoratology. Journal of Neurorestoratology. 2015;3:91–95. [Google Scholar]
- 4.Young W, AlZoubi ZM, Saberi H, Sharma A, Muresanu D, Feng S, Chen L, Huang H. Beijing declaration of international association of neurorestoratology. Journal of Neurorestoratology. 2015;3:121–122. [Google Scholar]
- 5.Meves JM, Zheng B. Extrinsic inhibitors in axon sprouting and functional recovery after spinal cord injury. Neural Regen Res. 2014;9:460–1. doi: 10.4103/1673-5374.130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vadivelu S, Stewart TJ, Qu Y, Horn K, Liu S, Li Q, Silver J, McDonald JW. NG2+ progenitors derived from embryonic stem cells penetrate glial scar and promote axonal outgrowth into white matter after spinal cord injury. Stem Cells Transl Med. 2015;4:401–11. doi: 10.5966/sctm.2014-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, Sun YE, Li X. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:13354–9. doi: 10.1073/pnas.1510194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutzman JH, Sahu SU, Kwas C. Non-muscle myosin IIA and IIB differentially regulate cell shape changes during zebrafish brain morphogenesis. Dev Biol. 2015;397:103–115. doi: 10.1016/j.ydbio.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia M, Leduc C, Lagardère M, Argento A, Sibarita JB, Thoumine O. Two-tiered coupling between flowing actin and immobilized N-cadherin/catenin complexes in neuronal growth cones. Proc Natl Acad Sci U S A. 2015;112:6997–7002. doi: 10.1073/pnas.1423455112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun T, Luo J, Jia M, Li H, Li K, Fu Z. Small interfering RNA-mediated knockdown of NF-kappaBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur j pharmacol. 2012;682:79–85. doi: 10.1016/j.ejphar.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Hur EM, Yang IH, Kim DH, Byun J, Saijilafu , Xu WL, Nicovich PR, Cheong R, Levchenko A, Thakor N, Zhou FQ. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci U S A. 2011;108:5057–62. doi: 10.1073/pnas.1011258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 21.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rhoassociated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 22.Das P, Saha S, Chandra S, Das A, Dey SK, Das MR, Sen S, Sarkar DP, Jana SS. Phosphorylation of nonmuscle myosin II-A regulatory light chain resists Sendai virus fusion with host cells. Sci Rep. 2015;5:10395. doi: 10.1038/srep10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Y, Atkinson SJ, Marrs JA, Gallagher PJ. Myosin II light chain phosphorylation regulates membrane localization and apoptotic signaling of tumor necrosis factor receptor-1. J Biol Chem. 2001;276:30342–30349. doi: 10.1074/jbc.M102404200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn PG, Helfman DM. Non-muscle myosin IIB helps mediate TNF cell death signaling independent of actomyosin contractility (AMC) J Cell Biochem. 2010;110:1365–75. doi: 10.1002/jcb.22653. [DOI] [PubMed] [Google Scholar]
- 25.Gao H, Hou F, Dong R, Wang Z, Zhao C, Tang W, Wu Y. Rho-Kinase inhibitor fasudil suppresses high glucose-induced H9c2 cell apoptosis through activation of autophagy. Cardiovasc Ther. 2016;34:352–9. doi: 10.1111/1755-5922.12206. [DOI] [PubMed] [Google Scholar]