Abstract
Background: There is a strong association between severity of injury and an increased susceptibility to infection after injury. T cell dysfunctions are central to the development of infections following a trauma. Trauma-induced myeloid-derived suppressor cells (MDSCs) can suppress T cell functions, and the process is associated with poor clinical outcome. In this study, we compared the dynamic changes in trauma-induced MDSCs in two trauma animal models. Methods: Rats were divided into three groups as follows: the control group, the femur fracture (FFx) group, and the polytrauma (PT) group. Animals were sacrificed at 2, 6, 12, 18, or 24 h postoperatively, and spleen was harvested for study. The MDSCs were identified by a flow cytometry and calculated. Incorporation of [3H]thymidine was used to measure T cell proliferation. The results showed that the number of MDSCs in the spleen reached a peak 2 h after the trauma in both the groups. The peak number of MDSCs in the PT group was about four times greater (P<0.001); and in the FFx group, about one and a half times more (P=0.003) than in the control group. The increased level of MDSCs returned to normal after 18 h in the PT group, and after 6 h in the FFx group, post-surgery. Incorporation of [3H]thymidine showed that MDSCs induced by trauma suppressed T cell proliferation. Conclusion: These results suggest that polytrauma stress represent a more extensive MDSCs expansion which may contribute to an increased susceptibility to infection.
Keywords: Polytrauma, myeloid-derived suppressor cells, injury severity
Introduction
Patients with traumatic injuries are at an in-creased risk for infections, which are known to be associated with severity of the injury [1,2]. There are several factors associated with the risk of infections following a trauma, including the interruption of tissue integrity, hemorrhage, tissue hypoperfusion [3], frequency of invasive procedures, and impaired host defense mechanisms [4,5].
Impaired host defenses, frequently observed as T cell dysfunctions, are central to the development of infections after trauma [5]. Expansion of myeloid-derived suppressor cells (MDSCs) can be induced by a physical injury in the spleen of a trauma model; and trauma-induced MDSCs are considered to suppress the functions of T cells [6,7]. MDSCs suppress T cell activation via multiple mechanisms: one such mechanism involves their uptake of arginine and high intracellular level of arginase, which depletes the surroundings of arginine, an essential amino acid for T cell activation [8]. Reactive oxygen species (ROS) produced by MDSCs inhibit T cells by catalyzing the nitration of T cell receptors (TCR), and thereby preventing T cell-peptide-MHC interactions [9]. Accordingly, delayed or extensive MDSC expansions suppress T cell functions more severely, which render the patients more susceptible to infections.
As the risk of infection is associated with the injury severity, we hypothesized that the expansion of trauma-induced MDSCs after polytrauma, would reach to a higher level and last longer. In this study, we built two kinds of animal trauma models and compared the dynamic changes of the trauma-induced MDSCs.
Materials and methods
Animals
The present study was approved by the animal research committee of Shanghai Jiao Tong University School of Medicine, Shanghai, China. 66 adult male Sprague-Dawley rats (250-300 g) were housed, with two per cage on a 12:12 h light-dark schedule, and habituated to the laboratory environment for one week prior to use.
Animal injury models
All rats were anesthetized using inhalational isoflurane and subjected to different trauma procedures. Rats were randomly assigned to one of the three groups as follows: the control group (n=6); the femur fracture (FFx) group (n=30); and the polytrauma (PT) group (n=30), comprising of a 1-cm laparotomy with cecectomy, combined with medial thigh dissection with femur fracture and muscle tissue damage. The rats were administered buprenorphine (0.2 mg/kg body weight) prior to arousal from anesthesia and every 12 h afterwards, until killing.
The FFx and PT models have been previously described by Gentile et al. [10]. In brief, for the femur fracture, careful blunt dissection of the soft tissues to expose the femur was performed and the femur was fractured with a wire saw. The bones were then realigned. In addition, for polytrauma, the superior muscle tissue was grasped with a clamp for 30 s. The skin was closed in a single layer. For the PT model with the addition of cecectomy, the cecum was identified after laparotomy, ligated twice with 3-0 silk suture, and resected. The abdominal incision was closed in two layers. Sham animals received anesthesia, but no trauma was applied.
Sample collection
Animals were sacrificed at 2, 6, 12, 18, or 24 h postoperatively, and a splenectomy was performed for cell harvest.
Isolation of cells
The harvested spleen was converted into single-cell suspensions. Erythrocytes were depleted using RBC lysing buffer (Sigma-Aldrich), and splenocytes were washed in MACS buffer (1 × PBS supplemented with 2 mM EDTA and 0.5% BSA). CD4+ T cells were isolated using corresponding MACS magnetic microbeads (Miltenyi Biotec, Cologne, Germany). The purity of separated cells ranged between 89% and 95%.
Flow cytometry analysis
The spleen tissue was forced through a 70 μm BD Falcon cell strainer (BD Biosciences, San Jose, CA, USA), and washed with PBS. Erythrocytes were depleted using RBC lysing buffer (Sigma-Aldrich, St. Louis, MO, USA), and splenocytes were washed in MACS buffer (1 × PBS supplemented with 2 mM EDTA and 0.5% BSA). Cell surface staining and FACS analysis were carried out, as described by Graf et al. [11]. Briefly, 1 × 106 cells were stained with FITC-His48 (anti-granulocytes) and PE-CD11b/c (OX42) (BD Pharminigen, San Diego, CA, USA) according to the standard procedure. All staining procedures were performed on ice. The stained cells were counted using a FACScan flow cytometer (Beckman Coulter, Fullerton, CA, USA).
T cell proliferation assay
The incorporation of [3H]thymidine was used as a measure of T cell proliferation [12]. Briefly, 1 × 106 CD11bc+/His48+ cells from polytrauma rats or 1 × 106 CD11bc+/His48+ cells from sham-operated rats were mixed with 1 × 106 CD4+ T cells. The mixed cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 0.05 mM 2-mercaptoethanol, HEPES (10 mM) and antibiotic/antimycotic solution (RPMI medium) and re-stimulated with 1 µg/mL anti-CD3 and 1 µg/mL anti-CD28 (BD Biosciences, San Jose, CA, USA). For the final 16 h of culture, 1 µCi [3H]thymidine (GE healthcare, Little Chalfont, UK) was added. Proliferation was determined by the incorporation of [3H]thymidine into the cells in co-culture.
Statistical analysis
Results were presented as mean ± SD. Continuous variables were first tested for normality and equality of variances. A one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls test as a post hoc test for multiple comparisons were performed to determine significant differences between experimental means. For a single comparison of two groups, Student’s t test was used. A P<0.05 was considered statistically significant.
Results
The dynamic changes in trauma-induced MDSCs in two trauma animal models
To quantify MDSCs, flow cytometry was performed with antibodies against CD11b/c and His48, which were expressed by rat MDSCs. There was a paucity of MDSCs (2.7±0.6%) in the control group. In contrast, in the PT group, at the time intervals, 2, 6, and 12 h, the percentage of MDSCs increased to 12.5±2.4% (P<0.001), 8.9±1.2% (P<0.001), and 5.2±0.9% (P=0.016), respectively; and returned to the baseline, 3.8±1.5% (P=0.345) at a time interval of 18 h. Nevertheless, in the FFx group, there was a transient increase in the level of MDSCs, of up to 4.3±0.8% (P=0.003) at a time interval of 2 h, which quickly returned to the baseline of 3.1±0.3% (P=0.607) by 6 h. Figure 1 demonstrates the postoperative kinetics of MDSCs in the two groups. Additionally, a representative data on MDSCs after surgery in the two groups at different time points are presented in Figure 2. The number of MDSCs in the spleen was observed to reach a peak 2 h after the trauma in both the groups. The peak number of MDSCs in the PT group was about four times greater than the number in the control group (12.5±2.4% vs. 2.7±0.6%, P<0.001). However, the peak number of MDSCs in the FFx group was approximately one and a half times more than that of the control group (4.3±0.8% vs. 2.7±0.6%, P=0.003). Besides, the increased level of MDSCs in the PT group returned to the normal level 18 h postoperatively, while in the FFx group, the level returned to normal 6 h after surgery.
Figure 1.
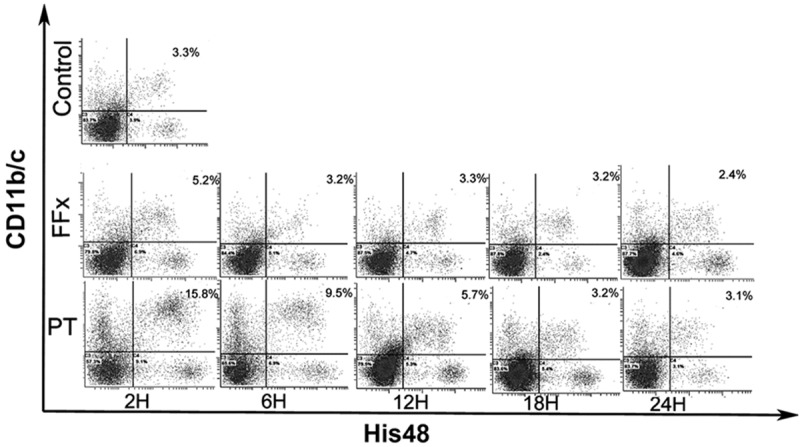
Representative kinetic changes in MDSCs in rats expressed as percentage of MDSCs in spleen of the polytrauma (PT) and femur fracture (FFx) groups at different time points postoperatively. Control group: level of MDSCs (2.9%) was found to be very low. PT group: at time intervals of 2, 6, and 12 h, the percentage of MDSCs increased to 15.8%, 9.5%, and 5.7%, respectively, and returned to the baseline after 18 h. FFx group: there was a transient increase of MDSCs (up to 5.2%) at a time interval of 2 h, and quickly returned to the baseline by 6 h.
Figure 2.
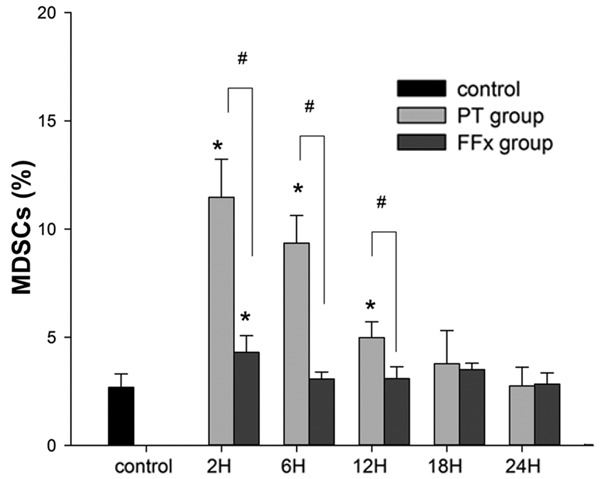
Average percentage of MDSCs measured in rats in the control, polytrauma (PT), and femur fracture (FFx) groups. Asterisk indicates significantly different vs. control (P<0.05); pound sign indicates significantly different PT vs. FFx (P<0.05).
MDSCs induced by polytrauma suppress T cell proliferation
T cells co-cultured in the presence of CD11bc+/His48+ cells induced by trauma exhibited a significant decrease in proliferation as measured by [3H]thymidine incorporation, compared with cells co-cultured with control CD11bc+/His48+ cells. Figure 3 shows that the most significant suppressive effect of CD11bc+/His48+ cells induced by polytrauma on T cell proliferation was observed at a 1:16 effector: target (E:T) ratio with a more than two-fold inhibition of T cell proliferation (30,333±2517 cpm for controls vs. 14,333±1528 cpm for polytrauma, P=0.007).
Figure 3.
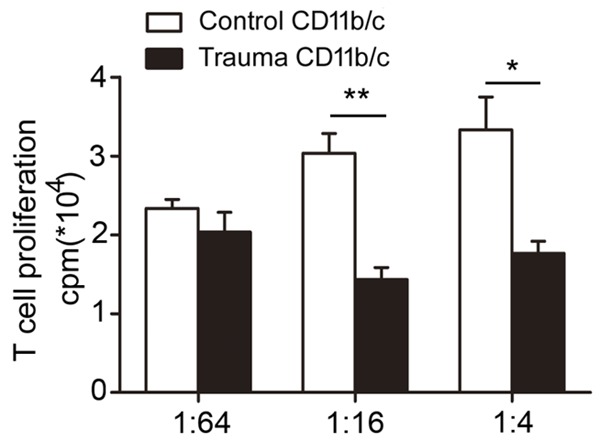
Myeloid-derived suppressor cells induced by polytrauma suppress T cell proliferation. T cells co-cultured in the presence of CD11bc+/His48+ cells induced by polytrauma exhibited a significant decrease in proliferation as measured by [3H]thymidine incorporation. The most significant suppressive effect of CD11bc+/His48+ cells induced by polytrauma on T cell proliferation was observed at an effector: target (E:T) ratio of 1:16 (P<0.05).
Discussion
Myeloid-derived suppressor cells were first described more than 30 years ago in cancer [13]. MDSCs are a heterogeneous population of cells consisting of precursors of granulocytes, macrophages, dendritic cells, and myeloid cells at their early stages of differentiation [14,15]. Recently, accumulating evidence has shown that MDSCs suppress the T-cell functions under conditions of traumatic stress [6,16], which is central to the development of infections following a trauma [5,17,18]. However, whether all kinds of traumas represent the same dynamic changes in MDSCs still remains unknown. In this study, the dynamic changes in MDSCs were studied in two trauma models and were correlated with the severity of injury.
In mice, MDSCs are characterized by the co-expression of the myeloid lineage differentiation antigen Gr1 with CD11b [19]. In humans, MDSCs are most commonly defined as CD14-CD16+ cells with low expression of the MHC-class-II molecule HLA-DR [20,21]. Graf et al. defined cells which express both monocyte (CD11b/c) and granulocyte (His48)-associated lineage markers as MDSCs in rats [22]. Accordingly, we used FITC-His48 and PE-CD11b/c+ to test changes in the MDSCs of bone marrow, blood and the spleen after polytrauma. To further test MDSC function, we co-cultured MDSCs following polytrauma with T cells and found that the proliferation of T cells was suppressed. This demonstrated that MDSCs induced by polytrauma in this study present a negative immunoregulatory function.
The spleen is rich in immune cells, including T cells and B cells. Normally, MDSCs make up only a small proportion (2-4%) of spleen cells in murines [15]. In pathological conditions, such as cancer, various infectious diseases, sepsis, trauma, or certain autoimmune disorders, the proportion of MDSCs in the spleen rises. Makarenkova et al. reported an increase in the level of trauma-induced MDSCs from 2±1% of all splenocytes at baseline to 7.8% of the cells by 6 h, which reached a peak of 15±4% by 12 h [6]. Our study presented a similar pattern of dynamic changes in the trauma-induced MDSCs in the PT group. However, in the FFx group, the trauma-induced MDSCs reached a peak by 2 h, and then returned to the baseline level. These may be attributed to differences in the severity of traumas.
The polytrauma patients have been found to be more susceptible to infections [1]. Xiao et al. observed that the critically injured patients, who developed an infection, represented a prolonged and dysregulated immune-inflammatory state [23]. Our data showed that the increased level of MDSCs in the PT group reached a higher level and lasted longer than in the FFx group. This may imply that polytrauma leads to a more severe immune disturbance, which in turn results in a vulnerable condition. The injury severity scores (ISS) of the trauma in the present study were 9 in the FFx group and 18 in the PT group. The number of trauma-induced MDSCs returned to the baseline level at 18 h post-injury. However, in clinical practice, there are more severe patients who develop an infection several days or weeks after a trauma. This could possibly be explained by the ISS of the polytrauma model in our study, which only reached the minimum standard for trauma studies in severely injured humans [24]. The immune response for this kind of injury can be quickly returned to normal.
In summary, the present study provides evidence that different traumatic stress represent different dynamic changes in MDSCs. The more severe the trauma, greater is the increase in the level of MDSCs. These findings may partly explain why polytrauma patients are more susceptible to infections.
Acknowledgements
We gratefully acknowledge Dr. Yiqing He, Dr. Jiajie Hu, and Dr. Yiwen Liu from Central Laboratory of Shanghai Sixth People’s Hospital for their excellent technical support. This study was supported by the Shanghai Committee of Science and Technology Research Projects (Grant no. 11JC1409400).
Disclosure of conflict of interest
None.
References
- 1.El-Masri MM, Joshi M, Hebden J, Korniewicz DM. Use of the injury severity score to predict nosocomial bloodstream infections among critically ill trauma patients. AACN Clin Issues. 2002;13:367–372. doi: 10.1097/00044067-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Jamulitrat S, Narong MN, Thongpiyapoom S. Trauma severity scoring systems as predictors of nosocomial infection. Infect Control Hosp Epidemiol. 2002;23:268–273. doi: 10.1086/502047. [DOI] [PubMed] [Google Scholar]
- 3.Chaudry IH, Ayala A. Mechanism of increased susceptibility to infection following hemorrhage. Amjsurg. 1993;165(Suppl):59S–67S. doi: 10.1016/s0002-9610(05)81208-5. [DOI] [PubMed] [Google Scholar]
- 4.Van Saene HK, Stoutenbeek CP, Zandstra DF, Gilbertston AA, Murray A, Hart CA. Nosocomial infections in severely traumatized patients: magnitude of problem, pathogenesis, prevention and therapy. Acta Anaesthesiol Belg. 1987;38:347–353. [PubMed] [Google Scholar]
- 5.Cheadle WG, Mercer-Jones M, Heinzelmann M, Polk HC Jr. Sepsis and septic complications in the surgical patient: who is at risk? Shock. 1996;6(Suppl 1):S6–9. [PubMed] [Google Scholar]
- 6.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 7.Munera V, Popovic PJ, Bryk J, Pribis J, Caba D, Matta BM, Zenati M, Ochoa JB. Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg. 2010;251:120–126. doi: 10.1097/SLA.0b013e3181bfda1c. [DOI] [PubMed] [Google Scholar]
- 8.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 9.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, Abouhamze AS, Lopez C, Baker HV, Moore FA, Ang DN, Efron PA. Identification and description of a novel murine model for polytrauma and shock. Crit Care Med. 2013;41:1075–1085. doi: 10.1097/CCM.0b013e318275d1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graf MR, Prins RM, Hawkins WT, Merchant RE. Irradiated tumor cell vaccine for treatment of an established glioma. I. Successful treatment with combined radiotherapy and cellular vaccination. Cancer Immunol Immunother. 2002;51:179–189. doi: 10.1007/s00262-002-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buessow SC, Paul RD, Lopez DM. Influence of mammary tumor progression on phenotype and function of spleen and in situ lymphocytes in mice. J Natl Cancer Inst. 1984;73:249–255. [PubMed] [Google Scholar]
- 14.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 18.Schwacha MG, Thobe BM, Daniel T, Hubbard WJ. Impact of thermal injury on wound infiltration and the dermal inflammatory response. J Surg Res. 2010;158:112–120. doi: 10.1016/j.jss.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 20.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 21.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 22.Graf MR, Sauer JT, Merchant RE. Tumor infiltration by myeloid suppressor cells in response to T cell activation in rat gliomas. J Neurooncol. 2005;73:29–36. doi: 10.1007/s11060-007-9442-z. [DOI] [PubMed] [Google Scholar]
- 23.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, López MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]


