Abstract
Primary pancreatic lymphoma (PPL), originating from the pancreatic parenchyma, is a rare type of lymphoma. The symptoms and radiographic findings of PPL are quite similar to pancreatic adenocarcinoma (PAC), and thus it is often misdiagnosed. In this study, we described the clinical features, radiographic findings, histological and immunohistochemical analysis, molecular detection and clinical treatment of two cases of PPL, aiming to distinguish PPL from PAC. The two cases were both low-grade PPL. One was follicular lymphoma and the other was small lymphocytic lymphoma. Imaging examination of the two cases both showed solid mass, thus highly suspecting of PAC. However, after surgery, PPL was diagnosed by the pathologists through histopathological observation, immunohistochemistry (IHC) assay and clonality analysis. Therefore, accurately diagnosing and classifying of PPL is essential for patient management, since PPL is a treatable malignant tumor.
Keywords: Primary pancreatic lymphoma, clinicopathological characteristics, diagnosis
Introduction
Primary pancreatic lymphoma (PPL) is an extremely rare disease and usually founded in 0.5% of pancreatic tumors [1]. Most cases of PPL are intermediate or high-grade non-Hodgkin’s lymphoma (NHL), with diffuse large B cell lymphoma (DLBCL) as the predominant histological type. Patients with PPL are usually around 50 s or 60 s, and there is a slight male-predominance [2]. The main clinical manifestations in PPL patients are abdominal pain, abdominal mass and weight loss. In addition, jaundice, nausea, vomiting, diarrhea, pancreatitis and bowel obstruction sometimes also can be observed in PPL [3]. These non-typical symptoms may be promiscuous with other pancreatic space-occupying lesion, like pancreatic adenocarcinoma (PAC).
To distinguish PPL from secondary involvement of the pancreas by NHL, Dawson et al. and Behrns et al. publish the criteria for the diagnosis of PPL: a predominant pancreatic mass with gross involvement of only the peripancreatic lymph nodes, no hepatic or splenic involvement, no palpable superficial lymphadenopathy, no enlargement of the mediastinal lymph nodes on chest radiograph and a normal leukocyte count [4,5]. In our study, we analyzed the clinical manifestations, image findings, serological tests, histological and molecular diagnosis of two Chinese patients with PPL, aiming to accumulate more information and diagnostic experience about this disease.
Materials and methods
Patients
Two cases of PPL treated at the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS), from July 2000 to June 2014, were selected. The tumors were radically removed by surgery (resection of pancreatic) as the clinical consideration of PAC. Pathological examinations, including morphology, immunophenotype and molecular pathology, were performed to make the definite diagnosis. Finally, both cases were discussed and diagnosed consistently by three pathological professors.
Immunohistochemistry
The specimens were fixed in 10% neutral buffered formalin for 24-48 hours. Immunohistochemistry (IHC) was performed on 4 μm-thick sections of formalin-fixed, paraffin-embedded (FFPE) tissue, using the Ventana Benchmark IHC automated slide strainers (Ventana Medical Systems, USA), according to manufacturer’s instructions. The primary antibodies used included CD3, CD5, CD10, CD20, CD23, CD34, CD43, CD79a, AE1/AE3, MPO, PAX-5, LCA, BCL-2, BCL-6, CyclinD1 and Ki-67. Negative and positive controls were included in each round of analysis.
Clonality analysis
DNA was extracted from FFPE tumor tissues using QIAamp DNA Mini Kit (Qiagen, Germany). Quality and concentration of the DNA samples were examined by NanoDrop ND-1000 Spectrophotometer (Thermo Fisher, MA, USA). Clonality analysis of lymphoid cells was performed using the BIOMED-2 clonality assays (IdentiClone, InVivo Scribe Technologies, USA), according to the manufacturer’s instructions. The DNA quality was checked for the two samples using the control gene PCR (Specimen Control Size Ladder master mix). The DNA was considered of adequate quality if ≥300 bp control PCR product was obtained. Negative and positive controls were included in the analysis.
Results
Clinical features
Case one was a 46-year old man who was admitted to the hospital with the complaint of finding an abdominal mass in physical examination. Case two was a 51-year old female who was admitted to the hospital with the complaint of abdominal dull pain and waist pain for 3 months. There was no obvious jaundice, nausea or vomiting for both of the patients.
Radiographic findings
For case one, abdominal CT scan showed that a class spherical solid mass located in the head of pancreas, with homogeneous density and clear border, without calcification and cystic changes. Diameter of the tumor was more than 5 cm (Figure 1A). For case two, the abdominal CT and MR showed that a huge homogeneous mass located in the tail of pancreas, with retroperitoneal lymphadenopathy (Figure 1B).
Figure 1.
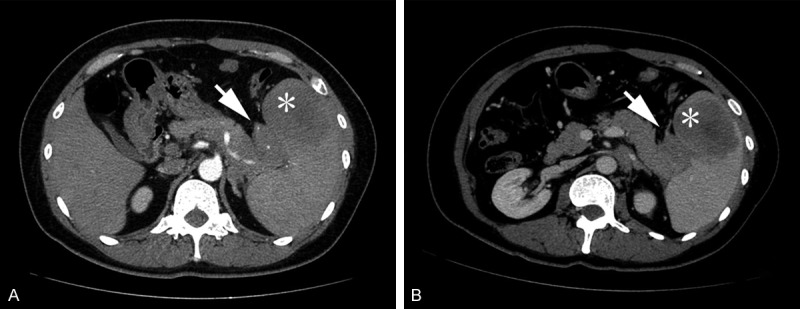
Preoperative computed tomography scan of both cases. A: For case one, an axial image showed a peripherally enhancing ill-defined mass with low-density mass (white arrow) in the head of pancreas. B: For case two, an axial image showed an irregular-shaped, peripherally enhancing low-density mass (arrow head) in the subcutaneous fat layer of the left cheek. The mass was surrounded by diffuse inflammatory tissue, and its overlying skin was thickened.
Serological tests
The levels of serum CA19-9, LDH and GGT were tested for both cases. The results showed that the levels of serum CA19-9, LDH and GGT for case one were normal. For case two, the level of serum CA19-9 was normal. However, elevated levels of LDH (463 U/L, reference range: 135-214 U/L) and GGT (128 U/L, reference range: 0-38 U/L) were observed.
Histological and IHC analysis
For case one, macroscopic examination showed that the lesion, measuring 4.4 cm × 5.2 cm × 2.8 cm, was homogeneous structure with a gray-yellow color. The tumor invaded part of the small intestine wall. Microscopically, the tumor was nodular growth. The neoplastic follicles were closely packed and lacked of mantle zones (Figure 2A). Lymphoma was considered by the histological analysis. The resection margins were free of disease. The adjacent lymph nodes in peripancreatic region were not infiltrated by the tumor. IHC analysis showed that tumor cells were strongly positive for CD20 and BCL-2 (Figure 2B and 2C). We also found that the tumor cells were positive for PAX-5, and faintly positive for CD79a and BCL-6. The immunostain was negative for CD10, CD3, CD5, CD34 and CyclinD1. The Ki-67 could be detected in about 25% of the tumor cell nuclei. Collectively, the pathology diagnosis was follicular lymphoma (Grade 1).
Figure 2.

Histological and IHC analysis of case one. A: H&E staining of PPL for case one showed nodular growth. B: Tumor cells were strongly positive for CD20. (SP, original magnification ×200). C: Tumor cells were strongly positive for BCL-2. (SP, original magnification ×200).
For case two, the size of resected tumor that located in the pancreatic tail was 4 cm × 3 cm × 2.5 cm. The tumor was soft in consistency and obviously adherent to peripancreatic fat tissues. The cut surface of the tumor demonstrated a grey-yellow solid homogeneous structure mass, without calcification and necrosis. Microscopically, the tumor tissue infiltrated the pancreas. The predominant cells were small lymphocytes, which were slightly larger than normal lymphocytes, with clumped chromatin and round nuclei (Figure 3A). A diagnosis of lymphoma without any lymph nodes involved was considered pathologically. Further, we found that the tumor cells were diffusely positive for CD20 and CD23 (Figure 3B and 3C). We also found that the tumor cells were diffusely positive for LCA and CD79a, faintly positive for CD5, but negative for AE1/AE3, MPO, CD3, CD10, CD43, CyclinD1 and BCL-6. Mitotic activity was very low, and less than 5% of cells were positive for Ki-67. Finally, a diagnosis of primary pancreatic B-small lymphocyte lymphoma was made pathologically.
Figure 3.

Histological and IHC analysis of case two. A: H&E staining of PPL for case two showed that small lymphocyte diffusely infiltrate the pancreas. B: The tumor cells were diffusely positive for CD20. (SP, original magnification ×200). C: The tumor cells were diffusely positive for CD23. (SP, original magnification ×400).
Molecular analysis
The two cases were analyzed for both B- and T-cell clonality. The monoclonal IGH rearrangements corresponding to monoclonal B-cell proliferations were detected in both of the two cases (Figure 4A and 4B). In addition, case one displayed BCL-2 rearrangements (Figure 5). All these results support the conclusion that the two cases of PPL are B-cell clonality.
Figure 4.
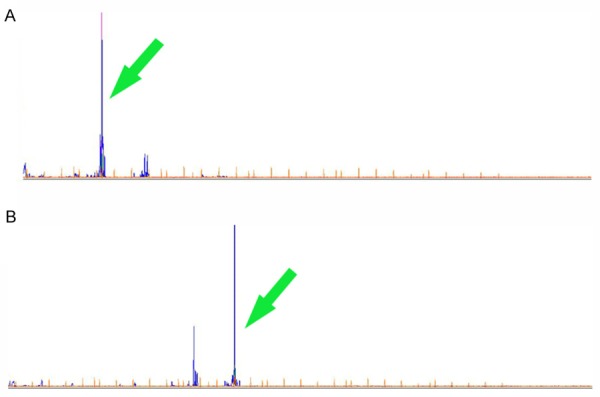
Molecular features of the two cases. The monoclonal IGH rearrangements were detected in both of A: Case one and B: Case two (arrow head).
Figure 5.
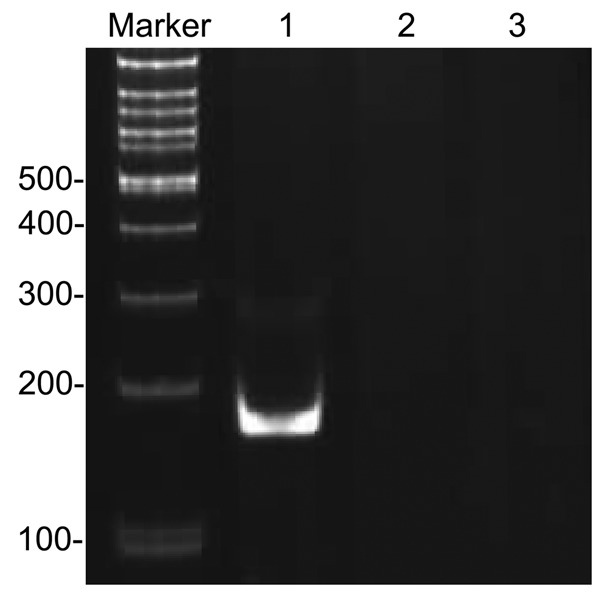
PCR detection of BCL2-IGH rearrangements. Lane 1: t (14; 18) tube A BCL2 MBR-JH; Lane 2: t (14; 18) tube B BCL2 3’MBR-JH; Lane 3: t (14; 18) tube C BCL2 MCR-JH.
Treatment and prognosis
The two patients were preceded with surgery and systematic chemotherapy of R-CHOP regimen for 4 cycles. The patients were alive without any evidence of recurrence by now.
Discussion
In this study, we retrospectively analyzed two cases of PPL. PPL is extremely rare. Most cases of PPL that have been reported are DLBCL [6,7]. Some T-cell non-Hodgkin primary pancreatic lymphoma cases have also been reported in Japanese [8]. Primary pancreatic follicular lymphoma and small lymphocytic lymphoma are less frequent than DLBCL [9]. Only nine cases of follicular lymphoma have been reported ever.
Patients with PPL are usually between the age of 35 and 75 (mean age: 55), with a male dominance. Abdominal pain is the most frequent symptom, followed by abdominal mass, weight loss, jaundice, acute pancreatitis, small bowel obstruction and diarrhea. Other clinical symptoms may include anorexia or early satiety. Obstructive jaundice seems to be less frequent than that in PAC. The classic symptoms of nodal non-Hodgkin’s lymphoma, such as fever, chills, and night sweats, are found in only 2% of PPL patients [10]. The clinical manifestation of PPL is non-specific, and thus it is very difficult to differentiate PPL from PAC [11]. Although the principle treatment for PPL is not uniform, the prognosis and survival rates are significantly better than PAC. A 30% 5-year survival rate is reported for patients with PPL, whereas for patients with PAC the 5-year survival rate is only 5% [12]. Some biochemical markers, together with suspicious clinical manifestations, may help focus the physician’s attention on the possibility of PPL. Lactate dehydrogenase (LDH) is considered to be a tumor marker in lymphoproliferative disorders and to have an important positive prognostic value. However, we only observed elevated LDH level in case two. Serum carbohydrate antigen 19-9 (CA 19-9) level in patients with PPL is usually not elevated. This is totally different from PAC, in which almost 80% of cases have high CA19-9 level. In our study, CA 19-9 level was normal in both of the two cases.
Imaging techniques are unable to distinguish PPL from PAC, especially in the early stage. PPL is often described as large homogeneous mass located in the head of pancreas, with or without associated lymphadenopathy. It is less observed in the body or tail, and more rarely diffuse involvement of the pancreas [13]. Ultrasound (US), endoscopic ultrasonography (EUS), CT and MR scan are well-established procedures to evaluate pancreatic masses and suggest a diagnosis of PPL. However, a definite diagnosis must be made after a mandatory histological diagnosis, which is crucial to a treatment planning. Two different morphologic patterns of pancreatic involvement are seen: one is the localized, well-circumscribed tumoral type, which shows no or mild ring or nodular enhancement after injection of contrast medium. The other is the diffuse enlargement infiltrating type or replacing most of the pancreatic gland. The huge homogeneous mass surrounding or involved pancreas, apparent enlargement of regional lymph nodes, especially lymph nodes under the renal vein, may be the typical imaging performance of PPL [14]. Merkle et al. have reported that for PPL, the presence of a bulky localized tumor in the pancreas is common, but it always appears without significant dilation of the main pancreatic duct [15]. The absence of main pancreatic duct dilation is a useful sign for diagnosing PPL [16,17]. We speculate that maybe it is related to the origin of PPL, as the tumor cells of PPL are not originated from the pancreatic ductal epidermis. PAC often demonstrates lacking of blood supply and violating the peripheral vascular. So for PAC, it is common to see vascular stenosis and significant dilation of the main pancreatic duct and bile duct [18].
Most of the time, it is difficult to obtain a final diagnosis of PPL by histomorphology and immunohistochemistry [19]. Thus, PCR-based molecular test clonality analysis is applied to detect gene rearrangement with high sensitivity and specificity, which may provide important diagnostic and prognostic genetic information for PPL. In this study, the monoclonal IGH rearrangements were detected in both of the two cases. It is considered that the results support the diagnosis of B cell lymphoma. Case one displayed BCL-2 rearrangements, supporting the diagnosis of follicular lymphoma.
In summary, PPL is a rare and manageable malignant tumor with non-specific symptoms and imaging examination results. It is important to differentiate between primary lymphoma and the more common adenocarcinoma of the pancreas, as treatment and prognosis differ significantly. FNA technique is recommended as a routine examination and tissue histology is fundamental for diagnosis. Chemotherapy or radiotherapy is preferred for treatment. Although the role of surgical resection remains controversial, it is generally agreed that surgery should be an option in the multimodal therapeutic regimen for respectable stage I or II pancreatic NHL. Compare to PAC, patients with PPL have a relatively favorable outcome.
Disclosure of conflict of interest
None.
References
- 1.Wallace D, Dang N, Dhawan M, Kulkarni A. Diagnosis of a patient with primary pancreatic lymphoma. Gastroenterol Hepatol (N Y) 2012;8:850–852. [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Zhang S, Vasdani N, Castillo E. Clues for diagnosing primary pancreatic lymphoma. Case Rep Gastroenterol. 2012;6:438–445. doi: 10.1159/000339968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saif MW. Primary pancreatic lymphomas. JOP. 2006;7:262–273. [PubMed] [Google Scholar]
- 4.Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80–89. doi: 10.1002/bjs.18004921319. [DOI] [PubMed] [Google Scholar]
- 5.Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9:662–667. [PubMed] [Google Scholar]
- 6.Lee MK, Jeon SW, Lee YD, Seo HE, Cho CM, Kim SG, Yoon YK. A case of primary pancreatic non-Hodgkin’s lymphoma. Korean J Intern Med. 2006;21:123–126. doi: 10.3904/kjim.2006.21.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Li SD, Hu XG, Li ZS. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol. 2006;12:5064–5067. doi: 10.3748/wjg.v12.i31.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura R, Takakuwa T, Hoshida Y, Tsujimoto M, Aozasa K. Primary pancreatic lymphoma: clinicopathological analysis of 19 cases from Japan and review of the literature. Oncology. 2001;60:322–329. doi: 10.1159/000058528. [DOI] [PubMed] [Google Scholar]
- 9.Grimison PS, Chin MT, Harrison ML, Goldstein D. Primary pancreatic lymphoma--pancreatic tumours that are potentially curable without resection, a retrospective review of four cases. BMC Cancer. 2006;6:117. doi: 10.1186/1471-2407-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Kuang TT, Tan YS, Chen Y, Zeng HY, Jin DY. Pancreatic primary lymphoma: a case report and review of the literature. Hepatobiliary Pancreat Dis Int. 2005;4:622–626. [PubMed] [Google Scholar]
- 12.Shahar KH, Carpenter LS, Jorgensen J, Truong L, Baker K, Teh BS. Role of radiation therapy in a patient with primary pancreatic lymphoma. Clin Lymphoma Myeloma. 2005;6:143–145. doi: 10.3816/CLM.2005.n.042. [DOI] [PubMed] [Google Scholar]
- 13.Salvatore JR, Cooper B, Shah I, Kummet T. Primary pancreatic lymphoma: a case report, literature review, and proposal for nomenclature. Med Oncol. 2000;17:237–247. doi: 10.1007/BF02780536. [DOI] [PubMed] [Google Scholar]
- 14.Van Beers B, Lalonde L, Soyer P, Grandin C, Trigaux JP, De Ronde T, Dive C, Pringot J. Dynamic CT in pancreatic lymphoma. J Comput Assist Tomogr. 1993;17:94–97. doi: 10.1097/00004728-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000;174:671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 16.Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fineneedle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–292. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 17.Volmar KE, Routbort MJ, Jones CK, Xie HB. Primary pancreatic lymphoma evaluated by fine-needle aspiration: findings in 14 cases. Am J Clin Pathol. 2004;121:898–903. doi: 10.1309/UAD9-PYFU-A82X-9R9U. [DOI] [PubMed] [Google Scholar]
- 18.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haioun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 19.Liakakos T, Misiakos EP, Tsapralis D, Nikolaou I, Karatzas G, Macheras A. A role for surgery in primary pancreatic B-cell lymphoma: a case report. J Med Case Rep. 2008;2:167. doi: 10.1186/1752-1947-2-167. [DOI] [PMC free article] [PubMed] [Google Scholar]


