Abstract
Extraskeletal osteosarcoma (EOS) is an aggressive malignant soft tissue tumor, producing neoplastic osteoid without attachment to the bone or periosteum. EOS predominantly occurs in the extremities and rarely involves the abdominal cavity. Only six cases of mesenteric EOS have been reported in the English literature. We describe an unusual case of a 70-year-old female patient, who was diagnosed to have a giant cell-rich variant of mesenteric EOS arising from small bowel mesentery. This patient presented with a mesenteric mass measuring 15.1×10.3×7.5 cm. Surgical resection of the tumor with small bowel was performed. The instrumental investigations didn’t reveal metastatic lesions. The patient refused further therapeutic regimen. EOS, regardless of its site or subtypes, has a very unfavorable prognosis and the patient died 2 months after discharge.
Keywords: Extraskeletal osteosarcoma, mesenteric osteosarcoma, giant cell-rich variant, intraabdominal osteosarcoma
Introduction
Extraskeletal osteosarcoma (EOS) is a malignant neoplasm composed of neoplastic cells secreting organic bone matrix with or without mineralization. Chondroblastic and fibroblastic differentiation may also be demonstrated. By definition, EOS is located in soft tissue without the primary involvement of bone or periosteum [1].
EOS is an uncommon malignant tumor, accounting for only 1-2% of all soft tissue sarcomas [2-4]. Only a small number of series studies and cases have been reported and the clinical features or natural history of EOS are not well appreciated as a result. The common sites of involvement are the deep soft tissue of thigh (47%), upper extremity (20%), and retroperitoneum (17%) [4]. It seldom involves the intraperitoneal visceral organs and only six cases of mesenteric EOS have been reported in the English literature [5-10]. The histological variants of EOS include osteoblastic, chondroblastic, fibroblastic, giant cell type, telangiectatic, small cell, or well-differentiated forms. The case reported is the first description of giant cell rich variant of EOS in small bowel mesentery.
Case report
A 70-year-old woman was referred for a huge abdominal mass. She complained of intermittent abdominal pain for last 6 months. She had no other medical history except diabetes medically well controlled.
Upper gastrointestinal endoscopy and colonoscopy were normal. Computed tomography scan disclosed a huge abdominal mass with mottled calcification in the left low quadrant (Figure 1) and overall image analysis suggested a malignant gastrointestinal stromal tumor. The subsequent investigation for metastasis of positron emission tomography-computed tomography scan showed no metastatic lesion throughout the body. The biochemical tests revealed no significant alterations. On the operation, the surgeon described a large calcified mass located in the mesentery of the small intestine with invasion to the root of superior mesenteric artery. Involvement of adjacent bony structure was not identified during the operation. En bloc tumor resection was performed with segmental resection of the small intestine.
Figure 1.

Computed tomography showing a heterogeneously enhancing mass (arrow) in the left low quadrant.
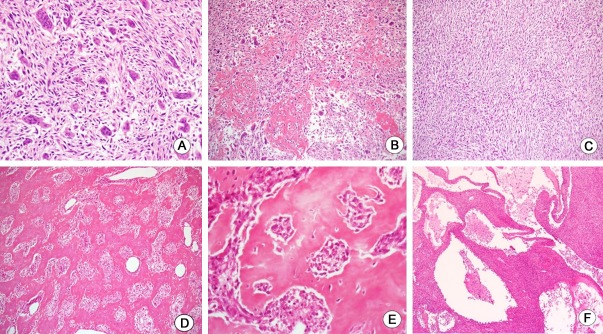
The resected tumor measured 15.1×10.3×7.5 cm. It was located in the mesentery without the involvement of intestinal wall (Figure 2A). It was stony hard in consistency with gritty sensation. A band saw was required to cut through the specimen completely. The cut surface is solid and ivory white with focal cystic degeneration and hemorrhage (Figure 2B). The tissue samples were decalcified and fixed in formalin, then routinely processed and embedded in paraffin, with cut sections of 4 µm. Microscopic examination showed predominantly osteoclast-like giant cells admixed with dyshesive malignant stromal cells (Figure 3A). Coarse deposit of osteoid separating groups of malignant cells was laid down (Figure 3B) whereas well developed trabecular bone was also produced by tumor osteoid (Figure 3D and 3E). Additionally, focal area of malignant spindle cell components was also appreciated with fasciculation (Figure 3C). The hemorrhagic foci of the tumor on the gross examination showed minor telangiectatic components consisting of multilocular cystic architecture divided by septa containing neoplastic cells and scattered multinucleate giant cells (Figure 3F). For immunohistochemical studies, the neoplastic cells are positive for vimentin and smooth muscle actin. Immunostains for C-kit, S-100, CD34, and CD99 showed the negative reaction. A final diagnosis of extraskeletal giant cell-rich osteosarcoma was rendered.
Figure 2.
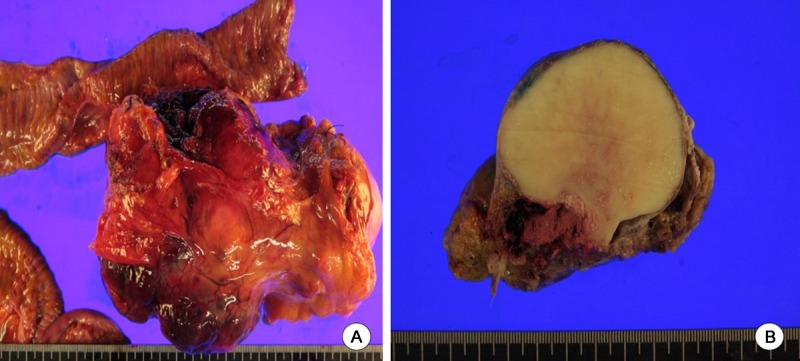
Macroscopic findings. A: A large lobulated mass, 15.1 cm in diameter, located in the mesentery without involvement of adjacent small intestine. B: Solid cut surface with ivory white in color and stony hard in consistency.
Figure 3.
Histologic findings. A: Numerous osteoclast-like giant cells, admixed with undifferentiated stromal tumor cells, reminiscent of giant cell tumor. B: Interconnected deposits of tumor osteoid separating clusters of tumor cells. C: Focal area of malignant spindle cells with fasciculation. D: Irregularly interconnected trabecular growth of bone. E: Higher power photomicrograph of (D) showing anaplastic tumor cells encircled by neoplastic osteoid. F: Low power view showing minor components of telangiectatic features.
Postoperative period was uneventful. Oncology team recommended chemotherapy, but the patient refused and requested for discharge. She died 2 months later due to cardiac and respiratory complications related to her oncological condition in a nursing hospital.
Discussion and conclusion
Extraskeletal osteosarcoma (EOS) is a highly aggressive tumor with rare incidence of 1-2% of all soft tissue sarcomas and 2-5% of all osteosarcomas [2-4]. EOS usually affects adults, typically in the sixth and seventh decades of life, compared with much younger age in the intraosseous primary osteosarcoma with a peak incidence in the second decade of life. Lee et al. [11] found that the predominance of men in primary osteosarcoma was also found in EOS, while recent studies [12] proposed that men and women are equally affected. All major variants of primary osteosarcoma are also found in EOS. The most common is the osteoblastic variant, followed by fibroblastic, chondroblastic, telangiectatic, small cell, and well-differentiated form.
The majority of EOS arise in the deep soft tissues, the most common location being the thigh (47%), upper extremity (20%), and retroperitoneum (17%) [4]. EOS in the abdominal cavity is uncommon, mesenteric EOS being even rarer. Only six mesenteric EOS have been reported in the English literature (Table 1). Intraabdominal EOS can also show any subtypes of primary osteosarcoma and should be considered in the differential diagnosis of any malignant mesenchymal tumors of the abdominal cavity. Metaplastic bone production can be found in many sarcomas including gastrointestinal stromal tumor, synovial sarcoma, epithelial sarcoma, liposarcoma, or malignant fibrous histiocytoma, but usually the amount of bone and osteoid is limited to a small portion of the tumor. A meticulous sampling, histological studies, and immunohistochemical investigations might be helpful to rule out other malignant mesenchymal tumors with metaplastic bone formation.
Table 1.
Clinicopathologic features of mesenteric extraskeletal osteosarcoma
| Case | Age (years) | Sex | Size (cm) | Histologic type | Surgery | Adjuvant chemotherapy | Metastasis or recur | Status | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 [5] | 56 | F | NA | NA | Incomplete tumor excision | No | Liver metastasis | Died | Died during hospitalization |
| 2 [6] | 45 | M | 15 | Osteoblastic | Tumor excision | Yes | No | NA | NA |
| 3 [7] | 61 | M | 20 | Osteoblastic | Palliative tumor resection | Yes | Peritoneal seeding | Died | 10 |
| 4 [8] | 67 | M | 18.5 | Telangiectatic | En bloc tumor excision, colon segmental resection and splenectomy | Yes | Peritoneal recurrence, liver and lung metastasis | Died | 4 |
| 5 [9] | 40 | M | 15 | Telangiectatic | Palliative en-bloc resection of tumor and small bowel, hepatic meastatectomy | Yes | Liver metastasis, peritoneal recurrence | NA | 14 |
| 6 [10] | 56 | M | NA | Osteoblastic | Tumor excision with ileal resection | No | NA | NA | NA |
| 7 (case) | 70 | F | 15.1 | giant cell-rich | en bloc resection of tumor and small intestine | No | No | Died | 2 |
NA: not available.
EOS has very poor prognosis with an overall survival rate at five years ranging from 25% to 66% [11,12]. Local recurrence is frequent with high rates of metastasis. The lungs are the most common site for metastasis. Most local recurrences and distant metastases occur within 3 years postoperatively [9]. Tumor size is the most important prognostic factor, as the patients with lesions greater than 5 cm have a worse clinical outcome [3]. There are controversies on the other clinico-pathologic prognostic factors such as histologic subtype, mitotic rates, cellularity, nuclear pleomorphism, tumor necrosis, initial treatment, and presence of metastases at diagnosis [3,4,11]. Based on the parameters, our patient was in a poor prognostic subgroup, with tumor larger than 5 cm at the time of diagnosis.
EOS currently lacks promising therapies. EOS is considered to be clinically and therapeutically distinct from primary osteosarcoma. In most published series, its treatment has been similar to that of other soft tissue sarcomas. A number of authors suggested that wide resection followed by adjuvant chemotherapy and radiotherapy is the available mode of management. However, there is an assertion that adjuvant chemotherapy after surgery may have been of little value in an advanced disease [13].
In conclusion, this report illustrates an unprecedented occurrence of a giant cell-rich variant of EOS in the mesentery and should be considered in the differentials of intraabdominal malignant mesenchymal tumors. EOS, mesenteric or not, have a very dismal outcome and a promising therapy is not currently available.
Disclosure of conflict of interest
None.
References
- 1.Goldblum J, Folpe A, Weiss S. Enzinger and Weiss’s Soft tissue tumors. 6th edition. Philadelphia: Elsevier-Saunders; 2014. pp. 936–44. [Google Scholar]
- 2.Allan C, Soule E. Osteogenic sarcoma of the somatic soft tissues; clinicopathologic study of 26 cases and review of literature. Cancer. 1971;27:1121–33. doi: 10.1002/1097-0142(197105)27:5<1121::aid-cncr2820270519>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Bane BL, Evans HL, Ro JY, Carrasco CH, Grignon DJ, Benjamin RS, Ayala AG. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65:2762–70. doi: 10.1002/1097-0142(19900615)65:12<2762::aid-cncr2820651226>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Chung E, Enzinger F. Extraskeletal osteosarcoma. Cancer. 1987;60:1132–42. doi: 10.1002/1097-0142(19870901)60:5<1132::aid-cncr2820600536>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PM, Chen DL. Extraosseous osteogenic sarcoma of the small bowel demonstrated by 18F scanning. J Nucl Med. 1973;14:295–7. [PubMed] [Google Scholar]
- 6.Choudur HN, Munk PL, Nielson TO, Ryan AG. Primary mesenteric extraskeletal osteosarcoma in the pelvic cavity. Skeletal Radiol. 2005;34:649–52. doi: 10.1007/s00256-005-0909-8. [DOI] [PubMed] [Google Scholar]
- 7.Heukamp LC, Knoblich A, Rausch E, Friedrichs N, Schildhaus HU, Kahl P, Tismer R, Schneider B, Büttner R, Houshdaran F. Extraosseous osteosarcoma arising from the small intestinal mesentery. Pathol Res Pract. 2007;203:473–7. doi: 10.1016/j.prp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Lee KH, Joo JK, Kim DY, Lee JS, Choi C, Lee JH. Mesenteric extraskeletal osteosarcoma with telangiectatic features: a case report. BMC Cancer. 2007;7:82. doi: 10.1186/1471-2407-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain MI, Al-Akeely MH, Alam MK, Jasser NA. Extraskeletal osteosarcoma, telangiectatic variant arising from the small bowel mesentery. Saudi Med J. 2011;32:958–61. [PubMed] [Google Scholar]
- 10.Ahmed SA, Calvin B, Garba ES, Mohammed U, Shehu MS. Primary mesenteric extraskeletal osteosarcoma. Niger J Basic Clin Sci [Internet] 2013;10:95–7. [Google Scholar]
- 11.Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76:2253–9. doi: 10.1002/1097-0142(19951201)76:11<2253::aid-cncr2820761112>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Torigoe T, Yazawa Y, Takagi T, Terakado A, Kurosawa H. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci. 2007;12:424–9. doi: 10.1007/s00776-007-1164-8. [DOI] [PubMed] [Google Scholar]
- 13.Sordillo PP, Hajdu SI, Magill GB, Golbey RB. Extraosseous osteogenic sarcoma: a review of 48 patients. Cancer. 1983;51:727–34. doi: 10.1002/1097-0142(19830215)51:4<727::aid-cncr2820510429>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]