Abstract
The Wnt/β-catenin signaling pathway, which is strictly controlled by multiple negative regulators, has been reported commonly hyper activated and closely related to the progression of bladder cancer. However, how tumor cells override the negative regulatory effects to maintain constitutive activation of Wnt/β-catenin signaling is still unclear. In the current study, we demonstrated that upregulation of miR-543-3p in bladder cancer activated Wnt/β-catenin signaling by directly targeting Wnt inhibitory factor 1 (WIF1) and Dickkopf 1 (DKK1), which are important antagonist molecules of the Wnt/β-catenin pathway. Expression of miR-543-3p was upregulated in both bladder cancer tissues and cells, and positively correlated with high-grade bladder cancer. Furthermore, ectopic overexpression of miR-543-3p promoted proliferation and inhibited apoptosis in bladder cancer cells. Notably, overexpression of miR-543-3p enhanced, while silencing miR-543-3p reduced, stem cell-like phenotype of bladder cancer cells. Therefore, our results suggest that miR-543-3p plays a significant role in promoting proliferation and stem cell-like phenotype in bladder cancer, which might be a potential target for anti-bladder cancer therapy.
Keywords: miR-543-3p, bladder cancer, cancer stem cells, proliferation, Wnt/β-catenin
Introduction
Bladder cancer is one of the leading causes of cancer-related death worldwide, with an estimated 429,800 new cases and 165,100 deaths around the world in 2012 [1]. Although therapeutic methods in operative therapy combined with radiotherapy and chemotherapy are developing, the prognosis for patients in advanced stages is still very poor [2]. Recent studies have shown that bladder cancer stem cells (CSCs) act as a reservoir of self-sustaining cells with the exclusive ability to self-renew and cause tumor growth, invasion, metastasis and recurrence [3,4]. However, the molecular mechanism for the maintenance of bladder cancer stem cell phenotype is still under exploration.
The Wnt/β-catenin signaling pathway, which is often aberrantly activated in various types of cancers, including bladder cancer, has been demonstrated as one of the most relevant pathways involved with CSCs [5,6]. Upon activation, ligand-receptor binding triggers the signaling cascades and releases β-catenin from the “destruction complex”, which includes Axin, adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase 3β (GSK3β) [7]. This phenomenon results in nucleus translocation and interaction between β-catenin and T-cell Factor (TCF)/lymphoid enhancer-binding protein (LEF) transcription factors and activates cancer stem cell-related genes [8]. On the other hand, it is considered that Wnt/β-catenin signaling is subject to multiple levels of negative regulation [9,10]. For instance, antagonists of the Wnt pathway, like Wnt inhibitory factor-1 (WIF1), Dickkopf 1 (DKK1), and Frizzled-related proteins (SFRPs), can suppress Wnt-ligands binding to Frizzled (Fz) receptors and prevent pathway from activation [11,12]. Moreover, β-catenin could be destroyed in the destruction complex through ubiquitin-proteasome pathway [13]. However, how tumor cells override these negative regulatory effects to maintain constitutive activation of Wnt/β-catenin signaling remains unknown.
MicroRNAs (miRNAs) are a class of highly conserved, small noncoding RNAs (17-25 nucleotides) which regulate gene expression at the post-transcriptional level [14]. Known from the bioinformatic predictions, miRNAs regulate more than 30% of the protein-coding genes [15]. Previous studies have shown that abnormity expression of miRNAis a common trait of cancer, which contribute to stemness maintenance and tumorigenicity of CSCs [16,17]. Thus, it is particularly interesting to identify miRNAs that might affect Wnt/β-catenin signaling and thereby lead to the self-renewal of bladder cancer stem cells. In the current research, we found that miR-543-3p was remarkably upregulated in bladder cancer and enhanced the stem cell-like phenotype of bladder cancer cells by directly targeting the negative modulators of Wnt/β-catenin pathway, including WIF1 and DKK1.
Materials and methods
Tissues and cells
Fresh bladder cancer tissue samples from bladder cancer patients, and pair-matched adjacent noncancerous bladder tissues were obtained from the Department of Urology, the Second Affiliated Hospital, Fujian Medical University, China. The samples had been clinically and histopathologically diagnosed based on the World Health Organization criteria. Hematoxylin and eosin staining was used to confirm the tumor and non-cancerous tissues histologically. All samples were collected from consenting individuals in accordance with the protocols approved by the Ethics Review Board at the Second Affiliated Hospital, Fujian Medical University. Human bladder cancer cell linesBIU87, T24, SW780, J82, RT4 and HT1376 were routinely maintained in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT).
Constructs, reagents and assays
pMSCV-miR-543-3p overexpressing human miR-543-3p was generated by subcloning the PCR-amplified human miR-543-3p precursor into pMSCV-puro-retro vector. The miR-5433p anti-sense was cloned into miRZip plasmid purchased from System Biosciences. Standard calcium phosphate transfection method was used to cotransfect pMSCV-miR-543-3p and pIK packaging plasmid into HEK293T cells. Puromycin (0.5 μg/ml) was used to select stably transduced cell after infections.
The 3’UTRs of the human WIF1 and DKK1 were cloned in between the SpeI and HindIII sites of pGL-3 (Promega, Madison, WI, USA). This vectors were named wild-type 3’UTR. By using a Quick Change Site-Directed Mutagenesis kit (SBS Genetech, Beijing, China), mutations of the 3’UTR sequence were created, the vectors containing them named as mutant 3’UTR. The reporter plasmids containing wild-type (CCTTTGATC; TOP flash) or mutant (CCTTTGGCC; FOP flash) TCF/LEF DNA binding sites were purchased from Upstate Biotechnology (New York, USA). All constructs were verified by DNA sequencing.
RNA extraction, reverse transcription, and real-time RT-PCR
Total miRNA from cultured cells and fresh surgical bladder tissues was isolated using TRIzol (Invitrogen). Detection of miR-543-3p and mRNA were performed as previously described [18]. GAPDH and U6 were used to normalize mRNA and miRNA respectively. c-Myc forward, 5’-TCAAGAGGCGAACACACAAC-3’; c-Myc reverse, 5’-GGCCTTTTCATTGTTTTCCA-3’; CCND1 forward, 5’-TTCTGCCTTTGATGTTAC-3’; CCND1 reverse, 5’-AGGCTGAATCAAT-GTCTT-3’; OCT4 forward, 5’-ATTCAGCCAAACGACCATCT-3’; OCT4 reverse, 5’-TCTCACTCGGTTCTCGATACTG-3’; SOX9 forward, 5’-GAACGCATACCAAGACGGAG-3’; SOX9 reverse, 5’-TCTCGTTGATTTCGCTGCTC-3’; CD133 forward, 5’-CAGATGCTCCTAA-GGCTTG-3’; CD133 reverse, 5’-GCAAAGCATTTCCTCAGG-3’; GAPDH forward, 5’-GACTCATGACCACAGTCCATGC-3’; and GAPDH reverse, 5’-AGAGGCAGGGATGATG-TTCTG-3’. Relative quantitation was calculated using the 2-ΔΔCt method. All experiments were carried out in triplicate.
Clone formation assay
Clone formation assay was performed as described [19]. Only Cell colonies with a diameter larger than 50 μm were counted.
TUNEL assay
TUNEL assay was performed as described [19]. TUNEL-positive cells were regarded as apoptotic cells. The ratio of TUNEL-positive cells to total cells was calculated and defined as the percentage of apoptotic cells. At least 10 different high-power fields of a fluorescent microscopein duplicate wells and five different experiments were selected for the calculation.
Western blot analysis
Western blotting was performed on the basis of a standard method, as previously described [20]. The following primary antibodies were used: anti-β-catenin (1:500, Cell Signaling Technology, Danvers, MA, USA), anti-WIF1 (1:1000, Cell Signaling Technology), anti-DKK1 (1:1000, Cell Signaling Technology), anti-Nuclear Matrix Protein p84 (1:500, Abcam, Cambridge, UK), and anti-GAPDH antibody (1:1000, Sigma-Aldrich, St. Louis, MO, USA). Nuclear extracts were prepared using the Nuclear Extraction Kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using a Magna ChIP kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol [21]. β-catenin (Abcam) and H3k9Ac (Abcam) antibodies were used to immunoprecipitate chromatin fragments.
Luciferase assays
Cells (4 × 104) were seeded onto 24-well plates in triplicate. 24-hours later, indicated luciferase reporter plasmids plus 5 ng pRL-TK Renilla plasmid (Promega) were transfected into the cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendation. Luciferase and Renilla signals were measured 48 hours after transfection using a Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
Bioinformatics analysis
The following online software programs were used for bioinformatics analysis: TargetScan6.2 (http://targetscan.org/vert_40/); and miRanda (http://www.microrna.org/microrna/getGeneForm.do).
Statistics
All statistical analyses were performed using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Statistical differences were determined by the two-tailed Student’s t-test between two groups of data; a p value <0.05 was considered statistically significant.
Results
miR-543-3p overexpression positively correlates with bladder cancer progression
To identify the role of miR-543-3p in the development of bladder cancer, real-time PCR analysis was performed and showed that miR-543-3p was ubiquitously overexpressed in all six bladder cancer cell lines (BIU87, T24, SW780, J82, RT4 and HT1376) and ten bladder cancer samples compared to human bladder epithelial cell (HCV29) and normal bladder adjacent tissues, respectively (Figure 1A and 1B). Further statistical analysis was performed to assess the clinicopathological significance of miR-543-3p expression in bladder cancer tissues at different stages and grades of cancer and along with normal tissue, we found that high miR-543-3p expression was positively correlated with histological high-grade bladder cancer (Figure 1C). Taken together, these results suggest that miR-543-3p is upregulated, which might be involved in human bladder cancer progression.
Figure 1.
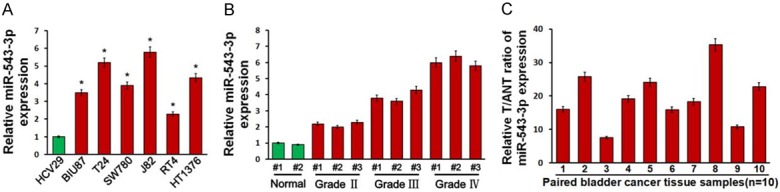
miR-543-3p is upregulated and positively correlated with bladder cancer progression. A. Real-time PCR analysis of miR-543-3p expression in normal bladder epithelial cell (HCV29) and six bladder cancer cell lines (BIU87, T24, SW780, J82, RT4 and HT1376). B. Real-time PCR analysis of miR-543-3p expression in 10 pairs of bladder cancer samples and adjacent normal tissues. C. miR-543-3p expression was positively correlated with tumor grades of bladder cancer. Transcript levels were normalized to U6 expression. Each bar represents the mean ± SD of three independent experiments. *P<0.05.
miR-543-3p promotes proliferation and inhibits apoptosis in bladder cancer
To determine the effect of miR-543-3p on bladder cancer cell proliferation, bladder cancer cell line J82 cells were engineered to overexpress or silence miR-543-3p by transfection of miR-543-3p or miR-543-3p inhibitor (Figure 2A) and then analyzed using clone formation and MTT assays. The numbers of J82 cell colonies were significantly increased by overexpression of miR-543-3p, while reduced by miR-543-3p inhibitor compared with the corresponding control cells (Figure 2B). In addition, the proliferation ability of J82 cell was significantly promoted by overexpression of miR-543-3p (Figure 2C).
Figure 2.
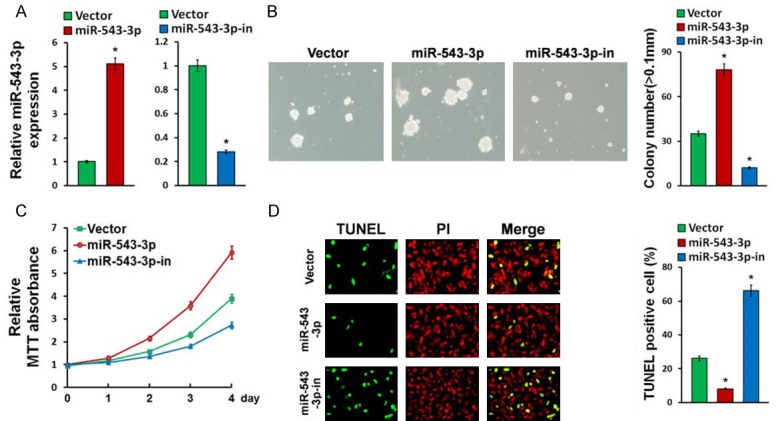
miR-543-3p promotes proliferation and inhibits apoptosis in bladder cancer. A. Real-time PCR analysis of miR-543-3p in Vector-transduced, miR-543-3p-overexpressing, miR-543-3p-silenced bladder cancer J82 cell lines. B. Representative images and statistical results of J82 cell colony number (>50 μm) after indicated treatments. C. Proliferation curves showing the effect of miR-543-3p-overexpression, miR-543-3p-inhibition, or control vector on J82 cell proliferation. D. TUNEL assays revealed the effect of miR-543-3p on bladder cancer cell apoptosis. Error bars represent mean ± SD from three independent experiments. *P<0.05.
To determine the effect of miR-543-3p on apoptosis, a TUNEL assay was performed. Compared with the corresponding control cells, overexpression of miR-543-3 produced, while downregulation of miR-543-3p increased the apoptosis of the bladder cancer cells (Figure 2D). Furthermore, we found that miR-543-3p overexpression significantly decreased, while miR-543-3p downregulation upregulated the mRNA expression levels of multiple apoptosis-related factors, including FASL, p21, Caspase-9, and Bax (Figure 3A).
Figure 3.
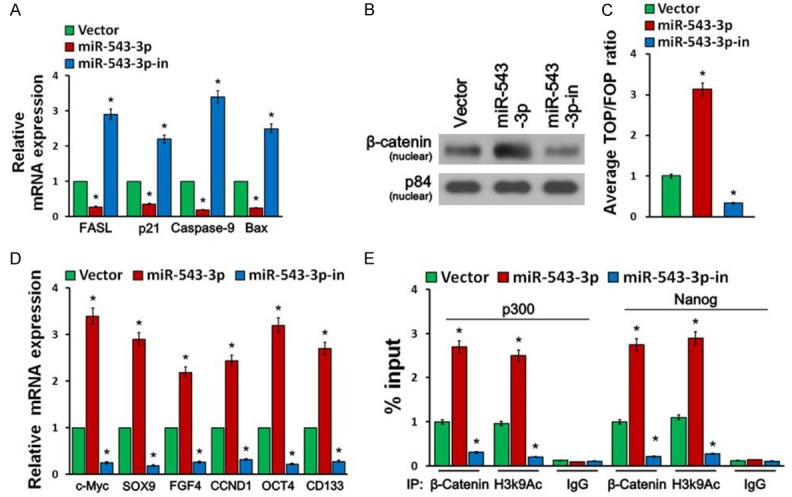
miR-543-3p activates the Wnt/β-catenin signaling and promotes CSC-likephenotype. A. Real-time PCR analysis revealed that miR-543-3p regulates the mRNA expression levels of multiple apoptosis-related factors. B. Western blotting of nuclear β-catenin expression. The nuclear protein p84 was used as the nuclear protein marker. C. Wnt/β-catenin signaling was monitored by β-catenin sensitive TOP/FOP luciferase reporter assay in the indicated cells. D. Real-time PCR analysis of mRNA expression of stem cell markers in the indicated cells. Transcript levels were normalized to GAPDH expression. E. CHIP assays revealed the effect of miR-543-3p on the binding of β-catenin to the promoters of core stemness transcription factors, p300 and Nanog. Error bars represent mean ± SD from three independent experiments. *P<0.05.
miR-543-3p activates the Wnt/β-catenin signaling and promotes CSC-like phenotype
Since wnt/β-catenin signaling is an important pathway that has significant roles in maintaining stem cell phenotype and is frequently activated in bladder cancer [5], we tested the role of miR-543-3p in wnt/β-catenin signaling pathway. As shown in Figure 3B, we found that overexpression of miR-543-3p increased nuclear accumulation of β-catenin, while silencing of miR-543-3p led to the opposite results. In addition, at 48 h after transfection, miR-543-3p overexpression significantly increased TOP/FOP luciferase reporter activity, whereas miR-543-3p silencing reduced reporter activity (Figure 3C).
Furthermore, we found that overexpression of miR-543-3p increased the mRNA expression levels of multiple pluripotency factors, including c-Myc, SOX9, FGF4, CCND1, OCT4, and CD133, while downregulation of miR-543-3p just the opposite (Figure 3D). Importantly, CHIP assays indicated that overexpression of miR-543-3p promoted the binding of β-catenin to the promoters of the core stemness transcription factors, p300 and Nanog (Figure 3E). Collectively, our data suggest that miR-543-3p promotes a stem cell-like phenotype in bladder cancer cells.
miR-543-3p activates Wnt/β-catenin pathway by targeting WIF1 and DKK1
To identify potential targets of miR-543-3p, we used the publicly available algorithms miRanda and TargetScan and identified the Wnt/β-catenin signaling negative regulators WIF1 and DKK1 (Figure 4A). Dual luciferase reporter assay showed that miR-543-3p overexpression attenuated reporter activities with the 3’UTR of these negative regulators, whereas miR-543-3p inhibition elevated these activities (Figure 4B). However, ectopic expression of the miR-543-3p did not exhibit repressive effects on the reporter activities driven by the mutant 3’UTRs of these transcripts (Figure 4B). Furthermore, revealed by the Western blot analysis that increased expression of miR-543-3p markedly reduced the expression levels of WIF1 and DKK1 (Figure 4C). MiR-543-3p inhibition on the contrary increased them, suggesting that miR-543-3p negatively regulated these proteins (Figure 4C). In addition, individual silencing of these targets potently rescued the TOP/FOP luciferase reporter activity in miR-543-3p-inhibited cells (Figure 4D), demonstrating that WIF1 and DKK1 were functional effectors of miR-543-3p on regulating wnt/β-catenin signaling activation. To further validate that miR-543-3p activates Wnt/β-catenin pathway, we investigated the relationship between miR-543-3p and β-catenin expression in tissues isolated from bladder cancer patients and found that β-catenin levels are inversely correlated with miR-543-3p expression levels (Figure 4E). Taken together, our results suggest that miR-543-3p activates wnt/β-catenin signaling by directly targeting WIF1 and DKK1.
Figure 4.
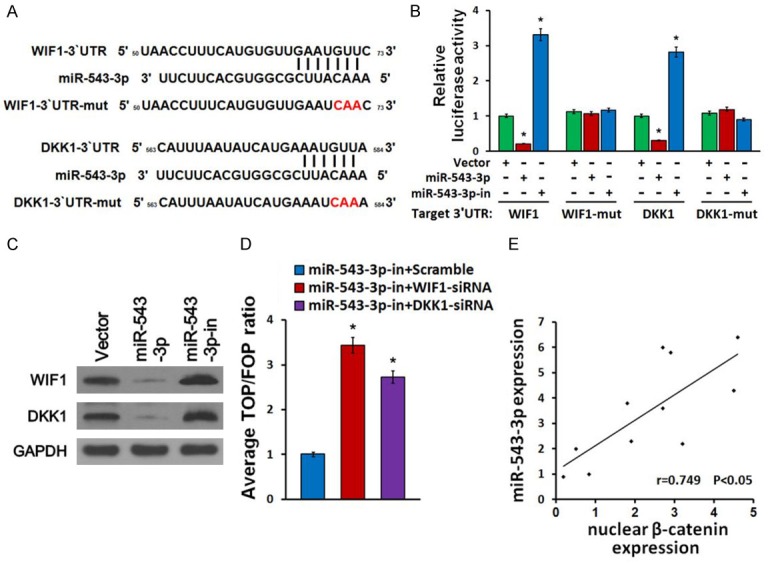
miR-543-3p activates Wnt/β-catenin pathway by targeting WIF1 and DKK1. A. Predicted miR-543-3p target sequence in 3’UTRs of WIF1 and DKK1. The mutated 3’UTRs of WIF1 and DKK1 containing three altered nucleotides were indicated. B. Luciferase assay of pGL3-WIF1-3’UTR, pGL3-WIF1-3’UTR-mut, pGL3-DKK1-3’UTR and pGL3- DKK1-3’UTR-mut reporter in the miR-543-3p-overexpressing, miR-543-3p-silenced and control cells. C. Western blot of WIF1 and DKK1 expression in the indicated cells. GAPDH served as the loading control. D. Silencing of WIF1 and DKK1 rescued the miR-543-3p-inhibitor mediated TOP/FOP luciferase reporter activity suppression. E. Pearson’s correlation scatter plot of the fold change of the levels of miR-543-3p and nuclear β-catenin in 11 bladder cancer samples. (R=0.749). *P<0.05.
Discussion
MicroRNAs have emerged as a novel molecular biomarker for cancer diagnosis, prognosis, and therapy, and function as regulators in a wide variety of oncogenic processes, such as CSC division and differentiation, invasion and metastasis, playing a role as either tumor suppressors or oncogenes [15-17]. Thus, it may provide valuable diagnostic and therapeutic strategies for malignancy by elucidating the underlying mechanism of miRNAs in tumor development. MiR-543-3p has been demonstrated to be up-regulated in diverse cancers, including lung cancer [22], colorectal cancer [23], gastric cancer [24] and hepatocellular carcinoma [25] and it promotes cell proliferation, migration and invasion via different mechanisms. We also found that miR-543-3p was markedly up-regulated in human bladder cancer, and was positively correlated with poor prognosis.
As a cohort of cells that are able to self-renew and to generate heterogeneous lineages of cancer cells, CSCs have been demonstrated to play crucial roles in tumor initiation, invasion, metastasis, chemo-resistance and recurrence [4,5]. Exploring the underlying mechanisms of CSCs maintaining a stem cell-like phenotype is critical to improve our understanding of cancer progression and discover potential therapeutic targets. Recent studies suggest that miRNAs play an important role in promotion or suppression of stemness maintenance of CSCs [16,17]. Geyan et al. showed that miR-1207 plays a significant role in promoting the stem cell-like phenotype in ovarian cancer [26]. Tang et al. revealed that miR-612 is able to suppress the stemness of hepatocellular carcinoma [27]. Herein, we found that ectopic overexpression of miR-543-3p in bladder cancer promoted proliferation and inhibited apoptosis. Moreover, overexpression of miR-543-3p enhanced, while silencing miR-543-3p inhibited, the CSC-like phenotypes.
The Wnt/β-catenin signaling pathway, a well-known pathway associated with cancer development and progression, has been found to be particularly activated and play a vital role in CSC maintenance [6,8]. In lung cancer, it has been reported that ablation of the β-catenin/TCF4 complex results in the inhibition of cancer stem cell properties [28]. In this respect, we found that miR-543-3p was substantially overexpressed in bladder cancer and induced hyper-activation of Wnt/β-catenin signaling through direct down-regulation different negative regulators of the pathway, including WIF1 and DKK1, hence enhancing bladder CSC-like traits. Furthermore, nuclear β-catenin positively correlated with the expression of miR-543-3p in bladder cancer clinical specimen. These findings reveal a novel mechanism in which Wnt/β-catenin signaling is constitutively activated in bladder cancers.
The dysregulation of WIF1 and DKK1, two important negative modulators of Wnt/β-catenin signaling, has been largely reported in numerous types of cancers [11,12]. For instance, WIF1 generally exhibited low levels of expression in gallbladder cancer, while rescuing expression of WIF1 in gallbladder cancer inhibits tumor growth and induces tumor cell apoptosis [29]. WIF1 has been demonstrated to act as a prognostic predictor of favorable outcomes in patients with colorectal carcinoma [30]. DKK1 is found to be down-regulated in gastric cancer that resulted in removal of the inhibitory effect of the Wnt/β-catenin signaling pathway, hence enhancing stem cell-like phenotype [31]. Our current study found that WIF1 and DKK1 were targeted and suppressed by miR-543-3p in bladder cancer, which promoted stem cell-like traits through highly activation of Wnt/β-catenin signaling. These data suggested a novel regulatory mechanism for WIF1 and DKK1, and also implies that suppression of WIF1 and DKK1 could enhance stemness of cancer cells.
In conclusion, this study demonstrated that by directly targeting multiple inhibitors of Wnt/β-catenin pathway, including WIF1 and DKK1, overexpression of miR-543-3p promoted cell proliferation and stem cell-like phenotype in bladder cancer cells. Therefore, these results reveal a new molecular mechanism that Wnt/β-catenin signaling pathway is hyper-activated in bladder cancer. This is an interesting finding which represents that miR-543-3p might serve as a potential therapeutic target for bladder cancer.
Acknowledgements
This work was supported by the Science and Technology Plan Project in Quanzhou (Z20150035).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortettieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Anghel RM, Gales LN, Trifanescu OG. Trifanescu, outcome of urinary bladder cancer after combined therapies. J Med Life. 2016;9:153–159. [PMC free article] [PubMed] [Google Scholar]
- 3.Ohishi T, Koga F, Migita T. Bladder cancer stem-like cells: their origin and therapeutic perspectives. Int J Mol Sci. 2015:17. doi: 10.3390/ijms17010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 2012;10:995–1009. doi: 10.1158/1541-7786.MCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 5.Pierzynski JA, Hildebrandt MA, Kamat AM, Lin J, Ye Y, Dinney CP, Wu X. Genetic variants in the Wnt/beta-Catenin signaling pathway as indicators of bladder cancer risk. J Urol. 2015;194:1771–6. doi: 10.1016/j.juro.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J, Guo D, Lu M, Liu F, Liu J, Ma C, Shi LL, Athiviraham A, He TC, Lee MJ. Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3:11–40. doi: 10.1016/j.gendis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/betacatenin signaling: components, mechanisms, and disease. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang K, Wang X, Zhang H, Wang Z, Nan G, Li Y, Zhang F, Mohammed MK, Haydon RC, Luu HH, Bi Y, He TC. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest. 2016;96:116–36. doi: 10.1038/labinvest.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazur M, Bujak A, Matloka M, Janowska S, Gunerka P, Bojarski L, Stanczak A, Klejman A, Bednarek A, Lamparska-Przybysz M, Wieczorek M. Cell-based assay for low- and highscale screening of the Wnt/beta-catenin signaling modulators. Anal Biochem. 2015;475:56–67. doi: 10.1016/j.ab.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014;10:512–25. doi: 10.1007/s12015-014-9515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsui Y, Yasumoto H, Nagami T, Hiraki M, Arichi N, Ishikawa N, Araki A, Maruyama R, Tanaka Y, Dahiya R, Shiina H. Extracellular activation of Wnt signaling through epigenetic dysregulation of Wnt inhibitory factor-1 (Wif-1) is associated with pathogenesis of adrenocortical tumor. Oncotarget. 2014;5:2198–207. doi: 10.18632/oncotarget.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogan Y, Halevi-Tobias KE, Hochman G, Baczmanska AK, Leyns L, Agur Z. A new validated mathematical model of the Wntsignalling pathway predicts effective combinational therapy by sFRP and Dkk. Biochem J. 2012;444:115–25. doi: 10.1042/BJ20111887. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz Y, Rateitschak K, Wolkenhauer O. Wolkenhauer, Analysing the impact of nucleocytoplasmic shuttling of beta-catenin and its antagonists APC, Axin and GSK3 on Wnt/betacatenin signalling. Cell Signal. 2013;25:2210–21. doi: 10.1016/j.cellsig.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie B, Ding Q, Han H, Wu D. miRCancer: a microRNA-cancer association database constructed by text mining on literature. Bioinformatics. 2013;29:638–44. doi: 10.1093/bioinformatics/btt014. [DOI] [PubMed] [Google Scholar]
- 16.Roden C, Lu J. MicroRNAs in control of stem cells in normal and malignant hematopoiesi. Curr Stem Cell Rep. 2016;2:183–196. doi: 10.1007/s40778-016-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osaki M, Okada F, Ochiya T. miRNA therapy targeting cancer stem cells: a new paradigm for cancer treatment and prevention of tumor recurrence. Ther Deliv. 2015;6:323–37. doi: 10.4155/tde.14.122. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Li Y, Wang N, Yang L, Zhao W, Zeng X. miR-130b targets NKD2 and regulates the Wnt signaling to promote proliferation and inhibit apoptosis in osteosarcoma cells. Biochem Biophys Res Commun. 2016;471:479–85. doi: 10.1016/j.bbrc.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Song JY, Park H, Jeong JY, Kwon AY, Heo JH, Kang H, Kim G, An HJ. miR-145, targeting high-mobility group A2, is a powerful predictor of patient outcome in ovarian carcinoma. Cancer Lett. 2015;356:937–45. doi: 10.1016/j.canlet.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Weng J, Wang C, Wang Y, Tang H, Liang J, Liu X, Huang H, Hou J. Beclin1 inhibits proliferation, migration and invasion in tongue squamous cell carcinoma cell lines. Oral Oncol. 2014;50:983–90. doi: 10.1016/j.oraloncology.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Shim JW, Park HS, Eum DY, Park MT, Mi Yi J, Choi SH, Kim SD, Son TG, Lu W, Kim ND, Yang K, Heo K. MacroH2A1 downregulation enhances the stem-like properties of bladder cancer cells by transactivation of Lin28B. Oncogene. 2016;35:1292–301. doi: 10.1038/onc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi M, Chen W, Yu H, Wang J, Ding F, Tang DJ, Tang C. miR-543 is up-regulated in gefitinib-resistant non-small cell lung cancer and promotes cell proliferation and invasion via phosphatase and tensin homolog. Biochem Biophys Res Commun. 2016;480:369–374. doi: 10.1016/j.bbrc.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 23.Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma H, Yu D, Maitikabili A, Xiao H, Zhang C, Liu F, Luo Q, Ouyang G. MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA2. Oncotarget. 2016;7:21825–39. doi: 10.18632/oncotarget.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Dong G, Wang B, Gao W, Yang Q. miR-543 promotes gastric cancer cell proliferation by targeting SIRT1. Biochem Biophys Res Commun. 2016;469:15–21. doi: 10.1016/j.bbrc.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Zhou L, Cheng Y, Sun L, Fan J, Liang J, Guo M, Liu N, Zhu L. MicroRNA-543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4:897–906. [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Liu A, Zhu J, Lei F, Wu S, Zhang X, Ye L, Cao L, He S. MiR-1207 overexpression promotes cancer stem cell-like traits in ovarian cancer by activating the Wnt/beta-catenin signaling pathway. Oncotarget. 2015;6:28882–94. doi: 10.18632/oncotarget.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J, Tao ZH, Wen D, Wan JL, Liu DL, Zhang S, Cui JF, Sun HC, Wang L, Zhou J, Fan J, Wu WZ. MiR-612 suppresses the stemness of liver cancer via Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2014;447:210–5. doi: 10.1016/j.bbrc.2014.03.135. [DOI] [PubMed] [Google Scholar]
- 28.Tong X, O’Kelly J, Xie D, Mori A, Lemp N, McKenna R, Miller CW, Koeffler HP. Cyr61 suppresses the growth of non-small-cell lung cancer cells via the beta-catenin-c-myc-p53 pathway. Oncogene. 2004;23:4847–55. doi: 10.1038/sj.onc.1207628. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Du Q, Wu W, She F, Chen Y. Rescued expression of WIF-1 in gallbladder cancer inhibits tumor growth and induces tumor cell apoptosis with altered expression of proteins. Mol Med Rep. 2016;14:2573–81. doi: 10.3892/mmr.2016.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Zhong X, Gao J, Song R, Wu H, Zi S, Yang S, Du P, Cui L, Yang C, Li Z. Coexpression of SFRP1 and WIF1 as a prognostic predictor of favorable outcomes in patients with colorectal carcinoma. Biomed Res Int. 2014;2014:256723. doi: 10.1155/2014/256723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan D, Ren B, Yang X, Liu J, Zhang Z. Upregulation of miR-501-5p activates the wnt/betacatenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J Exp Clin Cancer Res. 2016;35:177. doi: 10.1186/s13046-016-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


