Abstract
Background: This study aims to evaluate the roles of reactive oxygen species (ROS) generation and inflammasome formation in the development of diabetic rat wound inflammation. Materials and methods: Diabetes was induced in rats by a single intraperitoneal injection of STZ, and a skin wound (2×2 cm2) was produced on the back. Diabetic animals were treated with NAC and Bay 11-7082 to block ROS and the NLRP3 inflammasome, respectively. Total mRNA and protein were isolated from wound tissue and subjected to real-time polymerase chain reaction, Western blot analyses and ELISA. We also assessed the rate of wound closure and time to wound healing. Results: During healing, the diabetic rat exhibited increased ROS production, NLRP3 inflammasome activation and IL-1β secretion. NAC was responsible for the inhibition of ROS production and NLRP3 inflammasome activation in diabetic rat wounds. We also found that blocking ROS generation improved the impaired healing pattern in diabetic rats and decreased the time for complete skin healing. Conclusion: These data suggest that excessive ROS production contributes to activating NLRP3-IL-1β pathway events and impairs wound healing in a diabetic rat wound model.
Keywords: ROS, NLRP3 inflammasome, wound healing, diabetic rat
Introduction
Diabetic chronic wounds are one of the most complex pathological complications that affect the quality of life for diabetes mellitus (DM) patients and is the most common cause of hospitalization [1]. Healing impairment in diabetics is characterized by an increased and persistent inflammatory response, which in turn delays cellular infiltration and granulation tissue formation, consequently decreasing collagen organization and angiogenesis [2,3].
More recent studies have focused on innate immune-sensing receptors and their role in inflammation and the disease process. The NLRP3 inflammasome, a molecular platform that modulates innate immune functions by activation of caspase-1, catalyzes the proteolytic processing and secretion of IL-1β and IL-18 in immune cells [4]. This cascade triggers sustained inflammation, which has been associated with metabolic diseases [5,6]. In our previous study, we found a significantly higher expression of NLRP3, caspase-1, and secretion of IL-1β in human diabetic wounds [7]. This suggests that NLRP3 inflammasome activation might contribute to the hyperinflammation in wound healing.
Activation of the NLRP3 inflammasome can be induced by intracellular ROS generation in response to a variety of cellular stressors [8]. ROS formation is a normal byproduct of cellular metabolism and plays an important role in regulating cell signaling pathways [9]. Environmental stressors can dramatically increase ROS production, resulting in significant cell death [10]. Recently, an exciting role for ROS has been revealed in inflammatory disorders, especially in the activation and control of innate immune responses. However, the precise role of an excess of ROS production in the development of diabetic wound inflammation is not well understood.
N-acetylcysteine (NAC) is an antioxidant that acts as a free radical scavenger; it also stimulates glutathione synthesis, which acts intra- and extracellularly as an antioxidant eliminating ROS [11]. In this study, we detect the expression of the NLRP3 inflammasome and IL-1β in the wounds of diabetic rats and then observe the changes in influential components using NAC blocked ROS, aiming to evaluate the role of ROS generation and inflammasome formation in the development of diabetic rat wound inflammation. This may provide a new approach for improving the chronic wound healing process in diabetes mellitus.
Materials and methods
Animal
All male rats are purchased from Shanghai SLAC Laboratory Animal Co. Ltd. In total, 64 Sprague-Dawley rats (200-220 g, 6 weeks) are used in this study. During the experiments, the animals are housed one per cage under controlled environmental conditions (12 h light/dark cycle, 23°C) and provided with standard food and water ad libitum. All animal maintenance and experimental procedures are carried out in accordance with the US National Institute of Health Guidelines for the Use of Experimental Animals and approved by the Ethics Review Board of Shanghai Six People’s Hospital affiliated to Shanghai Jiaotong University.
We randomize rats into 4 groups (n=12 per group): Control (SD), diabetes mellitus (DM), DM + BAY 11-7082 and DM + NAC. The control group is fed the basal diet and the other groups are fed a high fat diet (HF diet, 16% fat and 0.25% cholesterol). Diabetes is induced by a single intraperitoneal injection of streptozotocin (STZ; 60 mg/kg i.p.) to rats [12] in the DM, DM + BAY 11-7082 and DM + NAC groups. Blood glucose levels are measured 72 h later, after rats have fasted overnight. Except for the control group, only rats with blood glucose levels ≥ 11.1 nmol/L are used in the study.
All rats in this study are anesthetized by pentobarbitone sodium (50 mg/kg i.p.). The hair on the back is shaved and the skin is sterilized with alcohol. Full-thickness of skin defects of 2×2 cm2 are made on rats.
Diabetic rats receive either BAY 11-7082 (20 mg/kg i.p.) or NAC (20 mg/kg i.p.) every 2 days until the wounds close completely. Four animals from each group are killed seven and fourteen days after wounding, and the wound is removed using a scalpel to cut the shape of an ellipse around the lesion. The tissue around the wound is collected for Western blot, gene expression and ELISA assays. The last 4 rats of each group are retained to measure the time needed to complete the wound closure (up to 40 days).
The following compounds are supplied as shown: BAY 11-7082 by Adipogene (San Diego, CA); NAC by Sigma Aldrich (Milan, Italy), and STZ by Sigma Aldrich (Milan, Italy).
Wound analysis
The wound images are collected using a digital camera every two days until the wounds heal. The morphometric analysis of the wounds is assessed using images of the wounds at days 0, 7, 14, and day 21 post-operation, the remaining wound area is measured using Image J software (NIH, US). The rate of wound closure that represents the percentage of wound reduction from the original wound size is calculated using the following formula: wound area day 0-wound area (day 7, 14, and 21)/wound area day 0×100. Values are expressed as percentages of the healed wounds. The time for complete wound closure is defined as the time needed for the wound bed to be completely re-epithelialize and fill with new tissue. It is shown by a closed linear healing ridge and is determined as described previously [13].
Real-time polymerase chain reaction
Total RNA is extracted from wound tissue by cell lysis with TRIzol (Invitrogen, Carlsbad, CA, USA). We use the reverse transcriptase kit (Promega, Madison, Wisconsin, USA) to synthesize the first strand of cDNA. The levels of NLRP3, caspase-1, ASC and IL-1β are measured by real-time PCR; β-actin is used as an endogenous control. Relative gene expression is determined using the 2-ΔCT method.
Western blotting
Western-blotting is employed for measuring the expression level of NLRP3, ASC and caspase-1 at days 7 and 14. We use antibodies against NLRP3, ASC and caspase-1 (Cell Signaling, Danvers, MA, USA). After washing, the membranes are incubated with the secondary peroxidase conjugated antibodies (Abcam, Cambridge, UK) at room temperature for 1 hour. Finally, the immunoreactive protein signals are visualized by chemiluminescence (ECL, Promega), and quantified by scanning densitometry. The results of the Western-blot, measured on stripped blots, are expressed as the integrated intensity relative to β-actin.
ELISA
The wound lysate (30 mg of wound tissue in 1 ml of PBS) is prepared. After centrifuging at 15,000×g for 15 min, the supernatant is used for ELISA assays. IL-1β is analyzed by using human-specific ELISA assay kits (Anogen, Mississauga, Ontario, Canada) as instructed by the manufacturer. The level of IL-1β is expressed as pg/mL.
Intracellular ROS measurement
The intracellular ROS level is measured using the probe 2’,7’-dichlorofluorescein diacetate (DCFH-DA; Sigma). The supernatant from skin homogenates is directly treated with 10 μM DCFH-DA at 37°C for 30 min. The fluorescence intensity is monitored using an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Statistical analyses
Quantitative data are presented as the mean ± standard deviations. Student’s t-test is used to compare two groups. A one-way analysis of variance (ANOVA) is used to assess the differences between multiple groups, followed by Tukey’s post hoc test. All statistical analyses are performed using SPSS 19.0 Windows edition (SPSS, Inc. Chicago, IL, USA). P < 0.05 is considered statistically significant.
Results
ROS and NLRP3 inflammation expression in diabetic and non-diabetic rat wounds
The data show increased and persistent ROS production in diabetic rats at days 7 and 14 (Figure 1I). The data also show higher levels of NLRP3, caspase-1 and ASC protein in the diabetic rat groups (Figure 1A-C, 1J). With the up-regulated proteolytic activity of NLRP3, the level of IL-1β is also increased (Figure 1D). At the gene level, we find a similar phenomenon. In the control group, lower mRNA expression of NLRP3, caspase-1 and ASC are found at all time points (Figure 1E-G), as well as with IL-1β mRNA (Figure 1H). These data indicate that ROS production, NLRP3 inflammation activation and IL-1β secretion in diabetic rat wounds are abnormally increased.
Figure 1.

ROS production and NLRP3 inflammasome expression in diabetic (DM) and non-diabetic (SD) rat wound. The protein level of NLRP3, caspase-1, ASC and IL-1β are measured by Western blot (A-C, J) and. Elisa (D), respectively. The mRNA level of NLRP3, ASC, Caspase1 and IL-1β are measured by real-time PCR (E-H). The intracellular ROS production is measured with fluorescence probe DCFH-DA (I). Data is presented as the means ± SD. *P < 0.05 versus control.
NAC is responsible for the inhibition of ROS production and NLRP3 inflammasome activation
We examine changes in the NLRP3 inflammasome activity and ROS production when wounds are treated with the ROS scavenger NAC. ROS production is inhibited at day 7 and day 14 (Figure 2I). NAC suppresses the increased mRNA levels of the NLRP3 inflammasome and of IL-1β induced by diabetes, but not for ASC mRNA level at day 7 (Figure 2E-H). Protein levels of the NLRP3 inflammasome and mature IL-1β are decreased after NAC treatment (Figure 2A-D, 2J).
Figure 2.
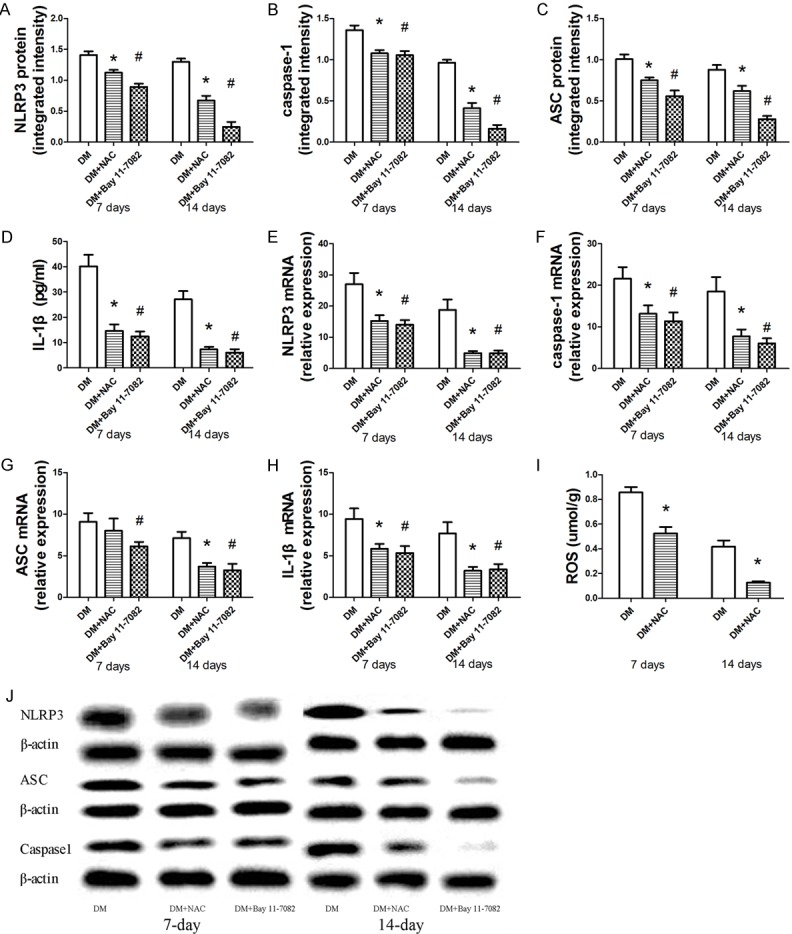
The role of NAC and BAY 11-7082 treatment of the expression of ROS generation and NLRP3 inflammasome activation in diabetic rat wound. The protein level of NLRP3, caspase-1, ASC and IL-1β are measured by Western blot (A-C, J) and. Elisa (D), respectively. The mRNA level of NLRP3, ASC, Caspase1 and IL-1β are measured by real-time PCR (E-H). The intracellular ROS production is measured with fluorescence probe DCFH-DA (I). Data is presented as the means ± SD. *P < 0.05 versus control; #P < 0.05 versus control.
Inflammasome blockade and activation of downstream pathways
The effectiveness of the NLRP3 inhibitor BAY 11-7082 in reducing NLRP3 mRNA and protein expression is verified with real-time PCR and Western blot analysis (Figure 2A, 2E, 2J). Meanwhile, the protein and mRNA expression of ASC and caspase-1 are significantly reduced in BAY 11-7082 treated diabetic rat wounds (Figure 2B, 2C, 2F, 2G, 2J). Declining levels of the active forms of IL-1β in wound lysate are sustained at day 7 and day 14 after BAY 11-7082 treatment (Figure 2D, 2H).
Wound closure
Images of the wounds are taken at days 0, 7, 14 and 21 (Figure 3A). Rats are observed for up to 40 days and complete wound healing is observed at 20 ± 1.4 in the control group, while wounds in diabetic rats close completely after 27 ± 2.2 days. Treatment with NAC or BAY 11-7082 improves the wound closure (22.0 ± 1.4 and 24 ± 1.1 days, respectively) of rat skin when compared with the diabetic group (Figure 3B). The mean rates of wound healing are higher in the NAC and BAY 11-7082 treatment groups when compared with the control group on days 7, 14 and 21 post-wounding (Figure 3B).
Figure 3.

Wound healing of the diabetic wound. A: Gross imaging of the wound healing at different time points as indicated. B: The areas of wounds are quantified at different time points as indicated and the mean ratios of wounds closure are expressed.
Discussion
In this study, we investigate the role of ROS production and the NLRP3 inflammasome signaling pathway in the wound healing of diabetes mellitus, and the results reveal that in a diabetic rat wound model, an increase in ROS generation triggers NLRP3 inflammasome complexation and activation leading to increases in bioactive IL-1β secretion and impaired wound healing.
Chronic wounds are typically associated with a persistent inflammatory response [14]. Findings from previous studies have reported elevated levels of IL-1β in wounds from humans [7] and diabetic mice [15]. In our study, we also find increased in bioactive IL-1β secretion in a diabetic rat model, which exhibits a persistent inflammatory response and impaired wound healing. Sustained IL-1β expression in diabetic wounds is associated with a pro-inflammatory macrophage phenotype [16]. Pro-inflammatory cytokines such as IL-1β and TNF-α act as primary chronic stimulators of cell metabolism and stimulate the innate immune response, activating downstream signal transduction pathways to produce large amounts of inflammatory mediators and lead to chronic inflammation. All these persistent inflammatory responses contribute to delayed re-epithelialization and granulation tissue formation, and impaired angiogenesis and wound healing [17].
The secretion of bioactive IL-1β is predominantly controlled by the activation of caspase-1 through the assembly of a multiprotein scaffold inflammasome composed of NLRP3, ASC, and procaspase-1 [18]. Our previous studies have demonstrated a significantly higher expression of the NLRP3 inflammasome and secretion of IL-1β in human diabetic wound and in high glucose-induced macrophages, suggesting that NLRP3 inflammasome activation might contribute to hyperinflammation in wound healing [7]. In this study, we report the activation of the NLRP3 inflammasome in a diabetic rat wound model. We also demonstrate the effectiveness of blocking the NLRP3 inflammasome on wound healing, due to reduced activation of the inflammatory cascade. Rats treated with BAY 11-7082 demonstrate a marked reduction in active caspase-1 expression and IL-1β secretion at the wound site. This emphasizes that the activation of the NLRP3 inflammasome is one of the mechanisms involved in impaired healing during glucotoxicity.
The mechanisms leading to NLRP3 inflammasome activation are a matter of debate. Previous literature has reported several models, including the K+ channel model, lysosomal damage model, and ROS model, although they may not be mutually exclusive [19,20]. More recent studies indicate that NLRP3 agonists trigger ROS generation, which leads to activation of the NLRP3 inflammasome via the ROS-sensitive NLRP3 ligand thioredoxin interacting protein (TXNIP) [21,22]. Mitochondria and NADPH oxidase are the two major sources of ROS that are induced by external stimuli, and the mitochondria respiratory chain is considered an important site of ROS production within most cells [23]. Under the condition of oxidative stress, ROS is overproduced leading to DNA fragmentation, lipid peroxidation and protein oxidation [24]. Shimada et al. have reported that mitochondrial dysfunction and apoptosis can lead to oxidized mtDNA, a ROS oxidation product, being released into the cytosol and directly binding to NLRP3, which is regarded as a new molecule, to trigger the secondary signal of NLRP3 activation [25]. Other researchers demonstrated that lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages can be inhibited by molecular hydrogen via targeting mitochondrial reactive oxygen species [26]. ROS-dependent activation of NLRP3 emphasizes the application of antioxidants with ROS-scavenging ability in the treatment of inflammatory pathological damage. Even ROS has been largely linked to various diseases including cancer, heart disease, and autoimmune disease, but there is not sufficient evidence to show how it impacts chronic wound healing in diabetes mellitus [27].
The role of ROS production in promoting this chain of events is proven by showing that the inhibitor NAC downregulates NLRP3 inflammasome activation and IL-1β secretion. In vivo the therapeutic effectiveness of NAC is validated by showing that NAC treatment improves the wound healing in diabetic rats. All these effects normalize skin remodeling, as suggested by the rate of wound healing and time to complete closure, which represents the main outcome for any therapeutic agent aimed at improving wound healing in diabetic rats. If the ROS-induced inflammasome activation is proven in humans to contribute to diabetic wound inflammation pathogenesis, targeting ROS generation with therapeutic drugs may be an effective approach to treat this condition.
In conclusion, we identify a functional role for excessive ROS production in the activation of the NLRP3-IL-1β pathway in a diabetic rat wound model. This finding implicates an essential priming role for the ROS-NLRP3-IL-1β pathway in chronic diabetic wounds, which reveals a potentially novel target for ROS inhibitors, which could target the priming source of this disease in rats. The mouse model symptoms are similar to what occurs in diabetic patients. This correspondence warrants further studies to assess in a clinical setting if treatment with ROS inhibitors improves wound healing in patients with diabetes.
Acknowledgements
This work was supported partially by the National Natural Science Foundation of China (No.81572122) and Foundation of Shanghai Sixth People’s Hospital (ynlc201505).
Disclosure of conflict of interest
None.
References
- 1.Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17060917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitto A, Altavilla D, Pizzino G, Irrera N, Pallio G, Colonna MR, Squadrito F. Inhibition of inflammasome activation improves the impaired pattern of healing in genetically diabetic mice. Br J Pharmacol. 2014;171:2300–2307. doi: 10.1111/bph.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodson WH 3rd, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res. 1977;22:221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- 4.Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabon MA, Rooney KT, Yoon JH, Ryter SW, Stout-Delgado H, Choi AM. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20:8525–8534. doi: 10.3748/wjg.v20.i26.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Dai J, Li L, Chen H, Chai Y. NLRP3 inflammasome expression and signaling in human diabetic wounds and in high glucose induced macrophages. J Diabetes Res. 2017;2017:5281358. doi: 10.1155/2017/5281358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, Lee YC. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014;5:e1498. doi: 10.1038/cddis.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct. 2012;2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey V, Ranjan S, Deeba F, Pandey AK, Singh R, Shirke PA, Pathre UV. Desiccationinduced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. J Plant Physiol. 2010;167:1351–1359. doi: 10.1016/j.jplph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 11.El-Lakkany NM, Seif El-Din SH, Sabra AA, Hammam OA, Ebeid FA. Co-administration of metformin and N-acetylcysteine with dietary control improves the biochemical and histological manifestations in rats with non-alcoholic fatty liver. Res Pharm Sci. 2016;11:374–382. doi: 10.4103/1735-5362.192487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen QY, Wang GG, Li W, Jiang YX, Lu XH, Zhou PP. Heme Oxygenase-1 promotes delayed wound healing in diabetic rats. J Diabetes Res. 2016;2016:9726503. doi: 10.1155/2016/9726503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altavilla D, Squadrito F, Polito F, Irrera N, Calo M, Lo Cascio P, Galeano M, La Cava L, Minutoli L, Marini H, Bitto A. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery. 2011;149:253–261. doi: 10.1016/j.surg.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–1114. doi: 10.2337/db13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healingassociated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGettrick AF, O’Neill LA. NLRP3 and IL-1beta in macrophages as critical regulators of metabolic diseases. Diabetes Obes Metab. 2013;15(Suppl 3):19–25. doi: 10.1111/dom.12169. [DOI] [PubMed] [Google Scholar]
- 17.Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One. 2015;10:e0119106. doi: 10.1371/journal.pone.0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RW, Dixit VD. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol. 2013;4:50. doi: 10.3389/fimmu.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 20.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen H, Miao EA, Ting JP. Mechanisms of NODlike receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 23.Nash KM, Ahmed S. Nanomedicine in the ROS-mediated pathophysiology: applications and clinical advances. Nanomedicine. 2015;11:2033–2040. doi: 10.1016/j.nano.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11:e0152925. doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren JD, Wu XB, Jiang R, Hao DP, Liu Y. Molecular hydrogen inhibits lipopolysaccharidetriggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochimica Et Biophysica Acta. 2016;1863:50–55. doi: 10.1016/j.bbamcr.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]


