Abstract
Cervical cancer is one of the most lethal gynecological malignancy. Aberrant expression of microRNAs (miRNAs) is associated with carcinogenesis of cervical cancer. The aim of current study was to elucidate the clinical significance of miR-22 in patients with cervical cancer. The expression levels of miR-22 in cervical intraepithelial neoplasia (CIN) and cervical cancer tissues as well as cervical cancer cell lines were quantified using real-time PCR. Then the potential prognostic value of the miR-22 was assessed. Our results showed that the expression level of miR-22 was significantly lower in cervical cancer tissues and cell lines. miR-22 levels were inversely correlated clinical stage both in CIN and cervical cancer. In addition, low expression of miR-22 was significantly associated with larger tumor size, presence of lymph node metastasis, and advanced tumor stage. Moreover, the patients expressing low levels of miR-22 had poorer five year overall survival and disease free survival. Multivariate analysis identified miR-22 as an independent prognostic factor for cervical cancer patients’ overall survival. Taken together, we demonstrate that the miR-22 may serve as a novel therapeutic target and prognostic marker in patients with cervical cancer.
Keywords: microRNA-22, cervical cancer, prognostic biomarker
Introduction
Cervical cancer is the third most common gynecological malignancy in female worldwide [1]. The progress in cervical cytology and cervical pathological biopsy has significantly improved the prognosis of cervical cancer in the past few decades [2]. Patients diagnosed at the early stage have favorable long term clinical outcome. However, the five year overall survival of cervical cancer patients at the advanced stage remain dismal [3]. Thus identifying molecular biomarkers predicating the prognosis of cervical cancer is beneficial to the individualized therapy.
MicroRNAs (miRNAs) are highly conserved, endogenous small non-protein-coded RNAs that negatively regulate gene expression at the post-transcriptional level by mRNA destabilization and translational inhibition [4]. Aberrant expression of miRNAs is involved in a number of physiological and pathological processes. Accumulating studies have demonstrated that miRNAs play an important role in the tumorigenesis and these molecules have become useful biomarkers for early detection, diagnosis and prognosis of various types of cancers including cervical cancer [5-7]. miR-425-5p was upregulated in the tissue and serum samples from patients with cervical cancer compared to the healthy controls. In addition, increased tissue and serum miR-425-5p levels were both correlated with unfavorable clinicopathological parameters in cervical cancer [8]. Zhou et al showed that the expression level of miR-519d was significantly higher in cervical cancer tissues than the adjacent normal tissues. Inhibition of miR-519d suppressed the proliferation, migration and invasion capacity of cancer cells and Smad7 was identified as the direct downstream target, indicating that miR-519d was an oncomiR in cervical cancer [9].
miR-22 is located in human chromosome 17 band p13.3. Deregulation of miR-22 has been found in many cancers such as acute myeloid leukemia, gastric cancer and hepatocellular carcinoma [10-12]. Interestingly, miR-22 might act as either an oncogene or a tumor suppressor gene, depending on the tumor type [13,14]. In the present study, we found that the expression level of miR-22 was significantly downregulated in cervical intraepithelial neoplasia (CIN) tissues and cervical cancer tissues. Also, reduced miR-22 levels were strongly associated with progression and worse clinical outcome of cervical cancer.
Materials and methods
Cell culture
Five cervical cancer cell lines SiHa, HeLa, Ca-Ski, HeLa229 and MS75 and one normal control cell line NCC-16 were used for this study. All the cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium (Gibco BRL, Rockville, USA) supplemented with 10% fetal bovine serum (Gibco BRL) 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a 5% CO2 humidified atmosphere.
Subjects and samples
This study was performed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital. A total of 105 cervical cancer patients and 40 CIN patients who underwent treatment in our department were recruited. Informed consent was obtained from all the participants. None of the patients had received chemoradiotherapy and surgery before enrollment. Cervical cancer was classified according to the classification system recommended by the International Federation of Gynecology and Obstetrics. Clinical characteristics were obtained from patients’ medical records. The detailed information of cervical patients was summarized in Table 1.
Table 1.
Relationship between miR-22 expression and clinicopathological characteristics
| Variables | Group | miR-22 expression | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age | <60 | 25 | 24 | 0.639 |
| ≥60 | 26 | 30 | ||
| Tumor size | <4 cm | 13 | 26 | 0.016 |
| ≥4 cm | 38 | 28 | ||
| Lymph node metastasis | No | 22 | 39 | 0.003 |
| Yes | 29 | 15 | ||
| Distant metastasis | No | 45 | 51 | 0.256 |
| Yes | 6 | 3 | ||
| FIGO stage | I/II | 14 | 34 | <0.001 |
| III/IV | 37 | 20 | ||
| Histological grade | Well/Moderate | 27 | 37 | 0.102 |
| Poor | 24 | 17 | ||
| HPV infection | No | 16 | 18 | 0.830 |
| Yes | 35 | 36 | ||
| Histological type | Squamous | 42 | 48 | 0.339 |
| Adenocarcinoma | 9 | 6 | ||
RNA isolation
Total RNA was isolated from tissue samples using the TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s protocol. Total RNA concentration was determined by a NanoDrop 2000 (Nanodrop Technologies, Wilmington, USA) and agarose gel electrophoresis was performed to check the RNA integrity. The pure RNA samples were stored at -80°C until further use.
Real-time polymerase chain reaction (qPCR)
The total RNA was reverse transcribed to complementary DNA using The SuperScript III reverse transcription kit (Invitrogen, Carlsbad, USA). miR-22 levels were quantified by using a Taqman MicroRNA Assay Kit (Applied Biosystems, Foster City, USA) and run on a ABI 7900 Real-Time PCR System (Applied Biosystems). Quantitative PCR was conducted at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. U6 small nuclear RNA was used as an internal control and the relative expression of miR-22 was evaluated by the 2-ΔΔCt method.
Statistical analyses
The expression levels of miR-22 were compared among different groups using the One way ANOVA or Kruskal-Wallis test. The association between miR-22 levels and clinicopathologic variables was analyzed by chi-square test. Survival analyses were performed using the Kaplan-Meier method in combination with the log-rank test. Multivariate analysis was performed to identify independent prognostic factors correlated with overall survival and disease free survival. All P values were two-sided and P<0.05 was considered statistically significant. Statistical analysis was performed with GraphPad Prism 5.0 (Graphpad Software Inc., San Diego, USA).
Results
miR-22 was downregulated in cervical cancer cell lines
We first compared the expression level of miR-22 between cervical cancer cell lines and the normal cell line NCC-16 using qPCR. Our results showed that miR-22 levels were significantly decreased in all five cervical cancer cell lines when compared to the normal control (**P<0.01) (Figure 1).
Figure 1.
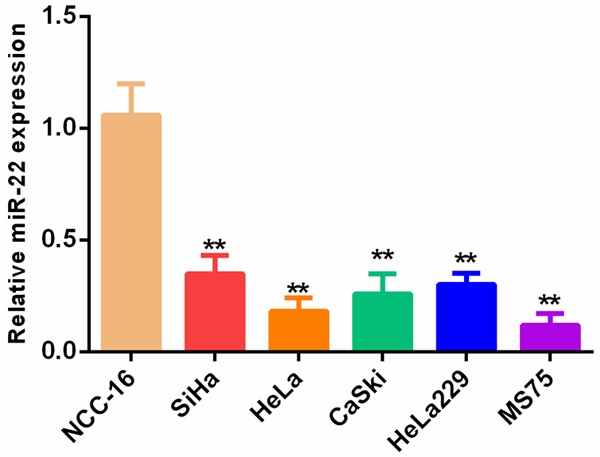
miR-22 expression was significantly downregulated in cervical cancer cells.
miR-22 levels were decreased in cervical cancer and CIN tissue specimens
qPCR was performed to compare the miR-22 levels among cervical cancer tissues, CIN tissues and normal controls. The results demonstrated that the expression level of miR-22 was highest in normal controls and progressively decreased from CIN tissues to cervical cancer tissues (**P<0.01, ***P<0.001) (Figure 2).
Figure 2.
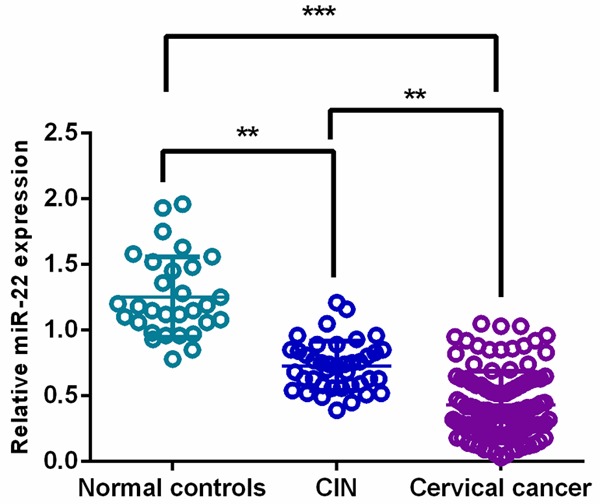
miR-22 levels were decreased in cervical cancer and CIN tissue specimens.
miR-22 expression was inversely correlated clinical stage both in CIN and cervical cancer
A negative correlation was found between miR-22 levels and clinical stage in CIN and cervical cancer. miR-22 levels were significantly decreased in CIN patients with higher clinical stage compared with those with lower clinical stage (**P<0.01) (Figure 3A). Similar findings was observed in patients with cervical cancer (*P<0.05, **P<0.01, ***P<0.001) (Figure 3B).
Figure 3.
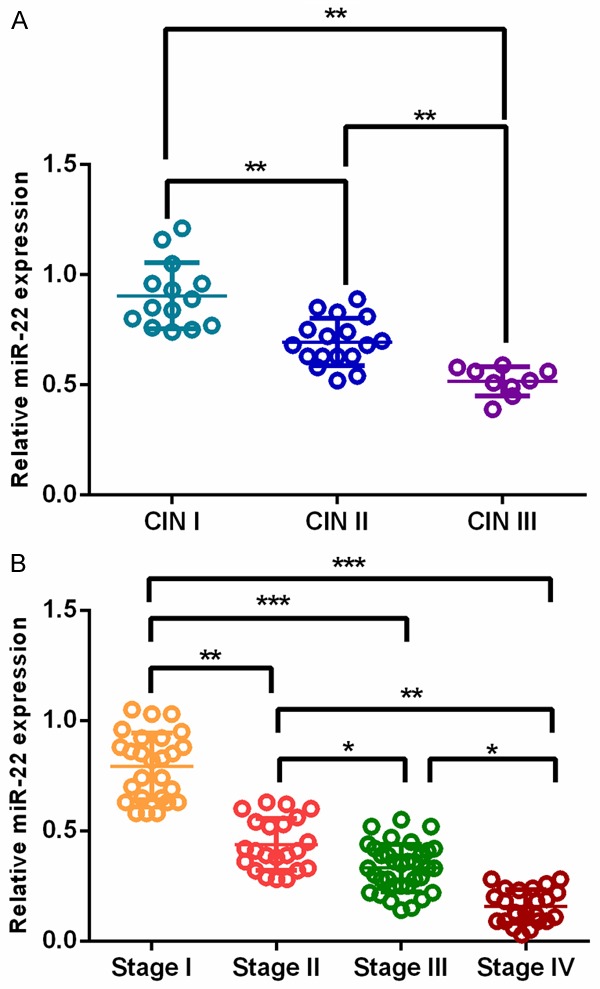
miR-22 expression was inversely correlated clinical stage both in CIN and cervical cancer.
Association between miR-22 expression levels and clinicopathological parameters
The median value of miR-22 expression was used as a threshold point to classify all the 105 cervical cancer patients into high miR-22 expression group (n=54) and low miR-22 expression group (n=51). Reduced miR-22 expression was associated with larger tumor size (P=0.016), presence of lymph node metastasis (P=0.003) and advanced clinical stage (P<0.001), while it was not correlated with other clinicopathological parameters such as age, distant metastasis, histological grade, histological type and HPV infection (Table 1).
The prognostic significance of miR-22 in cervical cancer
Kaplan-Meier survival analysis and Cox proportional hazard analysis were used to determine the prognostic value of miR-22 in cervical cancer. Our results showed that patients with lower levels of miR-22 had a 5-year overall survival of 43.1%, which was significantly poorer than that of those (75.9%) with higher expression of miR-22 (P=0.005) (Figure 4A). Patients in the low miR-22 group also suffered worse 5 year disease free survival (P=0.012) (Figure 4B).
Figure 4.
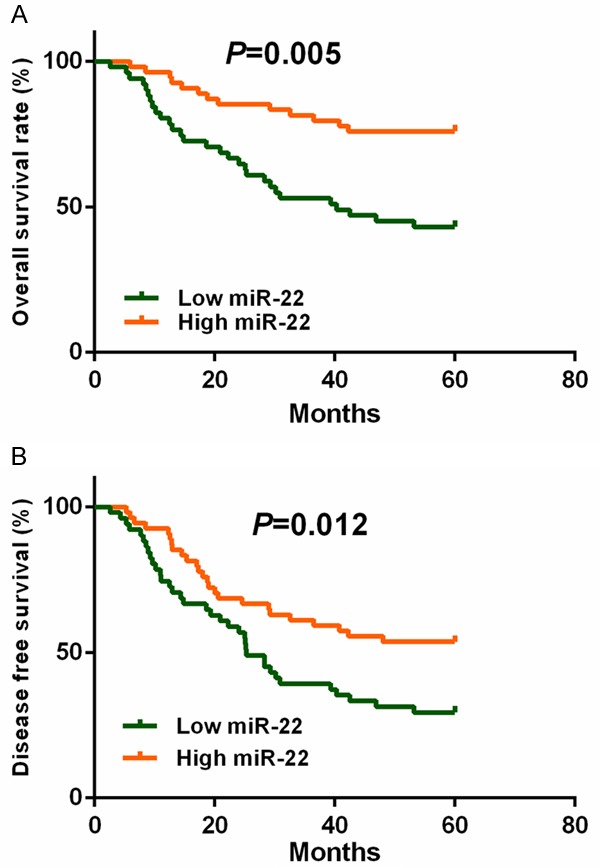
Survival analysis of cervical cancer patients with high or low miR-22 expression.
Multivariate analysis showed that lymph node metastasis (HR=1.752, 95% CI=1.135-2.602, P=0.021), clinical stage (HR=2.328, 95% CI=1.439-3.142, P=0.004) and miR-22 expression (HR=1.820, 95% CI=1.167-2.831, P=0.017) were independent prognostic factors associated with overall survival of cervical cancer (Table 2).
Table 2.
Multivariate Cox’s hazards model analysis for prognostic factors associated with overall survival
| Variables | Overall survival | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P | |
| Tumor size (≥4 cm vs <4 cm) | 1.416 | 0.971-2.283 | 0.086 |
| Lymph node metastasis (Yes vs No) | 1.752 | 1.135-2.602 | 0.021 |
| Clinical stage (III/IV vs I/II) | 2.328 | 1.439-3.142 | 0.004 |
| miR-22 expression (low vs high) | 1.820 | 1.167-2.831 | 0.017 |
HR indicates Hazard ratio; CI indicates confidence interval.
Discussion
Cervical cancer is a major health concern and remains a significant source of morbidity and mortality for women around the world [15,16]. In this study, we found that miR-22 levels were significantly decreased in cancer cell lines, CIN tissues and cervical cancer tissues compared to normal controls. In addition, a positive correlation was found between reduced miR-22 level and advanced TNM stage, presence of lymph node metastasis, larger tumor size and worse survival. Moreover, miR-22 was found to be an independent risk factor for cervical cancer.
Based on our results, miR-22 plays a tumor suppressive role in cervical cancer and might be employed as a useful prognostic biomarker for this malignancy. As miR-22 was progressively downregulated from CIN to cervical cancer, indicating that miR-22 might be implicating in the early stage of carcinogenesis. Similarly, miR-22 inhibited proliferation and invasion as well as promoted apoptosis of cervical cancer cells by targeting ATP citrate lyase. miR-22 was also downregulated in cervical cancer samples and could suppress tumor growth and metastasis in mouse model. Similar findings were observed in other types of cancers including osteosarcoma, prostate cancer and lung cancer [17]. Further studies of larger samples are required for the validation of our findings. Also we are very interesting in the diagnostic and prognostic value of circulating miR-22 for cervical cancer. What’s more, the underlying molecular mechanisms need further investigation.
miR-22 was also reported to function as a tumor suppressor gene in many types of cancers. For instance, the expression levels of miR-22 were remarkably reduced in lung cancer tissues and cell lines. Ectopic expression of miR-22 suppressed the proliferative and invasive capacity of cancer cells both in vitro and in vivo. ErbB3 was identified as a downstream target of miR-22 [18]. miR-22 was decreased in colon cancer tissues. Overexpression of miR-22 inhibited the expression of HIF-1α and the production of vascular endothelial growth factor (VEGF) and opposite findings was observed when miR-22 was underexpressed. More importantly, the miR-22 levels in cancer cells seemed to be associated with the function of endothelial cell, suggesting that miR-22 might have an anti-angiogenic effect [19].
To the best of our knowledge, only few studies demonstrated that miR-22 play an oncogenic role. miR-22 was overexpressed in myelodysplastic syndrome and associated with worse prognosis. In addition, upregulation of miR-22 promoted the development of hematological malignancies, and vice versa. TET2 was demonstrated to be a direct downstream target of miR-22 [13]. Jiang et al reported that miR-22 was an important promoter of HBV associated hepatocellular carcinoma. A negative relation was found between miR-22 and ERα, indicating miR-22 might accelerate the carcinogenesis by attenuating the protective effect of estrogen [11].
In conclusion, miR-22 is reduced in cervical cancer and associated with poor clinical outcome. The present results indicates that downregulation of miR-22 has the potential to be a promising prognostic biomarker for cervical cancer.
Acknowledgements
This study was supported partly by the grant from Department of Gynecology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sakuragi N. Refining insight into cervical cancer progression. Lancet Oncol. 2014;15:371–372. doi: 10.1016/S1470-2045(14)70085-3. [DOI] [PubMed] [Google Scholar]
- 3.Kong J, Di C, Piao J, Sun J, Han L, Chen L, Yan G, Lin Z. Ezrin contributes to cervical cancer progression through induction of epithelialmesenchymal transition. Oncotarget. 2016;7:19631–19642. doi: 10.18632/oncotarget.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelo B, Benedetti E, Cimini A, Giordano A. MicroRNAs: a puzzling tool in cancer diagnostics and therapy. Anticancer Res. 2016;36:5571–5575. doi: 10.21873/anticanres.11142. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Farwell MA. microRNAs: a new emerging class of players for disease diagnostics and gene therapy. J Cell Mol Med. 2008;12:3–21. doi: 10.1111/j.1582-4934.2007.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Li B, Xie X. The roles and clinical significance of microRNAs in cervical cancer. Histol Histopathol. 2016;31:131–139. [PubMed] [Google Scholar]
- 8.Sun L, Jiang R, Li J, Wang B, Ma C, Lv Y, Mu N. MicoRNA-425-5p is a potential prognostic biomarker for cervical cancer. Ann Clin Biochem. 2017;54:127–133. doi: 10.1177/0004563216649377. [DOI] [PubMed] [Google Scholar]
- 9.Zhou JY, Zheng SR, Liu J, Shi R, Yu HL, Wei M. MiR-519d facilitates the progression and metastasis of cervical cancer through direct targeting Smad7. Cancer Cell Int. 2016;16:21. doi: 10.1186/s12935-016-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Hu C, Arnovitz S, Bugno J, Yu M, Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, Weng H, Strong J, Wang Y, Li Y, Salat J, Li S, Elkahloun AG, Yang Y, Neilly MB, Larson RA, Le Beau MM, Herold T, Bohlander SK, Liu PP, Zhang J, Li Z, He C, Jin J, Hong S, Chen J. miR-22 has a potent anti-tumour role with therapeutic potential in acute myeloid leukaemia. Nat Commun. 2016;7:11452. doi: 10.1038/ncomms11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang R, Deng L, Zhao L, Li X, Zhang F, Xia Y, Gao Y, Wang X, Sun B. miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin Cancer Res. 2011;17:5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 12.Zuo QF, Cao LY, Yu T, Gong L, Wang LN, Zhao YL, Xiao B, Zou QM. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and snail. Cell Death Dis. 2015;6:e2000. doi: 10.1038/cddis.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, Jongen-Lavrencic M, Manova-Todorva K, Teruya-Feldstein J, Avigan DE, Delwel R, Pandolfi PP. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song SJ, Pandolfi PP. miR-22 in tumorigenesis. Cell Cycle. 2014;13:11–12. doi: 10.4161/cc.27027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031–1037. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin M, Qiao Z, Li J, Liu J, Song S, Zhao X, Miao P, Tang T, Wang L, Liu W, Yang X, Dai K, Huang G. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget. 2016;7:44252–44265. doi: 10.18632/oncotarget.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling B, Wang GX, Long G, Qiu JH, Hu ZL. Tumor suppressor miR-22 suppresses lung cancer cell progression through post-transcriptional regulation of ErbB3. J Cancer Res Clin Oncol. 2012;138:1355–1361. doi: 10.1007/s00432-012-1194-2. [DOI] [PubMed] [Google Scholar]
- 19.Yamakuchi M, Yagi S, Ito T, Lowenstein CJ. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One. 2011;6:e20291. doi: 10.1371/journal.pone.0020291. [DOI] [PMC free article] [PubMed] [Google Scholar]


