Abstract
Intravascular natural killer/T-cell lymphoma (IVNKTL) is a rare disorder and is reported gradually increased recently. We presented four cases including two extremely rare cases of primary lung IVNKTL with detailed clinicopathological features, therapy and prognosis, and reviewed the literature for 25 similar cases. H&E, Immunohistochemical staining and in situ hybridization (ISH) were used in the study. The medium-sized lymphoid cells were characterized by the selective growth within the kumina of vessels, particularly capillaries. The endothelial cells in the vessels exhibited positive CD34 staining. The lymphoid cells were positive for NK/T-cell markers, and cytotoxic proteins, and negative for B-cell markers. ISH demonstrated that the lymphoid cells expressed EBER. All the patients died of the disease a few months later. To conclude, the overall survival of patients with IVNKTL is very poor and the 1-year survival rate is only 31%. Patients with B symptoms and multiple organs involvement may be associated with the poor clinical prognosis. We deduce that the traditional chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) is inadequate for the treatment of IVNKTL. Early accurate diagnosis by biopsy for this lymphoma may be crucial for the patients’ medical prognosis due to the fatal disease course.
Keywords: Intravascular NK/T cell lymphoma, intravascular lymphoma, lung, CHOP, EBV
Introduction
IVL is a rare disorder characterized by the presence of large neoplastic lymphoid cells restricted to the lumens of small vessels, particularly capillaries [1]. Up to 90% of cases are of B-cell origin, but a T-cell lineage has been reported in 10% to 15% of cases [2]. However, increasing literatures of intravascular IVNKTL have been reported recently. Santucci et al [3] reported the first case in 2003. Recently, 21 other cases have been reported in English literatures [1,2,4-14] and 3 cases in Chinese literatures [15,16]. The disease has a rapidly progressive and fatal course. The disease is not classified as a separate entity in the 2008 World Health Organization (WHO) classification of hematopoietic and lymphoid tissue tumors [17], and the prognostic factors and more appropriate treatment strategies remained further validation by more cases.
In this study, we present four cases including two extremely rare cases of primary lung IVNKTL with detailed clinicopathological features, therapy and prognosis, and reviewed the literature for 25 similar cases. To the best of our knowledge, to date no cases of primary lung IVNKTL have been reported in the previous literatures.
Materials and methods
Patients
Four cases of IVNKTL were obtained from the Department of Pathology of Guangdong General Hospital. The follow-up times were 2 months, 2 months, 18 months and 3 months. Two expert pathologists confirmed a consensus diagnosis of IVNKTL according to the following findings: (1) the medium-large sized neoplastic lymphoid cells were restricted to the lumen of small vessels; (2) NK/T-cell immunophenotype (CD2+, CD3ε+ and CD56+); (3) expressing cytotoxic proteins (perforin, granzyme B and TIA-1); and (4) association with Epstein-Barr virus (EBV). This study was conducted according to the regulations of the local ethical committee.
Immunohistochemistry
Immunohistochemical staining was performed on 4-μm sections and using Real Envision Kit (K5007, DAKO, Carpinteria, CA, USA) on an automated immunostaining module (DAKO) according to the manufacturer’s instructions. Antibodies are detailed in Table 1. Appropriate positive and negative controls were used for each antibody. Only tumor tissues with distinct nuclear staining for PAX5 and Ki67, distinct membrane staining for CD34, CD2, CD5, CD20, and CD56, and distinct cytoplasm staining for CD3ε, TIA-1, and Granzyme B were recorded as positive.
Table 1.
Antibodies and probe used for immunohistochemistry and in situ hybridization
| Antibody or probe | Clone number | Source | Dilution |
|---|---|---|---|
| Immunohistochemistry | |||
| CD34 | QBEND 10 | DAKO, Glostrup, Denmark | 1:150 |
| CD2 | AB75 | DAKO, Glostrup, Denmark | 1:100 |
| CD3ε | Code:IS503 | DAKO, Glostrup, Denmark | 1:200 |
| CD5 | SP19 | ZETA, CA, USA | 1:100 |
| CD20 | L26 | DAKO, Glostrup, Denmark | 1:800 |
| PAX5 | DAK-Pax5 | DAKO, Glostrup, Denmark | 1:150 |
| CD56 | 1B6 | DAKO, Glostrup, Denmark | 1:200 |
| TIA-1 | 2G9A10F5 | DAKO, Glostrup, Denmark | 1:100 |
| Granzyme B | GrB-7 | DAKO, Glostrup, Denmark | 1:50 |
| Ki67 | MIB1 | DAKO, Glostrup, Denmark | 1:100 |
| In situ hybridization | |||
| EBER | EBER1/2(Y5200) | DAKO, Glostrup, Denmark | NA |
In situ hybridization
ISH for EBER was detected in all the four cases according to the manufacturer’s recommendations. Tumor cells that only appeared distinct nuclear staining were recorded as positive. The paraffin sections were detected for EBER by peptide nucleic acid probe (PNA probe, Dako-Y5200) (Table 1) labelled with fluorescein isothiocyanate, followed by anti-rabbit IgG with horse radish peroxidase. 3,3’-Diaminobenzidine was used to detect the hybridization signal of chromogen detection. EBV-positive nasopharyngeal carcinoma paraffin specimen was used for positive controls.
Statistical analysis
Statistical analysis was performed using SPSS software 13.0. Data are shown as mean ± SD. Correlation between clinical covariates and the overall survival was evaluated using the Kaplan-Meier method and the log-rank test. A Cox regression proportional hazards model was used for multivariate analyses to determine the independent significance of relevant clinical covariates. The hazard ratio (HR) with 95% confidence interval (CI) was measured to estimate the hazard risk for individual factors. Two-tailed P values of < 0.05 were considered statistically significant.
Results
Clinical features
The clinical features of all the cases were summarized in Table 2. In our case 1, a 38-year-old male presented with one-month history of cough in September 2008. Infiltrating pulmonary tuberculosis (TB) in the left upper lung was indicated by Chest X-ray at a local hospital. The patient underwent anti-TB treatment for two weeks, but the therapeutic efficacy was unsatisfactory. Then he got persistent fever (up to 39°C) for 10 days without weight loss and was admitted to our hospital. Chest X-ray revealed sheet blur in the left upper lung and considered inflammation. Chest computed tomography (CT) showed increased 2.6×1.7 density in the left upper lung. Blood routine and the other laboratory examinations showed no abnormalities. Positron emission CT (PET-CT) scan showed no obvious abnormalities, and there were no enlarged lymph nodes in the body surface, mediastinum or hilum. Bone marrow biopsy and aspirate revealed no evidence of tumor involvement. Percutaneous lung biopsy was performed after the consultation. The patient was treated with two cycles of CHOP chemotherapy after the diagnosis; however, he died of the disease two months later.
Table 2.
The clinical characteristics of 29 cases of intravascular NK/T-cell lymphoma
| Cases | Sex/age (y) | Involved organ(s) | B symptom | Chemotherapy | Follow up |
|---|---|---|---|---|---|
| 1 [3] | M/54, | Skin, CNS | Weight loss | CHOP | Died 17 months after diagnosis |
| 2 [1] | M/41 | Skin | None | CHOP and stem cell transplantation | Alive and event free at 12 months |
| 3 [1] | F/47 | CNS, bone marrow, kidneys, ovaries, cervix | High fever | None | Died half a month after diagnosis |
| 4 [4] | F/71 | Skin | None | None | Alive 5 months after diagnosis |
| 5 [5] | F/40 | Skin | None | CODOX-M and IVAC | Alive without recurrence at 7 months |
| 6 [6] | F/67 | Skin, CNS | None | None | Died 1 week after diagnosis |
| 7 [6] | M/63 | Skin | Weight loss, fever | None | Died 6 months after diagnosis |
| 8 [6] | M/63 | Skin | None | CHOP | Died 7 months after diagnosis |
| 9 [6] | M/87 | Skin | None | None | Died half a month after diagnosis |
| 10 [2] | M/62 | Skin | None | CHOP and DHAP | Alive and event free at 24 months |
| 11 [7] | F/42 | Skin | None | Radiotherapy, CHOP, proteasome inhibitor, EPOCH | Alive with disease at 14 months |
| 12 [15] | F/68 | Skin | Fever | CHOP-L | Died 2 months after diagnosis |
| 13 [15] | M/22 | Skin | Fever | CHOP-L | Died 2 months after diagnosis |
| 14 [8] | F/84 | Skin | Weight loss, fever | None | Alive 4 months after diagnosis |
| 15 [9] | F/38 | Skin, CNS | Fever | CHOP | Died of disease 13 months after diagnosis |
| 16 [10] | M/72 | Skin, bone marrow, CNS | None | Chlorambucil + urbasone | Died of sepsis due to pancytopenia 7 months after diagnosis |
| 17 [11] | F/48 | Skin | None | Combination chemotherapy | Alive with no evidence of disease at 18 months |
| 18 [12] | M/29 | Skin, liver | Low fever, weight loss | CVAD | Died 3 months after diagnosis |
| 19 [13] | M/45 | Skin | Weight loss, fever | None | Died of disease after 2 weeks |
| 20 [13] | F/52 | Skin, nasal cavity, sinus maxillaire | Fever | CHOP | Died of disease at 6 months |
| 21 [13] | M/32 | Skin | Fever | CHOP | Died of disease at 4 months |
| 22 [13] | F/18 | Skin | None | CHOP | Alive at 3 years |
| 23 [13] | M/51 | Skin | Fever, weight loss | CHOP + VP-16 | Died of disease at 6 months |
| 24 [14] | M/46 | Brain | None | None | Died of disease at 2 months |
| 25 [16] | M/57 | Testis | None | CHOP + tumor resection | Alive and event free at 22 months |
| 26 Our case 1 | M/38 | Lung | High fever | CHOP | Died of disease at 2 months |
| 27 Our case 2 | M/21 | Lung | High fever | None | Died of disease at 2 months |
| 28 Our case 3 | M/23 | Skin, bone marrow | High fever | P-GEMOX | Died of disease at 18 months |
| 29 Our case 4 | F/54 | Skin | None | None | Died of disease at 3 months |
MODS, multiple organ dysfunction syndrome; CODOX-M, cyclophosphamide, vincristine, doxorubicin, and methotrexate; IVAC, ifosfamide, mesna, etoposide, and cytarabine; DHAP, dexamethasone, cytarabine, cisplatin; EPOCH etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin; CHOP-L, cyclophosphamide, doxorubicin, vincristine, prednisone, and L-asparaginase; P-GEMOX, gemcitabine, oxaliplatin, and pegaspargase; CNS, central nervous system; CVAD, cyclophosphamide, Vincristine, Doxorubicin, Dexamethasone.
In our case 2, a 21-year-old male also presented with about one-month history of cough and high fever (up to 40°C) in November 2008. Chest X-ray revealed double lung inflammation and no occupation. Further hematologic analysis demonstrated a normal peripheral blood count (White blood cell count 3.41×109/L, HGB 106 g/L, Platelet count 101×109/L). Neither skin lesions nor enlarged liver or spleen was revealed by the physical examination and PET-CT scan. Bone marrow biopsy and aspirate also revealed no evidence of tumor involvement. The disease was finally diagnosed by percutaneous lung biopsy. The patient had no chance to have chemotherapy due to the rapidly progressive and fatal course and died of the disease at two months.
In our case 3, a 23-year-old male presented with two-month history of bilateral thigh erythema, nodules with pain in February 2015. Subsequently the patient appeared persistent fever (up to 39°C). Blood routine and the other laboratory examinations showed no abnormalities. A biopsy of the erythematous plaques on the left thigh was performed and revealed the secondary involvement of bone marrow. A physical examination and PET-CT scan revealed no enlarged liver or spleen. After the diagnosis, the patient was treated with six cycles of gemcitabine, oxaliplatin, and pegaspargase (P-GEMOX) therapy; resulting in regression of the lesions. However, he died of the disease 18 months later.
In our case 4, a 54-year-old female presented with more than two-month history of abdominal rash with partial induration in October 2016. Subsequently the patient appeared bilateral thigh, hip, chest, and back rash. There was no fever, night sweats, and weight loss. Head MRI, chest X-ray, and bone marrow examination yielded normal findings. A biopsy of the erythematous plaques on the left thigh was performed. The patient refused chemotherapy and discharged home. The patient died of the disease at 3 months after the diagnosis.
Pathologic findings
The results of immunostaining and ISH of our four cases with IVNKTL were detailed in Supplementary Table 1, Figures 1 and 2. Both our case 1 and 2 were finally diagnosed by percutaneous lung biopsy and exhibited the similar histopathological findings and immunophenotype. The medium-large sized lymphoid cells were characterized by the selective growth within the kumina of vessels, particularly capillaries in the alveolar septa (Figure 1A, 1B). The endothelial cells in the vessels exhibited positive CD34 staining (Figure 1C). The lymphoid cells were positive for CD2 (Figure 1D), CD3ε, CD56 (Figure 1E) and cytotoxic proteins including TIA-1 (Figure 1F) and granzyme B (Figure 1G), and negative for CD20 and PAX5. ISH demonstrated that the cells expressed EBER (Figure 1H). The cells also showed high Ki67 proliferation index (Figure 1I).
Figure 1.

The lymphoid cells were characterized by the selective growth within the kumina of vessels, particularly capillaries in the alveolar septa (A, H&E×40; B, H&E×200). The endothelial cells in the vessels exhibited positive CD34 staining (C). The lymphoid cells were positive for CD2 (D), CD56 (E), TIA-1 (F), granzyme B (G), EBER (H), and Ki67 (I) by IHC staining (200×).
Figure 2.
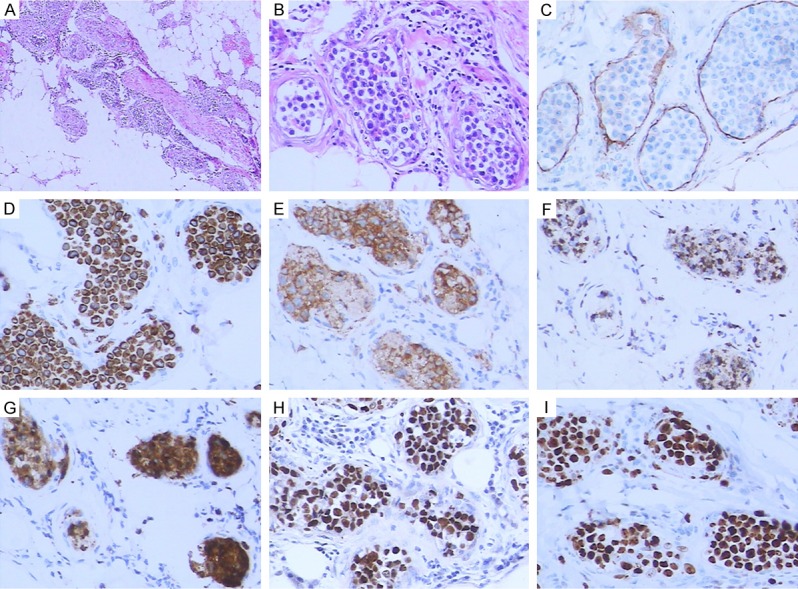
The lymphoid cells featured the proliferation of abnormal lymphocytes in dilated vessels of the dermis and subcutaneous tissues (A, H&E×40; B, H&E×200). The endothelial cells in the vessels exhibited positive CD34 staining (C). The lymphoid cells were positive for CD3ε (D), CD56 (E), TIA-1 (F), granzyme B (G), EBER (H), and Ki67 (I) by IHC staining (200×).
Our case 3 and 4 featured the proliferation of abnormal lymphocytes in the dilated vessels of the dermis and subcutaneous tissues (Figure 2A, 2B). The endothelial cells in the vessels exhibited positive CD34 staining (Figure 2C). The medium-sized lymphoid cells were positive for CD3ε (Figure 2D), CD56 (Figure 2E) and cytotoxic proteins including TIA-1 (Figure 2F) and granzyme B (Figure 2G), and negative for CD5, CD20 and PAX5. ISH demonstrated that the cells expressed EBER (Figure 2H). The cells also showed high Ki67 proliferation index (Figure 2I).
Prognostic significance
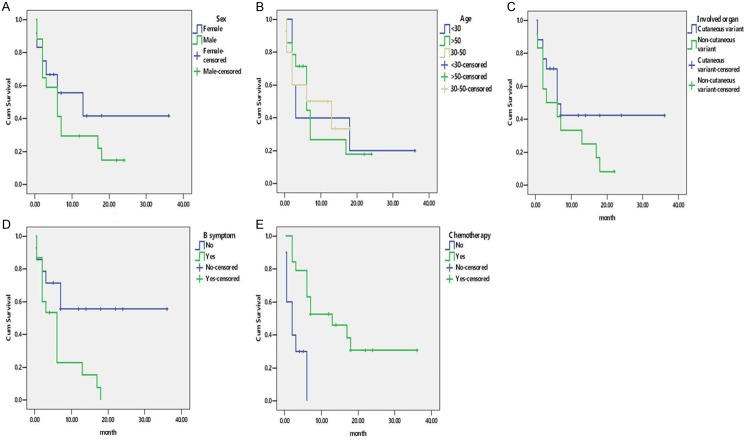
Among the study subjects of 29 cases including 25 reported cases and our 4 cases with IVNKTL, 17 were male and 12 were female (male:female ratio: 1.42:1). The mean age was 49 years (range, 18-87 years). The involved organs included 24 cases of skin lesions, 6 cases of CNS, 4 cases of bone marrow, 2 cases of lung, and 1 case of nasal, kidney, ovary, cervix, liver and testis, respectively. The involved organs of the lymphoma cells were divided into two subgroups: 17 cases of cutaneous variant (skin manifestations only) and 12 cases of non-cutaneous variant (multiple organs involvement or non-skin manifestations only). 15 out of 29 cases were with B symptoms. 19 out of 29 cases were treated with chemotherapy or combination chemotherapy. The median survival time was 6 months and the 1-year survival rate was only 31%.
Kaplan Meier analysis indicated that patients with B symptoms (P = 0.011) and chemotherapy (P = 0.001) were associated with clinical prognosis. The clinical outcome of the patients with multiple organs involvement or non-skin manifestations only was worse than that with skin manifestations only. The difference however was not statistically significant (P = 0.150). In contrast, little relationship was observed between Sex (P = 0.991) and age (P = 0.940). (Table 3; Figure 3).
Table 3.
Kaplan-Meier curve analyses for clinical factors
| Variables | Mean ± SD (m) | χ2 | P |
|---|---|---|---|
| Sex (Male vs. Female) | (8.691 ± 2.009) vs. (17.951 ± 5.010) | 0.991 | 0.320 |
| Age (y) (< 30 vs. 30-50 vs. >50) | (12.200 ± 5.976) vs. (9.267 ± 2.379) vs. (9.071 ± 2.333) | 0.123 | 0.940 |
| Involved organ (Cutaneous variant* vs. Non-cutaneous variant) | (17.482 ± 4.097) vs. (7.729 ± 2.133) | 2.073 | 0.150 |
| B symptom (Yes vs. No) | (6.286 ± 1.580) vs. (21.522 ± 4.449) | 6.489 | 0.011 |
| Chemotherapy (Yes vs. No) | (16.752 ± 3.305) vs. (2.675 ± 0.787) | 11.253 | 0.001 |
Cutaneous variant (Patients with disease limited to the skin).
Figure 3.
Kaplan-Meier curve analyses for clinical factors. A. Sex; B. Age; C. Involved organ; D. B symptom; E. Chemotherapy.
On univariate analysis, Cox proportional hazards model showed that patients without B symptoms (P = 0.022) and chemotherapy group (P = 0.004) correlated with an increased overall survival (Table 4). However, multivariate analysis revealed that only chemotherapy group was associated with improved survival (P = 0.004) (Table 4). The median survival time of chemotherapy group was prolonged by 11 months compared with non-chemotherapy group.
Table 4.
Univariate and multivariate cox regression analyses for clinical factors
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex (Male vs. Female) | 0.634 (0.243-1.652) | 0.351 | 0.803 (0.297-2.170) | 0.665 |
| Age (< 30 vs. 30-50 vs. ≥ 50 years) | 1.030 (0.576-1.841) | 0.920 | 1.073 (0.589-1.955) | 0.818 |
| Involved organ (Cutaneous variant* vs. Non-cutaneous variant#) | 1.830 (0.756-4.427) | 0.180 | 1.781 (0.622-5.103) | 0.282 |
| B symptom (Yes vs. No) | 3.148 (1.181-8.389) | 0.022 | 2.343 (0.833-6.588) | 0.107 |
| Chemotherapy (Yes vs. No) | 0.200 (0.068-0.594) | 0.004 | 0.189 (0.061-0.587) | 0.004 |
HR, Hazard ratio; CI, confidence interval;
skin manifestations only;
multiple organs involvement or non-skin manifestations only.
Discussion
Among the study subjects of 29 cases including 25 reported cases and our 4 cases with IVNKTL, skin lesions with tender erythematous subcutaneous nodules represented the commonest clinical manifestations; and were less common in other organs such as CNS, bone marrow, nasal, lung, and so on. 15 out of 29 (52%) patients with IVNKTL exhibited B symptoms. The pathologic diagnosis of IVNKTL was often not difficult. However, the disease did not present tumor occupation by imaging examination, which was different from the usual lymphomas presenting with solid tumor or enlarged lymphoid organ, making diagnosis challenging.
Both our patient 1 and 2 got persistent fever and presented the pulmonary infection symptom as the initial presentation. Infiltrating pulmonary TB or lung inflammation and no occupation were revealed by Chest X-ray. The treatment effect of anti-TB or anti-infection was unsatisfactory. The disease was finally diagnosed by percutaneous lung biopsy. Both the patients with or without CHOP therapy died of the disease at two months. It indicated that the primary lung IVNKTL was an extremely rare and fatal disease. The clinical symptoms of the disease were also not specific and it was very easy to be overlooked or misdiagnosis for pulmonary infection. Thus, if the patients with persistent high fever could not be explained by other diseases and the treatment effect of anti-infection were unsatisfactory, we should try to make an early accurate diagnosis by lung biopsy. Clinicians and pathologists should be aware that IVNKTL could occur in the lung.
Because of the similar morphology, immunophenotype, and EBV infection status of extra-nodal NK/T-cell lymphoma, nasal type (ENKTCL) and aggressive NK-cell leukemia [18,19], IVNKTL should also be distinguished from the two subtypes. Although ENKTCL can also occur in the skin but presents with multiple nodules with ulceration and the tumor cells are distributed in tissues and show vascular invasion. Patients with IVNKTL have no nasal abnormalities and tumor cells are confined to the endovascular system. In aggressive NK-cell leukemia, tumor cells are diffusely scattered in the extravascular tissue rather than deposited in blood vessels [12]. Patients with IVNKTL had no nasal symptoms and obvious abnormalities in the peripheral blood but had the hallmark of intravascular dissemination of tumor cells [12]. In our case 3, although the tumor cells were revealed the secondary involvement of bone marrow by biopsy, blood routine and the other examinations showed no abnormalities and no enlarged liver or spleen. Hence, we believed that it might best be included in the spectrum of IVNKTL. The other differential diagnoses of IVNKTL include IVL of other lineages (in which the tumor cells have typical immunophenotype, such as being positive for B or T-cell markers), metastatic neoplasms (for example melanoma or breast cancer, which are validated by medical history and immunochemical staining) [12].
Conventional staging procedures were generally associated with a high proportion of false negatives because of the lack of detectable tumor masses [17]. In a series of 38 patients with IVL [20], patients with disease limited to the skin (‘cutaneous variant’; 26% of cases) were invariably females with a normal platelet count, and exhibited a significantly better outcome than the remaining patients, which deserved further investigation. One patient had an indolent course with apparently localized cutaneous disease, 24 months after the onset of symptoms [2]. However, in our case 4, the patient of IVNKTL with skin manifestations only died of the disease at only 3 months after the diagnosis. In our small serial study of 29 cases, although the clinical outcome of the patients with multiple organs involvement or non-skin manifestations only (Non-cutaneous variant) was worse than that with skin manifestations only (Cutaneous variant), multivariate analysis revealed the difference was not statistically significant. Surprisingly, univariate analysis showed that patients with B symptoms correlated with a decreased overall survival, despite the difference was not statistically significant by multivariate analysis. Patients with B symptoms appeared to be a potential factor for predicting poor prognosis of patients with IVNKTL.
IVL was an aggressive lymphoma which responded poorly to chemotherapy [17]. IVNKTL therapy was unsatisfactory and there was no standard chemotherapy regimen at present. In the small serial study of 29 cases, multivariate analysis revealed that only chemotherapy was associated with improved survival. The median survival time of chemotherapy group was prolonged by 11 months compared with non-chemotherapy group. However, the overall survival of patients with IVNKTL was very poor and the 1-year survival rate was only 31%. 19 out of 29 (66%) patients were treated with chemotherapy and the major chemotherapy regimen was the traditional chemotherapy with CHOP. 8 out of 9 patients treated with CHOP alone were died at short notice after diagnosis, and only one was fortunately alive for three years. Furthermore, both our patient 1 and 2 with or without CHOP therapy died of the disease at two months. Based on these findings, we could deduce that the traditional CHOP was inadequate for the treatment of IVNKTL. In our case 3, the patient was treated with six cycles of P-GEMOX therapy; resulting in regression of the lesions. However, he died of the disease 18 months later. Recently, a combination treatment of CHOP and stem cell transplantation [1], salvage chemotherapy (dexamethasone, cytarabine, cisplatin; DHAP) [2], radiotherapy and proteasome inhibitor therapy [7], or complete tumor resection [16] for IVNKTL therapy seemed more effective in different individuals. Of course, further studies were warranted to provide more definitive evidence. In addition, I thought patients who did not chemotherapy had very poor general condition or rapidly deteriorating disease course, therefore they had no chance to have chemotherapy. Thus, early accurate diagnosis by biopsy for this lymphoma may be crucial for the patients’ medical prognosis.
To conclude, the overall survival of patients with IVNKTL is very poor and the 1-year survival rate is only 31%. Primary lung IVNKTL is an extremely rare and fatal disease presents the pulmonary infection symptom as the initial presentation, which may be overlooked or misdiagnosed as infiltrating pulmonary TB or lung inflammation. Patients with B symptoms and multiple organs involvement or non-skin manifestations only may be associated with the poor clinical prognosis. We deduce that the traditional CHOP is inadequate for the treatment of IVNKTL. Early accurate diagnosis by biopsy for this lymphoma may be crucial for the patients’ medical prognosis due to the fatal disease course.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81172244) and National Clinical Key Subject Construction Project Fund of China.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wu H, Said JW, Ames ED, Chen C, McWhorter V, Chen P, Ghali V, Pinkus GS. First reported cases of intravascular large cell lymphoma of the NK cell type. Am J Clin Pathol. 2005;123:603–611. doi: 10.1309/X597-G3QM-XAFB-CM5V. [DOI] [PubMed] [Google Scholar]
- 2.Gleason BC, Brinster NK, Granter SR, Pinkus GS, Lindeman NI, Miller DM. Intravascular cytotoxic T-cell lymphoma: a case report and review of the literature. J Am Acad Dermatol. 2008;58:290–294. doi: 10.1016/j.jaad.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Santucci M, Pimpinelli N, Massi D, Kadin ME, Meijer CJ, Muller-Hermelink HK, Paulli M, Wechsler J, Willemze R, Audring H, Bernengo MG, Cerroni L, Chimenti S, Chott A, Diaz-Perez JL, Dippel E, Duncan LM, Feller AC, Geerts ML, Hallermann C, Kempf W, Russell-Jones R, Sander C, Berti E. Cytotoxic/natural killer cell cutaneous lymphomas. Report of EORTC cutaneous lymphoma task force workshop. Cancer. 2003;97:610–627. doi: 10.1002/cncr.11107. [DOI] [PubMed] [Google Scholar]
- 4.Kuo TT, Chen MJ, Kuo MC. Cutaneous intravascular NK-cell lymphoma: report of a rare variant associated with Epstein-Barr virus. Am J Surg Pathol. 2006;30:1197–1201. doi: 10.1097/01.pas.0000213263.99973.09. [DOI] [PubMed] [Google Scholar]
- 5.Song DE, Lee MW, Ryu MH, Kang DW, Kim SJ, Huh J. Intravascular large cell lymphoma of the natural killer cell type. J. Clin. Oncol. 2007;25:1279–1282. doi: 10.1200/JCO.2006.09.9259. [DOI] [PubMed] [Google Scholar]
- 6.Cerroni L, Massone C, Kutzner H, Mentzel T, Umbert P, Kerl H. Intravascular large T-cell or NK-cell lymphoma: a rare variant of intravascular large cell lymphoma with frequent cytotoxic phenotype and association with Epstein-Barr virus infection. Am J Surg Pathol. 2008;32:891–898. doi: 10.1097/PAS.0b013e31815d29c9. [DOI] [PubMed] [Google Scholar]
- 7.Wu CS, Liao JB, Hsieh PP, Hwang YC, Lin SL. Cutaneous intravascular natural killer-cell lymphoma: a rare case and review of the literature. Acta Dermato Venereologica. 2011;91:472–473. doi: 10.2340/00015555-1083. [DOI] [PubMed] [Google Scholar]
- 8.Yanning X, Chen H, Si H, Liu Y, Min Z. Cutaneous intravascular NK-cell lymphoma. Eur J Dermatol. 2013;23:252–253. doi: 10.1684/ejd.2013.1990. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang W, An J, Li H, Liu S. Cutaneous intravascular natural killer-cell lymphoma: a case report and review of the literature. Am J Clin Pathol. 2014;142:243–247. doi: 10.1309/AJCP1JLYXLGDNOCH. [DOI] [PubMed] [Google Scholar]
- 10.Gebauer N, Nissen EJ, Driesch P, Feller AC, Merz H. Intravascular natural killer cell lymphoma mimicking mycosis fungoides: a case report and review of the literature. Am J Dermatopathol. 2014;36:e100–e104. doi: 10.1097/DAD.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 11.Alhumidi A. Cutaneous Intravascular NK/T-cell lymphoma mimic panniculitis clinically, case report and literature brief review. Diagn Pathol. 2015;10:107. doi: 10.1186/s13000-015-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi Y, Huo Z, Liang Z, Meng Y, Jia C, Shi X, Song L, Luo Y, Ling Q, Liu T. Intravascular NK-cell lymphoma: a case report and review of the literature. Diagn Pathol. 2015;10:84. doi: 10.1186/s13000-015-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Chen S, Ma H, Shi D, Huang C, Lu C, Gao T, Wang G. Intravascular NK/T-cell lymphoma: a report of five cases with cutaneous manifestation from China. J Cutan Pathol. 2015;42:610–617. doi: 10.1111/cup.12515. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Zhou X, Zhang X, Zheng Y, Yue B. Primary intravascular natural killer/T cell lymphoma of the central nervous system. Leuk Lymphoma. 2015;56:1154–1156. doi: 10.3109/10428194.2014.951847. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Xie JL, Zhou XG. [Intravascular NK-cell lymphoma: a clinicopathologic study and literature review] . Zhonghua Bing Li Xue Za Zhi. 2011;40:689–693. [PubMed] [Google Scholar]
- 16.Jiao X, Wang WC, Bao JJ, Xiao W, Yu H, Wang CF. [Intravascular NK/T-cell lymphoma of testis: report of a case] . Zhonghua Bing Li Xue Za Zhi. 2016;45:717–718. doi: 10.3760/cma.j.issn.0529-5807.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Cehn SS. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008. [PubMed] [Google Scholar]
- 18.Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, Konoplev S, Bueso-Ramos CE, Vega F, Medeiros LJ, Yin CC. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson cancer center. Am J Surg Pathol. 2013;37:14–23. doi: 10.1097/PAS.0b013e31826731b5. [DOI] [PubMed] [Google Scholar]
- 19.Kwong YL. The diagnosis and management of extranodal NK/T-cell lymphoma, nasal-type and aggressive NK-cell leukemia. J Clin Exp Hematop. 2011;51:21–28. doi: 10.3960/jslrt.51.21. [DOI] [PubMed] [Google Scholar]
- 20.Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, Zucca E, Rossi G, Lopez-Guillermo A, Pavlovsky MA, Geerts ML, Candoni A, Lestani M, Asioli S, Milani M, Piris MA, Pileri S, Facchetti F, Cavalli F, Ponzoni M. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127:173–183. doi: 10.1111/j.1365-2141.2004.05177.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.