Abstract
Inhibiting inflammation is helpful in relieving the absorption of alveolar bone and promoting periodontal bone regeneration. In a previous study, we showed that transforming growth factor-beta1 (TGF-β1)-induced Treg cells inhibit the absorption of tissue-engineered cartilage caused by endogenous IFN-γ and TNF-α. In this study, we investigated the effect of inhibiting local inflammatory responses on Geistlich Bio-Oss® osteogenesis promotion in vivo. TGF-β1+BMMSCs (bone marrow mesenchymal stem cells) were cultured in Geistlich Bio-Oss® medium, and biocompatibility was evaluated. Alveolar bone defects in New Zealand rabbits repaired by application of Geistlich Bio-Oss® were compared to the effects of added TGF-β1+BMMSCs. There was no significant difference between the untreated Geistlich Bio-Oss® medium-control group and the group treated with the addition of TGF-β1+BMMSCs. Pro-inflammatory cytokines IFN-γ and TNF-α delayed Geistlich Bio-Oss®-induced osteogenesis, but no significant difference in osteogenesis was seen with the addition of TGF-β1+BMMSCs. Geistlich Bio-Oss® has good compatibility with TGF-β1+BMMSCs. However, the dual role of in vivo TGF-β1+BMMSCs in regenerating periodontal bone and limiting local inflammation is not clear.
Keywords: Transforming growth factor-beta1, interferon-γ, tumor necrosis factor-α, Geistlich Bio-Oss®
Introduction
It has been widely reported that periodontitis-induced tooth loss can be partly repaired by implant treatment and bone graft technology. However, the persistent periodontal pathogens in the oral cavity can increase the incidence of peri-implantitis and reduce alveolar bone resorption compared to periodontally healthy patients, which leads to the instability of implants [1-3]. Therefore, the design of an effective periodontal regeneration strategy that reduces alveolar bone loss has practical significance for improving the quality of life of patients with periodontitis and the long-term success of implanted prostheses.
Infections are the leading causes of failing dental implants [4,5]. At present, eradicating pathogenic bacteria and pro-inflammatory factors are the main surgical managements in treating peri-implant inflammation. Restoring original organizational structure contributes to the stability of the dental implant by enhancing bone regeneration or re-osseointegration [6-8]. However, the bone regeneration process after alveolar bone defect repair is not stable. In addition, the processes of peri-implant inflammation and bone regeneration can be affected by host immune regulation [9].
In our previous study [10], we demonstrated that TGF-β1 can stimulate the newly forming regulatory T cells (Treg) cells and induce the differentiation of BMMSCs. induced Treg (iTreg) cells, converted from CD4+ T cells, can suppress the inflammatory function of IFN-γ and TNF-α. Therefore, we hypothesized that TGF-β1 could promote bone generation by inhibiting local inflammatory reaction.
In the present study, TGF-β1+BMMSCs were cultured in Geistlich Bio-Oss® medium, and the biocompatibility between Geistlich Bio-Oss® and TGF-β1+BMMSCs was evaluated by detection of TGF-β1+BMMSC cell growth. To investigate the effect of inflammation on bone generation, TGF-β1+BMMSCs were used to inhibit the peri-implant inflammation resulting from Geistlich Bio-Oss®. New Zealand rabbits with alveolar bone defects were treated with Geistlich Bio-Oss®, and the effect of addition of TGF-β1+BMMSCs on subsequent osteogenesis was measured.
Materials and methods
New Zealand rabbits
Twenty-four male New Zealand rabbits (2.0 kg) were obtained from and maintained in the animal facility of the Animal Laboratory of Shanghai Medical College. All animal experiments were performed under the institutionally approved animal research protocols of Zhongshan Hospital of Fudan University. All efforts were made to minimize both the suffering of the animals and the number of animals used.
Isolation of BMMSCs
Bone marrow cells were flushed out from the bone cavities of femurs and tibias of mice with 2% heat-inactivated fetal bovine serum (FBS; Equitech-Bio) in PBS. A single-cell suspension of all nucleated cells was obtained by passing the bone marrow cells through a 70-μm cell strainer. The single cells were then seeded at a density of 1×106 cells per 10 cm culture dishes and initially incubated for 48 h at 37°C under 5% CO2. To eliminate non-adherent cells, the cultures were washed with PBS twice on the second day. The attached cells were cultured for 16 days with alpha minimum essential medium (α-MEM) supplemented with 20% FBS, 2 mM L-glutamine, 55 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA). To confirm the mesenchymal stem cell character of the cells, we performed a flow cytometric analysis to show that the BMMSCs were positive for CD29, CD44 and CD90 and negative for CD31, CD34 and CD45.
TGF-β1 gene transfection of BMMSCs
Second-passage BMMSCs were transfected using Lipofectamine 2000 (Gibco, USA) in 6-well plates in accordance with the protocol provided by the manufacturer. Briefly, Lipofectamine (10 μl) diluted in 250 μl of OptiMEM medium (Gibco) was mixed with 4 μg of purified pcDNA3.1 or pcDNA3.1-TGF-β1 (maintained by our team) diluted in 250 μl of OptiMEM medium. The DNA-Lipofectamine mixtures were incubated for 20 min at room temperature and then added to each well at a final volume of 2 ml. Following incubation for 24 h at 37°C in a 5% CO2 incubator, the cells were sub-cultured at a 1:20 dilution in fresh growth medium. G418 (400 μg/ml; Gibco) was added the following day. High TGF-β1-expressing clones were selected from neomycin-resistant BMMSCs via enzyme-linked immunosorbent assay (ELISA).
ELISA for TGF-β1 protein levels
The expression level of the TGF-β1 protein in cell culture medium was quantified using a commercial ELISA kit (ELISA; R&D Systems, USA) following the manufacturers protocol. TGF-β1 production was determined either after activation with 0.2 M HCl, or without prior activation, to determine the concentration of active TGF-β1 in the medium. The culture medium was replaced with serum-free medium prior to the assay, and the supernatant was then collected at different time points for evaluation.
Adherence assay for TGF-β1+BMMSCs and Geistlich Bio-Oss®
Transfected BMMSCs were divided into two groups: Group Geistlich Bio-Oss®+TGF-β1+BMMSCs (experimental group) and Group TGF-β1+BMMSCs (control group). One milligram of Geistlich Bio-Oss® was slightly crushed by a sterile metal hammer and placed in a 6-well plate. Then, 2×106/ml TGF-β1+BMMSCs (3-5 passage) were seeded on the Geistlich Bio-Oss® and maintained in α-MEM at 37°C in a humidified incubator of 5% CO2 for 4 h. Images of cells cultured for 3, 5 and 7 days were captured by an inverted microscope.
Establishment of alveolar defect model in rabbit
Surgical methods: New Zealand rabbits were anesthetized by 5 ml/kg of lidocaine. Incision location: Unilateral 1-2 cm critical-size defects were created at 60 degrees above the front direction in the mandible. Then, soft tissue was dissected, and the bone exposed by gentle retraction of the muscles. A dental implant machine was used to drill into the bone marrow cavity. Drilling speed was controlled at 1000 rpm/min, and the drill diameter was 3.3 mm (Figure 1A). During drilling, 0.9% sterile physiological saline solution was used to cool the gap. Alveolar defect rabbits were divided into 3 groups (group A, B and C). After that, Geistlich Bio-Oss® repair materials were fitted into the gap, followed by slightly compaction and immersion in blood, and then covered by a 10 mm×10 mm Geistlich Bio-Gide membrane (Figure 1B). The periosteum, muscle and skin were sutured by layer. Following surgery, rabbits were fed with a standard laboratory diet and water. The weight of the rabbits was recorded daily, and any changes in dietary habits or activity were monitored closely. After 7 days, the stitches were taken out.
Figure 1.
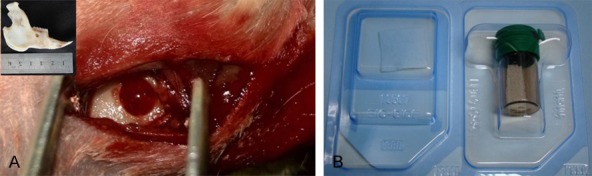
A. Establishment of the Alveolar Defect Model in Rabbit (φ3.3 mm). The top left corner shows soft tissues was removed. B. Geistlich Bio-Gide (left, 25×25 mm) and Geistlich Bio-Oss® (right, 0.5 g).
Group A: Rabbits with alveolar defects were repaired by Geistlich Bio-Oss® (80 mg/hole) and TGF-β1+BMMSCs (1×106/hole).
Group B: Rabbits with alveolar defects were repaired by Geistlich Bio-Oss® (80 mg/hole), TGF-β1+BMMSCs (1×106/hole), and IFN-γ plus TNF-α (1:1, 200 ng/ml, 0,1 ml/hole).
Group C: The control group, rabbits with alveol ar defects were repaired only by Geistlich Bio-Oss® (80 mg/hole).
Preparation of the samples and histomorphometric analysis
Rabbits were sacrificed at 30, 60 and 90 days after surgery. Retrieved alveolar bone defect specimens were obtained by removing soft tissues. The retrieved specimens were fixed in 10% neutral buffered formalin for subsequent hard-tissue slicing analysis. Consecutive slicing was performed using a hard tissue slicer (Leica, Germany) at a slice thickness of 30-40 μm and stained with Van Gieson’s picro-fuchsin methods. Examination was conducted using an inverted microscope.
Statistical analysis
SPSS 13.0 was used for statistical analyses. The data were expressed as the mean ± SEM. Analysis of variance (ANOVA) or Student’s t-test was used for two-treatment comparisons. A P-value of less than 0.05 was considered to indicate a significant difference.
Results
Production of TGF-β1
The amount of TGF-β1 produced by the transduced cells was determined through ELISA analysis of samples collected from the media at 7, 14, 21 and 28 days. The cells transduced with TGF-β1 synthesized 1419 ± 193, 1832 ± 588, 1640 ± 377 and 1300 ± 310 ng/l of active TGF-β1 at 7, 14, 21 and 28 days, respectively. The maximum concentration of active TGF-β1 detected in the media was at 14 days and gradually decreased with time. However, even after 28 days, the transduced cells produced significantly more TGF-β1 than the control groups (Figure 2).
Figure 2.
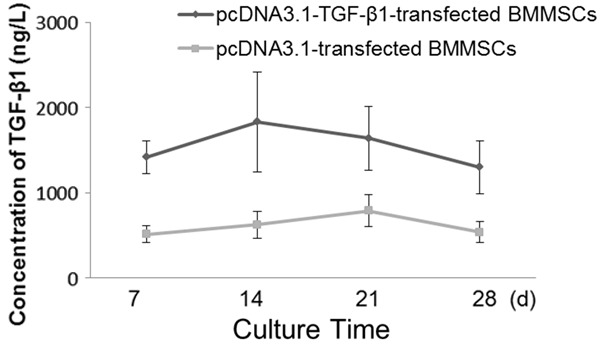
The synthesis of active TGF-β1 in transduced cells. The highest concentration of TGF-β1 was detected on day 14. The data are expressed as the means, and error bars represents the standard deviation (SD), n = 5. The cell-conditioned media were collected on the 7th, 14th, 21st and 28th day respectively, and then TGF-β1 concentrations in these media were determined by ELISA.
Adhesion between TGF-β1+BMMSCs and Geistlich Bio-Oss®
After 3 days of culturing, the medium was changed for the first time, and we observed the scattered distribution and varied morphology of cells on the flask. After 7 days of culturing, adherent cells exhibited rod-like or short spindle morphology, and showed clear boundaries between cytoplasm and nuclei. In general, the majority of cells possessed a single nucleus, and the nuclei were located near the center of the cell expansion regions, though irregular protrusions were detected on the cell bodies. Most of the cells showed an orderly arrangement along the long axis of the cell body, which was attached to the surface of the repair materials, and there was no significant difference compared with the control group (Figure 3). These phenomena indicated good compatibility between the repair material and the cells.
Figure 3.
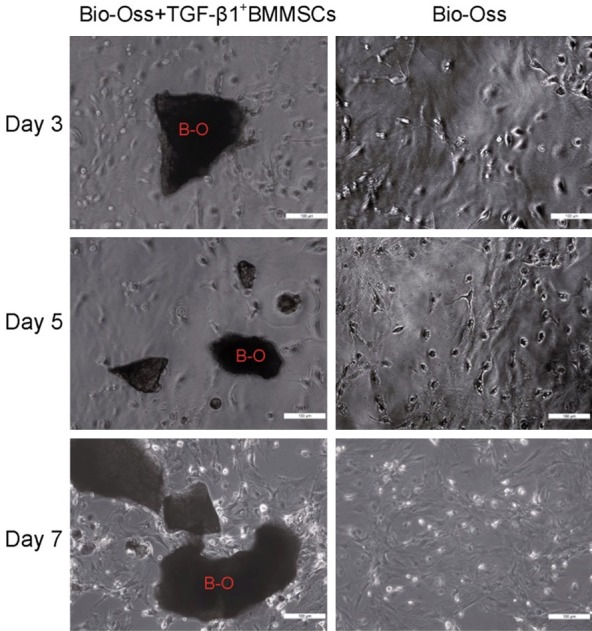
2×106/ml of TGF-β1+BMMSCs (3-5 passage) were seeded on the Geistlich Bio-Oss®. After 7 days of culturing, no significant differences were detected in the cells compared with the control group, indicating that the material has a good compatibility with the cells. (400×).
Status of animals after surgery
Within 3 days, rabbits with repaired alveolar defects were eating less compared to the sham-operated group. After 3 days, they ate normally compared to the sham-operated group. The incision was dry, and hemorrhagic and purulent fluid was not detected. On the 7th day, the sutures were removed, and wounds were well healed. The postoperative masseter was much thinner than the contralateral one. No significant differences were found between the animals in each group.
Postoperative pathological analysis of rabbit alveolar
Thirty days after surgery, a white and tough film was detected on the repair parts, which indicated that the Geistlich Bio-Gide film was not fully absorbed. After removing the Geistlich Bio-Gide membrane, we found that the Geistlich Bio-Oss® showed a granular shape, which was similar to the original form. The clear boundary between Geistlich Bio-Oss® and alveolar bone tissue could be observed. In addition, the soft Geistlich Bio-Oss® was easy to strip away (Figure 4A). There were no obvious differences in the animal specimens between experimental groups.
Figure 4.
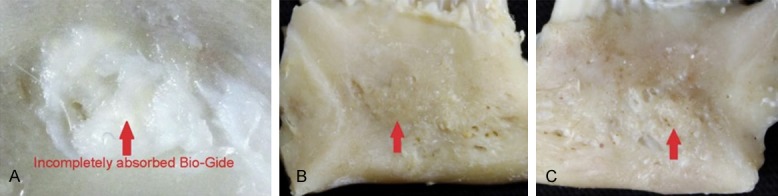
A. Thirty days after surgery, Geistlich Bio-Oss® still shows a granular shape and slightly soft quality and easy to be stripped from the bone. The red arrow indicates incompletely absorbed Geistlich Bio-Gide. B. Sixty days after surgery, Geistlich Bio-Gide membrane is completely absorbed. Geistlich Bio-Oss® showed small granular shape, and the alveolar bone tissue surrounding it was not obvious. Geistlich Bio-Oss® was hard and not easy to peel. C. Ninety days after surgery, fine granular boundaries were observed between Geistlich Bio-Oss® and the surrounding alveolar bone. Geistlich Bio-Oss® was hard and could not be stripped off.
Sixty days after surgery, no white film was observed on the repaired parts, which indicates that the Geistlich Bio-Gide membrane has been completely absorbed. Geistlich Bio-Oss®, in small granular form, was mixed with the surrounding alveolar bone tissue and was hard to cut and not easy to peel. There were no obvious differences in the animal specimens between experimental groups (Figure 4B).
Ninety days after surgery, no white film was observed attached to the repaired parts. Geistlich Bio-Oss®, in fine granular form, had a texture and color like the surrounding alveolar bone and was hard to cut and could not be stripped off. There were no obvious differences in the animal specimens between experimental groups (Figure 4C).
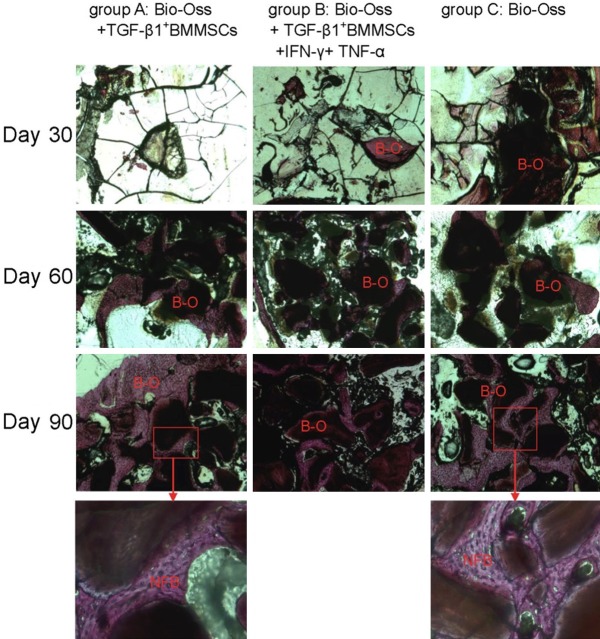
Hard-tissue slices and Van Gieson’s Picro-Fuchsin stain
Thirty days after surgery, most Geistlich Bio-Oss® fell off during the tissue fixation and dehydration process so that only a little residue was detected in the bottom of the defect parts. Therefore, it is difficult to observe the degree of ossification of the Geistlich Bio-Oss in tissue sections. Sixty days after surgery, Geistlich Bio-Oss® in each group showed a granular particle shape, and its outer ossification and fusion was not obvious. There were no significant differences in the degree of ossification in each group. On the 90th day, ossification was observed on the outer layer of Geistlich Bio-Oss® particles in group A and C, and some of these particles had merged with each other. In addition, we detected that the newborn cells increased significantly on the outer layer of Geistlich Bio-Oss® particles. In group B, more significant ossification was detected on the outer Geistlich Bio-Oss® compared with that on day 60, but most of the Geistlich Bio-Oss® appeared as single granular particles and without obvious fusion (Figure 5).
Figure 5.
Thirty days after surgery, Geistlich Bio-Oss® (B-O) was easy to remove during the staining process. The ossification of the Geistlich Bio-Oss in tissue sections was difficult to accurately observe. Sixty days after surgery, Geistlich Bio-Oss® (B-O) in each group showed granular particles, and the ossification and fusion in its outer layer was not obvious. On the 90th day in group A and C, the merging of ossification is detected in the outer layer of Geistlich Bio-Oss® (B-O). In addition, the newly formed cells (NFB) increased significantly. In group B, compared with that on the 60th day, more significant ossification from the outer Geistlich Bio-Oss® was detected. Geistlich Bio-Oss® showed single granular shape and fusion was not obvious. (50×, frame 200×).
Discussion
Geistlich Bio-Oss® is a perfect material for bone repair, and in combination with membrane-guided bone regeneration techniques can effectively treat alveolar bone defects and bone defects around dental implants [11-13]. After filling a bone defect with Geistlich Bio-Oss®, chemotaxis of bone cells is induced, promoting proliferation, differentiation and secretion of the extracellular matrix; eventually, the graft material is replaced by new bone tissue, and the bone defect is repaired [14]. Given the observed good biological compatibility and guiding role in bone regeneration, Geistlich Bio-Oss® with membrane-guided bone regeneration techniques is a reliable combination. BMMSCs are a group of cells with the ability of regeneration and differentiation. Because they are easy to obtain, BMMSCs are widely studied. BMMSCs can be directed to differentiate into a variety of tissue cells, including periodontal cells [15,16]. The use of growth factors, such as recombinant human platelet-derived growth factor-BB (rhPDGF) or TGF-β1 with biocompatible matrices to promote tissue regeneration, represents a promising approach in the disciplines of periodontology and implantology [17]. TGF-β1 could affect the metabolism and growth of bone cells [18]. In these complex processes, it is vital to create the most suitable regeneration environment to support tissue regeneration [19]. In the present study, we transfected the TGF-β1 gene into BMMSCs. The level of TGF-β1 expression in transfected cells was highest on day 14 and was maintained at this level during the experimental period. The continuous high expression of the TGF-β1 protein is associated with a long protection time for osteogenic processes. A previous study showed that a concentration of 1-10 μg/l (1000-10,000 ng/l) of TGF-β1 can induce BMMSCs to differentiate into chondrocytes and thus promote greater treatment efficiency.
Continuous inflammation, especially inflammation involving a variety of inflammatory factors, plays a leading the role in inducing implant and alveolar bone resorption. It is reported that inflammatory T cells can down-regulate the runt-related transcription factor 2 (Runx-2) pathway in stem cells by secreting IFN-γ and up-regulate the TNF-α signal pathway by activating caspase 8 and caspase 3, resulting in apoptosis of transplanted cells [20,21]. Our previous study demonstrated that CD4+ T cells can induce apoptosis in BMMSCs and tissue-engineered cartilage absorption by secreting endogenous IFN-γ and TNF-α. However, TGF-β1 can induce CD4+ T cells to differentiate into iTreg cells and then inhibit apoptosis of BMMSCs and reduce tissue-engineered cartilage absorption. Thus, TGF-β1 not only plays a role in inducing cartilage but also promotes CD4+ T cells to differentiate into iTreg cells. Therefore, we speculate that combining Geistlich Bio-Oss® and TGF-β1+BMMSCs achieves dual functions in promoting bone guidance and controlling local inflammation. Suppressing local inflammation to provide environmental protection for the ossification of Geistlich Bio-Oss® promotes the formation and maturation of new bone, which also has important clinical significance in preserving the height and thickness of the alveolar ridg e.
In this study, we have established a rabbit model of alveolar bone defects, and Geistlich Bio-Oss® with and without pro-inflammatory cytokines and TGF-β1 complex were used to repair these defects. Up to 60 days after surgery, no significant differences in repair were detected between groups. These observations indicate that pro-inflammatory cytokines and TGF-β1 had no significant effects on the ossification of Geistlich Bio-Oss® at early stages and suggest that stem cells cannot effectively promote bone tissue regeneration. Ninety days after surgery, tissue sections from the TGF-β1+BMMSCs+Geistlich Bio-Oss® group and the Geistlich Bio-Oss® group showed bony unions between nascent alveolar bone and newly formed alveolar bone, reflecting an increased amount of new bone. However, these differences were not significant, which indicates that TGF-β1+BMMSCs did not effectively increase bone formation induced by Geistlich Bio-Oss®. In group B, pro-inflammatory cytokines significantly decreased the osteogenesis, indicating that pro-inflammatory cytokines can cause inflammation and inhibit the osteogenesis of Geistlich Bio-Oss®. These results suggest that, compared to their in vitro effects, TGF-β1+BMMSCs did not effectively secrete TGF-β1 to inhibit inflammation in vivo.
Acknowledgements
Chichi Li and Wei Bi contributed equally to this work. The authors gratefully acknowledge funding support from the National Natural Science Foundation of China (Grant No.81670956), the State Key Program (Grant No.14JC1490600) and Shanghai Cooperative International Project (Grant No.16520710400) of Shanghai Committee of Science and Technology, China.
Disclosure of conflict of interest
None.
References
- 1.Corbella S, Weinstein R, Francetti L, Taschieri S, Del Fabbro M. Periodontal regeneration in aggressive periodontitis patients: a systematic review of the literature. J Investig Clin Dent. 2016 doi: 10.1111/jicd.12245. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Tolstunov L. Maxillary tuberosity block bone graft: innovative technique and case report. J Oral Maxillofac Surg. 2009;67:1723–1729. doi: 10.1016/j.joms.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol 2000. 2015;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 4.Chrcanovic BR, Albrektsson T, Wennerberg A. Prophylactic antibiotic regimen and dental implant failure: a meta-analysis. J Oral Rehabil. 2014;41:941–956. doi: 10.1111/joor.12211. [DOI] [PubMed] [Google Scholar]
- 5.Robitaille N, Reed DN, Walters JD, Kumar PS. Periodontal and peri-implant diseases: identical or fraternal infections? Mol Oral Microbiol. 2016;31:285–301. doi: 10.1111/omi.12124. [DOI] [PubMed] [Google Scholar]
- 6.Carlino P, Pepe V, Pollice G, Grassi FR. Immediate transmucosal implant placement in fresh maxillary and mandibular molar extraction sockets: description of technique and preliminary results. Minerva Stomatol. 2008;57:471–483. [PubMed] [Google Scholar]
- 7.Chen X, Hirt H, Li Y, Gorr SU, Aparicio C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of streptococcus gordonii and prevented formation and growth of biofilms. PLoS One. 2014;9:e111579. doi: 10.1371/journal.pone.0111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaky AE, Sawair FA, Al-Karadsheh OA, Eimar HA, Algarugly SA, Baqain ZH. Antibiotic prophylaxis and early dental implant failure: a quasirandom controlled clinical trial. Eur J Oral Implantol. 2011;4:31–38. [PubMed] [Google Scholar]
- 9.Koutouzis T, Catania D, Neiva K, Wallet SM. Innate immune receptor expression in peri-implant tissues of patients with different susceptibility to periodontal diseases. J Periodontol. 2013;84:221–229. doi: 10.1902/jop.2012.120061. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Sun J, Gong Y, Ding X, Ruan H, Ye L, Yu Y. Transforming growth factor-beta1-induced Treg cells inhibit the absorption of tissue-engineered cartilage caused by endogenous IFNgamma and TNF-alpha. Expert Opin Biol Ther. 2014;14:573–581. doi: 10.1517/14712598.2014.896333. [DOI] [PubMed] [Google Scholar]
- 11.Mladenovic Z, Sahlin-Platt A, Andersson B, Johansson A, Bjorn E, Ransjo M. In vitro study of the biological interface of Bio-Oss: implications of the experimental setup. Clin Oral Implants Res. 2013;24:329–335. doi: 10.1111/j.1600-0501.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- 12.Stavropoulos A, Karring T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J Clin Periodontol. 2010;37:200–210. doi: 10.1111/j.1600-051X.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu HY, Zheng H, Hou XP, Zhong WJ, Ying XX, Chai SL, Ma GW. Bio-Oss((R)) for delayed osseointegration of implants in dogs: a histological study. Br J Oral Maxillofac Surg. 2014;52:729–734. doi: 10.1016/j.bjoms.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Payer M, Lohberger B, Strunk D, Reich KM, Acham S, Jakse N. Effects of directly autotransplanted tibial bone marrow aspirates on bone regeneration and osseointegration of dental implants. Clin Oral Implants Res. 2014;25:468–474. doi: 10.1111/clr.12172. [DOI] [PubMed] [Google Scholar]
- 15.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: from basic research to clinical case study. Cell Transplant. 2004;13:343–355. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhou L, Li C, Xie H, Lu Y, Wu Y, Liu H. Bone marrow-derived cells homing for self-repair of periodontal tissues: a histological characterization and expression analysis. Int J Clin Exp Pathol. 2015;8:12379–12389. [PMC free article] [PubMed] [Google Scholar]
- 18.Kaigler D, Avila G, Wisner-Lynch L, Nevins ML, Nevins M, Rasperini G, Lynch SE, Giannobile WV. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11:375–385. doi: 10.1517/14712598.2011.554814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon S, Smith AJ, Lumley PJ, Berdal A, Smith G, Finney S, Cooper PR. Molecular characterization of young and mature odontoblasts. Bone. 2009;45:693–703. doi: 10.1016/j.bone.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Singhatanadgit W, Salih V, Olsen I. Up-regulation of bone morphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. J Cell Physiol. 2006;209:912–922. doi: 10.1002/jcp.20799. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFNgamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]