Abstract
Aims: Spinal cord injuries (SCIs) can cause severe disability or death. The principal treatments for traumatic SCI include surgical stabilization and decompression. Using muscle as a scaffold is a new approach. The aim of this work is to evaluate the clinical efficacy of muscle graft as a scaffold for the growing axons organizing their growth, preventing gliosis in the damaged area and enhancing neural recovery in canine model of traumatic spinal cord injury. Methods: 14 dogs were divided into group I (Control group) 4 control dogs subjected to Sham operation, group II (Trauma control group) 5 dogs subjected to dorsal laminectomy with excision of 1 cm segment of the spinal cord and group III (Muscle graft group) 5 dogs subjected to dorsal laminectomy then muscle graft was taken from the longissimus thoraces and inserted into the spinal cord gap. The animals of all groups were euthanatized after 8 weeks. Olby and modified Tarlov scores were used to clinically evaluate the therapeutic effects. Spinal cord specimens were subjected to histological, morphometric and statistical studies. Results: Olby and modified Tarlov scores revealed significant clinical improvement in the muscle graft group. Histological sections showed overgrowth of axons on the muscle graft and the sections started to organize as central gray matter and peripheral white matter. CD44 & CD105 stains were positive for endogenous stem cells. Conclusions: This study proved the clinical efficacy of muscle grafting as a tool for induction of neuroregeneration after traumatic spinal cord injury.
Keywords: Spinal cord, dorsal laminectomy, muscle graft, stem cells
Introduction
Spinal cord injuries (SCIs) can cause severe disability or death. The most common causes of SCI are motor vehicle accidents (38%), falls (22%), violence (13.5%), and sport/recreational accidents (9%) [1]. Traumatic SCI often results in the death of a number of neurons that can neither be recovered nor regenerated. The SCI process is characterized by demyelination, neural apoptosis and posttraumatic inflammatory reactions [2]. In SCI, structural and functional damage of the spinal cord occurs by primary and secondary injury. This results in loss of movement, sensation and autonomic nerve dysfunction below the damaged level.
These effects can damage the patient’s mental health and place a huge burden on society from a public health perspective [3]. There is evidence that T lymphocytes and macrophages which infiltrate the injured spinal cord, are directly involved in the pathogenesis and extension of SCI [4]. The principal treatments for traumatic SCI include surgical stabilization and decompression. Physiotherapy and neurotrophic rehabilitation attenuate further damage, relieve spinal cord ischemia and hypoxia, save the damaged area, rescue necrotic neurons, and promote neuronal function [5]. Mesenchymal stem cells (MSCs) are a promising potential therapeutic approach to SCI promotes neuronal regeneration, reduces glial scar formation and ultimately improves locomotor recovery [6]. Some researchers used tissue engineering to develop stem cells approach by using silk as a scaffold and adding growth factors [7]. Others suggested the efficacy of utilizing autogenous skeletal muscle instead of using a nerve segment as a graft in peripheral nerve injury [8].
The aim of this work is to evaluate the clinical efficacy of muscle graft as a scaffold for the growing axons organizing their growth, preventing gliosis in the damaged area and enhancing neural recovery in canine model of traumatic spinal cord injury.
Materials and methods
Experimental design
This study was conducted on 14 adult male mongrel dogs with average body weight 7-9 kilograms, housed in the Animal House, Faculty of Veterinary Medicine, Cairo University, according to the guidelines for the care and use of experimental animals of Cairo University and were divided into the following 3 groups:
Group I: (Control Group): It included 4 dogs that were subjected to Sham operation [9] where animals underwent dorsal laminectomy only at the midline and extended two to three vertebrae cranial and caudal to the vertebrae to be exposed. The animals were sacrificed with the corresponding experimental groups.
Group II (Trauma control group): It included 5 dogs that were subjected to dorsal laminectomy [10]. Dorsal laminectomy was performed with longitudinal incision of the dura then excision of one cm segment of the spinal cord was performed at the mid thoracic region then the dura was repaired to prevent cerebrospinal fluid (CSF) leakage. The animals were kept for 8 weeks after operation without therapy.
Group III (Muscle graft group): It included 5 dogs that underwent to dorsal laminectomy and excision of the spinal cord as in group II, then muscle graft was taken from the longissimus thoraces during surgery. The muscle graft was prepared to fit inside the gap between the proximal end and the distal ends of the spinal cord. The dura was repaired to prevent CSF leakage. The animals were kept for 8 weeks after operation.
Anesthesia
Atropine sulphate (0.1 mg/kg SC), dexamethasone (1.5 mg/kg IV) and xylazine hydrochloride (1 mg/kg IM) were used. Induction of anesthesia was performed using Propofol (4-6 mg/kg IV) and the maintenance was applied using Isoflurane [11,12].
Surgical technique
The dogs were positioned in sternal recumbence. The skin was surgically incised at the dorsal midline and extended two to three vertebrae cranial and caudal to the vertebrae to be exposed [10]. The subcutaneous tissue was desiccated to the muscle fascia and retracted laterally with the skin. The dorsal thoracic fascia was bluntly desiccated slightly away from the dorsal thoracic spinous processes.
Lateral retraction of the dorsal thoracic fascia exposed the longissimus lumborum and multifidus muscles caudally and spinalis and semispinalis muscles cranially. The multifidus, interspinalis, and rotators longi muscles were elevated from the spinous processes and vertebral arches one vertebra cranial and caudal to the intended vertebrae with a periosteal elevator. The muscle elevation was continued to the lateral aspect and ventral to the articular processes. In the thoracic region fascicles of the longissimus thoraces and lumborum muscle were divided. A small blood vessel was severed at each articular process [11].
Dorsal laminectomy was initiated by removing of the dorsal thoracic spinous processes. The spinal cord was exposed by removal of bone from the bilateral portions of the pedicles and dorsal laminae of the involved vertebrae. Lembert and Kerrison rongeurs and small bone curettes were used for completing the laminectomy. After the outer white cortical bone was brushed away, a thick layer of reddish brown trabecular bone was encountered next, followed by a thin inner layer of white cortical bone and finally a translucent inner periosteal layer. Careful lavage and suction were needed to maintain a clear field throughout the laminectomy process. Incision of the Dura matter was carefully performed then a segment of one cm length from the spinal cord was transected. The venous plexus was visible on the floor of the vertebral canal. To avoid damaging the venous structures, care should be taken [13].
A suitable segment was cut from the longissimus thoracis muscle and located instead of the spinal cord defect. The Closure was started by suturing the dorsal fascia of the thoracic musculature. The subcutaneous tissue and skin were closed in separate layers with avoiding of dead space [11].
Clinical scoring (initial assessment and follow up)
All dogs were subjected to neurological assessment by a neurologist to exclude any motor or sensory deficits before the experiment, the gait of each animal in the different groups was assessed and videotaped before and after surgery, assessment was done using Olby score (a 14-point functional scoring system for observational gait analysis) and revised modified Tarlov scale. Both were established and commonly used to evaluate functional differences in dogs with acute spinal cord injury by examining the pain sensation and motor function including, tail movement, weight bearing and movement of limbs. Statistical analysis and calculations were performed using Statistical Package for the Social Sciences (SPSS) version 16. The comparison between the different groups was analyzed using T test. P-values <0.05 were considered statistically significant (Table 1). Two different independent observers blinded to cases and control rated the animals by reviewing the videotapes of different groups. Assessment was done weekly for 8 weeks [14].
Table 1.
Comparison of Olby score and Revised Modified Tarlov scale
| Olby score | Revised Modified Tarlov scale | |||
|---|---|---|---|---|
| No pelvic limb movement. | No deep pain sensation. | 0 | 1 | Flaccid hind limbs |
| With deep pain sensation. | 1 | |||
| But voluntary tail movement. | 2 | |||
| Non-weight-bearing protraction of pelvic limb with more than one joint involved. | Minimal movement of one joint. | 3 | 2 | Tone in hind limb |
| Less than 50% of the time. | 4 | 3 | Purposeful hind limb motion | |
| More than 50% of the time. | 5 | |||
| Weight-bearing protraction of pelvic limb. | Less than 10% of the time. | 6 | 4 | Stands with assistance |
| 10-50% of the time. | 7 | |||
| More than 50% of the time. | 8 | |||
| Weight-bearing protraction 100% of time with reduced strength of pelvic limb. | Mistake >90% of the rime. | 9 | 5 | Stands unassisted |
| Mistake 50%-90% of the rime. | 10 | 6 | Limited ambulation | |
| Mistake <50% of the rime. | 11 | |||
| Ataxic pelvic limb gait with normal strength. | But mistakes made >50% of time. | 12 | 7 | Full ambulation |
| But mistakes made <50% of time. | 13 | 8 | Climbs a 20 incline ramp half-way | |
| Normal pelvic limb gait. | 14 | 9 | Climbs 20 incline ramp | |
The animals were euthanatized by injection of 20% solution of pentobarbital sodium and full saturated solution of potassium chloride intravenous [15]. Spinal cord specimens were fixed in 10% formalin saline for 24 hours. Paraffin blocks were prepared and 5 μm thick serial sections were subjected to the following studies in the Histology Department, Faculty of Medicine, Cairo University:
Histological Study: Hematoxylin and eosin (H&E) [16].
Histochemical stain: using silver impregnation for demonstrating neurofibrils and glial cells [17].
Immunohistochemical Study: CD44 (IW-PA1021) for endogenous mesenchymal stem cells. 0.1 ml primary rabbit polyclonal antibodies were applied to sections for 60 minutes [18]. and CD105 (559286 Ab, BD Biosciences, San Jose, California, USA) immunostaining, for detecting endogenous undifferentiated MSCs 0.1 ml diluted goat polyclonal 1ry antibodies were applied to sections for 60 minutes [19]. Tonsil sections were considered positive control and the reaction is membranous.
Morphometric study
Computer assisted image analysis was performed using Olympus camera connected to Olympus microscope, assessment of the numbers of axons in the transverse sections of the white matter in H&E stained sections. The number of oligodendroglia in the white matter in silver stained sections. The areas of CD44+ and CD105+ cells were measured. Using interactive measurements menu the parameters were assessed in 10 high power fields.
Statistical methods [20]
Statistical analysis and calculations were performed using Statistical Package for the Social Sciences (SPSS) version 16. The comparison between the different groups was analyzed using ANOVA test, followed by Bonferroni post-hoc test to detect which pairs of groups caused the significant difference. P-values <0.05 were considered statistically significant [20].
Results
Death of 2 dogs in the trauma control group was recorded which were compensated. In the sham control group, neuronal morphology was normal; the general structure and structural integrity were preserved. The most marked degenerative changes were observed in the trauma control group.
Clinical results
There was a statistically significant difference in Olby scoring between muscle graft dogs and trauma control ones regarding the motor functions; in each group we compared the performance in week 1 to that in week 8 as shown in Tables 2, 3, which illustrated that the muscle graft dogs showed clinical improvement regarding the motor functions with a P value 0.002. The results on Tarlov scale were equal before and after in all the trauma group equally with 1 grade in this scale so the standard error equal zero.
Table 2.
Olby score/revised modified tarlov scale
| 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | 7th week | 8th week | |
|---|---|---|---|---|---|---|---|---|
| Trauma control 1 | 1/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| Trauma control 2 | 1/1 | 1/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| Trauma control 3 | 0/1 | 1/1 | 1/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| Trauma control 4 | 1/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| Trauma control 5 | 0/1 | 1/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 | 2/1 |
| Graft 1 | 1/1 | 0/1 | 1/1 | 3/2 | 4/3 | 6/4 | 7/4 | 8/4 |
| Graft 2 | 1/1 | 1/1 | 1/1 | 3/2 | 4/3 | 6/4 | 7/4 | 8/4 |
| Graft 3 | 1/1 | 1/1 | 3/2 | 5/3 | 6/4 | 7/4 | 8/4 | 9/5 |
| Graft 4 | 1/1 | 1/1 | 2/1 | 4/3 | 5/3 | 6/4 | 7/4 | 8/4 |
| Graft 5 | 1/1 | 1/1 | 2/1 | 3/2 | 4/3 | 5/4 | 6/4 | 7/4 |
Table 3.
Mean difference between 1st week (pre-intervention) and 8th week (post-intervention) with standard deviation (SD) and P-value
| Trauma control group | Graft group | |
|---|---|---|
| Olby score (mean ± SD) | 1.333 ± 0.58 (P value =0.06) | 7.33 ± 0.57 (P value =0.002)* |
| Tarlov scale (mean ± SD) | ----- | 3.33 ± 0.57 (P value =0.01) |
significant p value.
Histological results
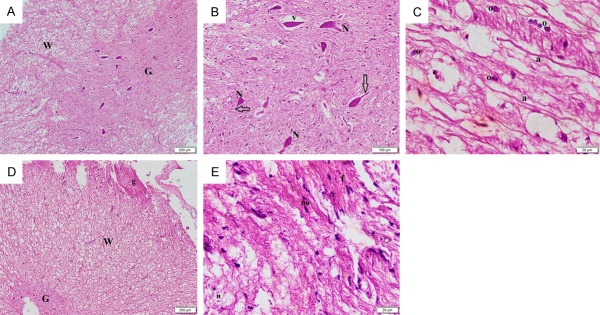
Sections stained with H&E of the dog spinal cord in the control group showed central canal, grey matter and white matter all with apparently normal structure (Figure 1A). Multiple neurons were seen in the grey matter and nerve fibers with axons were detected in the cross sections of the white matter (Figure 1B). The longitudinal sections of the white matter showed multiple continuous axons. Oligodendroglia and microglia could be seen (Figure 1C). In group II, vacuolation were detected in the grey and white matter (Figure 1D). The grey matter showed neurons with lost processes surrounded by vacuolated areas. Few nerves exhibited an axon in the white matter (Figure 1E). Disrupted axons were detected in the white matter with few oligodendroglia and some microglia (Figure 1F). Group III showed grey matter with multiple neurons and white matter with apparently normal nerve fibers (Figure 2A). Multiple neurons exhibiting processes could be seen with few neurons surrounded by vacuolation (Figure 2B). Some continuous axons in the white matter were seen with multiple oligodendroglia (Figure 2C). Residual graft tissue was detected attached to the peripheral part of the white matter (Figure 2D). At higher magnification, the graft tissue exhibited flat nuclei and elongated fibers (Figure 2D).
Figure 1.
Photomicrographs of the dog spinal cord sections (H&E, ×) showing in Group I: (A) central canal (C), grey matter (G) and white matter (W) all demonstrating normal structures. (40) (B) multiple neurons (N) in the grey matter and nerve fibers each containing an axon (n) in the white matter (100) (C) multiple axons (a) in the white matter. Note oligodendroglia (o) and microglia (m) (400). In Group II: (D) vacuolations (v) in the grey (G) and white (W) matter (40) (E) neurons (N) each surrounded by a vacuolated area in the grey matter. The white matter shows multiple vacuolations (v). Note few nerves exhibiting an axon (n) in the white matter (100) (F) disrupted axons (dr) in the white matter. Note few oligodendroglia (o) and some microglia (m) (400).
Figure 2.
Photomicrographs of the dog spinal cord sections showing in Group III (H&E, ×): (A) grey matter (G) with multiple neurons and white matter (W) with apparently normal nerve fibers (40) (B) multiple neurons (N) exhibiting processes (arrows). Few neurons surrounded by vacuolations (v) (100) (C) some continuous axons (a) in the white matter. Note multiple oligodendroglia (o) (D) residual graft tissue (g) attached to the peripheral part of the white matter (W) and central grey matter (G) (40) (E) the graft tissue with flat nuclei (nu) and elongated fibers (f). Note a nerve fiber exhibiting an axon (n) in the white matter (400).
Silver impregnation results: Spinal cord sections in control group demonstrated neurofibrils in multiple nerve cell bodies, processes, in the neuropil, multiple axons and some oligodendroglia (Figure 3A, 3D). Neurofibrils were seen in deformed neurons with lost processes, disrupted axons and few oligodendroglia in group II (Figure 3B, 3E). Neurofibrils were evident in fewer nerve cell bodies, processes, in the neuropil, multiple scattered and arrayed oligodendroglia in group III (Figure 3C, 3F).
Figure 3.

Photomicrographs of the dog spinal cord sections demonstrating the grey matter in (A-C) (silver impregnation, ×100) and the white matter in (D-F) (silver impregnation, ×400) of Group I, II & III respectively showing neurofibrils in (a) multiple nerve cell bodies (n), processes (arrow) and in the neuropil (arrowhead) (B) deformed neurons with lost processes (white arrows) (C) fewer nerve cell bodies (n), processes (arrow) and in the neuropil (arrowhead) (D) multiple axons (a) and some oligodendroglia (o) (E) disrupted axons (dn) and few oligodendroglia (o) (F) multiple scattered and arrayed of oligodendroglia (o).
Immunohistochemical results
CD44 Immunostained group I showed negative immunoexpression (Figure 4A). While in group II some positive spindle cells were found among the nerve fibers (Figure 4B). In group III multiple positive cells were detected among the nerve fibers and in the remaining graft tissue (Figure 4C).
Figure 4.

Photomicrographs of the dog spinal cord sections showing in: (A) Group I -ve immunoexpression (B) Group II some +ve spindle cells among the nerve fibers (lines) (C) Group III multiple +ve cells among the nerve fibers (arrow) and in the remaining graft tissue (red arrow) (CD44 immunostaining, ×200). (D) Group II some +ve spindle cells among the nerve fibers (lines) (E) Group III multiple +ve cells among the nerve fibers (arrow) and in the remaining graft tissue (arrows) (CD105 immunostaining, ×200).
CD105 Immunostained sections in group II some positive spindle cells were found among the nerve fibers and in group III multiple positive cells were detected among the nerve fibers and in the remaining graft tissue (Figure 4D, 4E).
Morphometric results
A significant increase was found in group I compared to groups II and III in addition to a significant increase in group III compared to group II concerning count of axons. In group III a significant increase was found compared to groups I and II as regards count of oligodendrocytes. In group III a significant increase was found compared to group II concerning area of CD44 & CD105 positive cells (Table 4).
Table 4.
Mean count of axons, mean count of Oligodendrocytes and mean area of CD44 in control and experimental groups ± SD and (P value)
| Count of axons | Count of Oligodendrocytes | Area of CD44+ ve cells | Area of CD105+ ve cells | |
|---|---|---|---|---|
| Control group I | 30.9 ± 3.42* | 7.8 ± 1.58 | - | - |
| Trauma control group II | 7.6 ± 1.32 | 6.1 ± 1.28 | 124.21 ± 22.64 | 111.24 ± 20.74 |
| Graft group III | 17.6 ± 3.04** (P value =0.01) | 15.5 ± 2.08# (P value =0.03) | 236.02 ± 31.87^ (P value =0.01) | 226.03 ± 25.97^ (P value =0.01) |
significant increase compared to groups II and III;
significant increase compared to group II;
significant increase compared to groups I and II;
significant increase compared to group II.
Discussion
Dogs have been chosen to study spinal cord injuries because neurological examinations can be performed easily, and the mechanisms of SCI in dogs are similar to those in human patients mainly due to vertebral fracture-luxation and disc extrusions with mixed contusion-compression, as previously reported [21].
It’s known that human spinal cord injury is complex, so no one model can represent all aspects of injury. Transection models whether complete or partial are used to investigate neuronal regeneration following injury with the ability to assess axonal regeneration and functional recovery [22]. In the present study dorsal laminectomy was done with removal of 1 cm long of the spinal cord thoracic segment. Spinal cord injury was proved by clinical assessment of Olby score and modified Tarlov scale [14].
Histological examinations of trauma control group (g II) revealed vacuolation in grey and white matter. The grey matter showed neurons surrounded by vacuolated areas. Disrupted axons were detected in the white matter with few oligodendroglia and some microglia. The previously mentioned findings may occur due to failure of regeneration after SCI due to a combination of factors including myelin-derived inhibitors, inhibitory molecules expressed by reactive astrocytes near the injury site, poor intrinsic regenerative capacity of spinal cord neurons and a shortage of substance promoting neuron regeneration [23]. These findings coincide with Song et al, [24] who observed a small number of neurons survived in the gray matter together with swollen axons and many vacuoles in the remaining white matter in after SCI. Cells and molecules associated with scar formation were activated to form a glial scar that can limit axon regeneration.
The necrotic and degenerated tissues were removed by phagocytes and replaced by neuroglial cells at the injured site within 6 weeks after the injury. Then, the physical separation and neural demyelination interrupted the physiological signal transduction pathway, which was marked clinically by a partial or total loss of sensory, motor, urine, and voluntary control of urination and defecation [25].
Neurofibrils were seen in deformed neurons with lost processes, disrupted axons and few oligodendroglia in silver impregnation in group II. The previously mentioned results denoted degenerative changes. It was documented that reduced cell-cell adhesion, disruption of the blood-spinal cord barrier, up-regulation of pro-inflammatory cytokines, and demyelination were found in SCI [26]. It was confirmed that IL-8 as an early mediator of inflammation was found to be increased, compared to control dogs, especially in acute SCI [27].
In group II, some CD44+ spindle cells and CD105+ spindle cells were seen among the nerve fibers. Many studies have reported that ependymal cells (ECs) in the central canal of the adult spinal cord serve as neural progenitor cells [28]. It was recorded that at the site of lesion, the functionalized cells were probably generated from endogenous neuronal progenitor cells [6]. Many experimental studies have examined the effect of different types of grafts including neural stem cell graft [29], scaffolds seeded with bone marrow stromal cells graft [30]; collagen scaffolds seeded with human umbilical cord-derived mesenchymal stem cells hUC-MSCs [6] on repairing of spinal cord injuries. However, it was recorded that adult neurons can regenerate their axons to some extent when provided with an optimal tissue environment but growth is limited due to neuron-intrinsic mechanisms [31]. Using muscle as a graft has been previously described in repairing peripheral nerve injuries [8,32]. To our knowledge, there is no previous study used muscle as a graft in repairing spinal cord injuries.
In the view of its results the current study showed the efficacy of muscle grafting in neuro regeneration of the acutely injured spinal cord, this was observed clinically as muscle graft dogs had better motor functions and showed improvement of gait even before the 8th week, However the bladder functions were not recovered, this can be explained by dyssynergia, resulted from loss of supraspinal regulation, incoordination of detrusor and sphincter contraction which causes a functional obstruction that reduces the ability to void, In human urinary control is complex and recovery took several months. Loss of control over urine voiding is a common complication after SCI and may be permanent [33]. As reported by Olby et al, management of overflow incontinence consequent to dyssynergia can be difficult in dogs and can lead to urinary tract infection [34].
Histological examination of group III showed grey matter with multiple neurons and white matter with apparently normal nerve fibers. Multiple neurons exhibiting processes could be seen with significant increase in the number of continuous axons compared with group II. Few neurons surrounded by vacuolation. Some continuous axons in the white matter were seen with multiple oligodendroglia. These results indicated regression of degenerative changes compared to group II. It was documented that muscle graft reduced migration of microglia and decreased the expression of oxidative metabolites [35]. It was also reported that histological and morphometric data did not differ significantly between skeletal muscle graft and a conventional nerve graft in repairing segmentary peripheral nerve defect in an experimental animal [36].
In group III, residual graft tissue was detected attached to the peripheral part of the white matter. The graft tissue exhibited flat nuclei and elongated fibers. This may be explained by degradation of muscle tissue after 8 weeks and its replacement by nervous tissue. It was documented that the skeletal muscle when used as a graft is a useful tool for the advancement of regenerated axons bridge. The muscle has been described as the most effective histological structure for the local release of the factors when injected [37].
Neurofibrils were evident with silver impregnation in fewer nerve cell bodies, processes, in the neuropil, multiple scattered and arrayed oligodendroglia with significant increase in the number of oligodendroglia in group III compared to groups I and II. These finding may be explained by regenerative capacity of muscle graft. In previous studies, it was declared that the criteria of a useful graft used in SCI was to provide mechanical support for axonal growth and regeneration and to provide attachment sites for cells via cell surface proteins [38]. The graft has to be guide for growth of axons across a site of injury and to be degradable [39].
Multiple positive cells among the nerve fibers with significant increase in the mean area% of CD44 and the mean area% of CD105 was recorded in group III compared to group II contributed to endogenous stem cells activation. This may be explained by progenitor cells activation that were present in the CT or myocytes of muscle graft or can be stimulated from endogenous sources. Endomysium acts as anatomical and biomechanical support to the growth of sprouting out axons towards the distal stump, motivated by the presence of Schwann cells in both ends initially [8]. It was reported that regenerating axons and Schwann cells of the injured sciatic nerve does not require the presence of the basal lamina of Schwann cells, but only the existence of any cellular basal lamina, including that coming from the skeletal muscle fibers [40]. It has become theoretically possible to create grafts that reconstruct nerves from tubes parallel of basal membrane with exactly the same structure as skeletal muscles [32].
It can be concluded that skeletal muscle graft can be successfully used as a biological scaffold promoting regeneration of the injured cells and providing some neurotrophic factors for the growth of the neurons.
Acknowledgements
We thank the Animal House, Faculty of Veterinary Medicine, Cairo University for hosting of dogs and the Histology Department, Faculty of Medicine, Cairo University for histological staining and immunohistochemical study.
Disclosure of conflict of interest
None.
References
- 1.Gomes-Osman J, Cortes M, Guest J, Pascual-Leone A. A systematic review of experimental strategies aimed at improving motor function after acute and chronic spinal cord injury. J Neurotrauma. 2016;33:425–438. doi: 10.1089/neu.2014.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2010;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito E, Bruscoli S, Mazzon E, Paterniti I, Coppo M, Velardi E, Cuzzocrea S, Riccardi C. Glucocorticoidinduced leucine zipper (GILZ) over-expression in T lymphocytes inhibits inflammation and tissue damage in spinal cord injury. Neurotherapeutics. 2012;9:210–225. doi: 10.1007/s13311-011-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Tan J, Xiao ZF, Zhao YN, Han S, Liu D, Yin W, Li J, Li J, Wanggou S, Chen B, Ren C, Jiang X, Dai J. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in dogs with chronic spinal cord injury. Sci Rep. 2017;7:43559. doi: 10.1038/srep43559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhardwaj N, Kundu SC. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials. 2012;33:2848–57. doi: 10.1016/j.biomaterials.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 8.García-Medrano B, Pérez CS, Sanz PB, García MG, Martín-Ferrero M, et al. The role of the basal lamina in nerve regeneration. J Cytol Histol. 2016;7:438. [Google Scholar]
- 9.Kara H, Degirmenci S, Ak A, Bayir A, Kayis SA, Uyar M, Akinci M, Acar D, Kocacan M, Akyurek F. Neuroprotective effects of sildenafil in experimental spinal cord injury in rabbits. Bosn J Basic Med Sci. 2015;15:38–44. doi: 10.17305/bjbms.2015.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankin EJ, Jerram RM, Walker AM, King MD, Warman CG. Transarticular facet screw stabilization and dorsal laminectomy in 26 dogs with degenerative lumbosacral stenosis with instability. Vet Surg. 2012;41:611–9. doi: 10.1111/j.1532-950X.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 11.Fossum TW. In: Small animal surgery of the thoracolumbar spine. 4th edition. Mosby , editor. St Louis, United States: Elsevier-health sciences division; 2013. Chapter 40. [Google Scholar]
- 12.Duke-Novakovski T, Vries de M, Seymour C. BSAVA manual of dog and feline anaesthesia and analgesia, British small animal veterinary association. 3rd edition. Dorset, UK: Lookers, Upton; 2016. Chapter 1. [Google Scholar]
- 13.Johnson AL, Houlton JEF, Vannini R. Switzerland, Clavadelerstrasse. Davos Platz: CH-7270, AO Publishing; 2005. AO principles of fracture management in the dog and cat; p. 137. [Google Scholar]
- 14.Ryu HH, Kang BJ, Park SS, Kim Y, Sung GJ, Woo HM, Kweon OK. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74:1617–1630. doi: 10.1292/jvms.12-0065. [DOI] [PubMed] [Google Scholar]
- 15.The Human Society of the United States. Euthanasia references manual. 2nd edition. 2013. ISBN 978-934785-03-4. [Google Scholar]
- 16.Bancroft JD, Gamble M. Theory and practice of histological technique, Elsevier Health Science Churchill living stone. 6th edition. Edinburgh, London, Oxford, New York, Philadelphia, St Louis, Sydney and Toronto: 2008. Connective tissue stains; pp. 150–166. [Google Scholar]
- 17.Otify DY, Youssef EA, Nagy NB, Marei MK, Youssef MI. Transdifferentiation of bone marrow mesenchymal stem cells into neural cells via cerebrospinal fluid. Biomedicine and Biotechnology. 2014;2:66–79. [Google Scholar]
- 18.Cai Y, Liu T, Fang F, Xiong C, Shen S. Comparisons of mouse mesenchymal stem cells in primary adherent culture of compact bone fragments and whole bone marrow. Stem Cells Int. 2015;2015:708906. doi: 10.1155/2015/708906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinert AF, Kunz M, Prager P, Göbel S, Klein-Hitpass L, Ebert R, Nöth U, Jakob F, Gohlke F. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther. 2015;6:114–127. doi: 10.1186/s13287-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emsley R, Dunn G, White I. Mediation and moderation of treatment effects in randomized controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery N, Smith P, Lakatos A, Ibanez C, Ito D, Franklin R. Clinical dog spinal cord injury provides an opportunity to examine the issues in translating laboratory techniques into practical therapy. Spinal Cord. 2006;44:584–93. doi: 10.1038/sj.sc.3101912. [DOI] [PubMed] [Google Scholar]
- 22.Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, Kirsch T, Errico TJ. Spinal cord injury models: a review. Spinal Cord. 2014;52:588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 23.Levine JM, Cohen ND, Heller M, Fajt VR, Levine GJ, Kerwin SC, Trivedi AA, Fandel TM, Werb Z, Modestino A, Noble-Haeusslein LJ. Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One. 2014;9:e96408. doi: 10.1371/journal.pone.0096408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Wang Z, Shen J, Xu S, Hu Z. Nerve growth factor delivery by ultrasound-mediated nanobubble destruction as a treatment for acute spinal cord injury in rats. Int J Nanomedicine. 2017;12:1717–1729. doi: 10.2147/IJN.S128848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anna Z, Katarzyna JW, Joanna C, Barczewska M, Joanna W, Wojciech M. Therapeutic potential of olfactory ensheathing cells and mesenchymal stem cells in spinal cord injuries. Stem Cells Int. 2017;2017:3978595. doi: 10.1155/2017/3978595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. J Neurosci. 2011;31:15894–15903. doi: 10.1523/JNEUROSCI.3943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AR, Welsh CJ, Young C, Spoor E, Kerwin SC, Griffin JF, Levine GJ, Cohen ND, Levine JM. Cerebrospinal fluid inflammatory cytokines and chemokines in naturally occurring dog spinal cord injury. J Neurotrauma. 2014;31:1561–1569. doi: 10.1089/neu.2014.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton LK, Truong MK, Bednarczyk MR, Aumont A, Fernandes KJ. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–1056. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014;257:186–204. doi: 10.1016/j.expneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N, Xie H, Xi C, Zhang H, Yan J. A study to compare the efficacy of polyether ether ketone rod device with titanium devices in posterior spinal fusion in a dog model. J Orthop Surg Res. 2017;12:40. doi: 10.1186/s13018-017-0543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palhares A, Viterbo F, Cardoso RG. Muscle graft as a substitute for peripheral nerve graft in rats. Acta Cir Bras. 2009;24:221–5. doi: 10.1590/s0102-86502009000300011. [DOI] [PubMed] [Google Scholar]
- 33.Tai C, Roppolo JR, de Groat WC. Spinal reflex control of micturition after spinal cord injury. Restor Neurol Neurosci. 2006;24:69–78. [PMC free article] [PubMed] [Google Scholar]
- 34.Olby NJ, MacKillop E, Cerda-Gonzalez S, Moore S, Muñana KR, Grafinger M, Osborne JA, Vaden SL. Prevalence of urinary tract infection in dogs after surgery for thoracolumbar intervertebral disc extrusion. J Vet Intern Med. 2010;24:1106–1111. doi: 10.1111/j.1939-1676.2010.0567.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, Jo S, Kim WH, Kweon O. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015;6:229. doi: 10.1186/s13287-015-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira EF, Mazzer N, Barbieri CH, DelBel EA. The use of a muscle graft to repair a segmentary nerve defect: an experimental study using the sciatic nerve of rats as model. J Neurosci Methods. 2004;133:19–26. doi: 10.1016/j.jneumeth.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Moimas S, Novati F, Ronchi G, Zacchigna S, Fregnan F, Zentilin L, Papa G, Giacca M, Geuna S, Perroteau I, Arnež ZM, Raimondo S. Effect of vascular endothelial growth factor gene therapy on post-traumatic peripheral nerve regeneration and denervation-related muscle atrophy. Gene Ther. 2013;30:1–8. doi: 10.1038/gt.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Zhang X, Cao R, Yu B, Liang H, Zhou M, Li D, Wang Y, Liu E. Allografts of the acellular sciatic nerve and brain-derived neurotrophic factor repair spinal cord injury in adult rats. PLoS One. 2012;7:e42813. doi: 10.1371/journal.pone.0042813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, Gao P, Hu G, Jin Y. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose derived mesenchymal stem cells. Biomaterials. 2010;31:5312–24. doi: 10.1016/j.biomaterials.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Meek MF, Varejão AS, Geuna S. Use of skeletal muscle tissue in peripheral nerve repair: review of the literature. Tissue Eng. 2004;10:1027–1036. doi: 10.1089/ten.2004.10.1027. [DOI] [PubMed] [Google Scholar]