Abstract
Purpose: Previous studies have shown that celastrol has anti-inflammatory, anti-oxidative and anti-tumor activities, but little is known about its protective effects on ventilator induced lung injury (VILI). This study is aimed to investigate the effects of celastrol on VILI and explore its potential mechanism. Methods: A total of 40 ICR male mice aged 7-9 weeks were randomly divided into 4 groups (n=10 per group): control group (Con), control + celastrol group (Con+Ce), mechanical ventilation group (Ven) and mechanical ventilation + celastrol group (Ven+Ce). The lungs were collected for histological examination, detection of W/D, and MPO, MDA, SOD, inflammatory cytokines (IL-1β, IL-6, IL-10 and TNF-α) by ELISA, p-P38 and p-JNK 1/2 protein by Western blotting, and collagen-1 and TGF-β mRNA expression by RT-PCR. Results: The W/D in the Ven group was significantly higher than the W/D in the Con group and the Ven+Ce group (both P<0.01). Mechanical ventilation for 4 h markedly increased lung MPO and MDA activity, TNF-α, IL-1β and IL-6, but dramatically reduced SOD and IL-10 (all P<0.01). However, celastrol pre-treatment compromised the increased MPO, MDA, TNF-α, IL-1β, IL-6 (all P<0.01) and significantly increased SOD (P=0.035<0.05) and IL-10 (P<0.01). In addition, mRNA level of collagen-1 and TGF-β as well as p-P38 and p-JNK 1/2 protein expression increased significantly (P<0.01) after mechanical ventilation, which however were markedly reduced in the presence of celastrol pre-treatment. Conclusion: Celastrol pre-treatment may exert anti-oxidative and anti-inflammatory effects and related lung fibrosis to attenuate VILI in mice, which may be related to the inhibition of p-P38 and p-JNK 1/2 by MAPK pathway.
Keywords: Ventilator induced lung injury, celastrol, MAPK pathway, collagen-1, TGF-β
Introduction
Mechanical ventilation [1] is the most common respiratory support technique during the peri-operative period and has been regarded as an indispensable tool in the management of critical illnesses. Mechanical ventilation assures sufficient oxygenation in patients. However, it is not a physiological ventilation pattern. The shear force due to positive pressure ventilation in mechanical ventilation may not only induce inflammatory cell activation and the generation of inflammatory mediators and cytokines [2], which are closely related to the clinical outcomes, but also cause pulmonary fibrosis [3], resulting in permanent lung injury [4]. There is evidence showing that prolonged mechanical ventilation will significantly increase mortality [3]. Generally, the lung injury second to mechanical ventilation is known as ventilator induced lung injury (VILI). VILI includes barotrauma, volutrauma, biotrauma and atelectasis [5]. Mechanical ventilation as a pro-inflammatory stimulation may activate inflammatory cascade, causing biological injury via molecular biological and cytological responses [1,2,6-9]. Reduction in tidal volume and ventilation pressure has been employed for the effective prevention and/or therapy of VILI in clinical practice [10,11]. However, reduction in tidal volume and/or ventilation pressure is usually infeasible for some patients due to some limitations [12]. Thus, investigators attempt to use strategies to inhibit the activation of inflammatory cells and reduce the generation of inflammatory cytokines, exerting pulmonary protective effects. To date, anti-oxidant glutathione and anti-inflammatory ulinastatin have been used for lung protection [13,14].
Celastrol (also known as tripterine) is an effective compound extracted from the traditional Chinese medicine, Tripterygium wilfordii, and is a member of triterpenes [5]. Studies have shown that celastrol has multiple biological activities such as anti-inflammatory, anti-tumor and immunosuppressive activities [15-19]. Tripterygium wilfordii is a perennial creeping plant, known as Thunder of God Vine, belongs to the family Celastraceae. Celastrol is a most widely studied and promising compound isolated from Tripterygium wilfordii. Celastrol was initially shown to have potent anti-inflammatory capability, and thus it is widely used in the treatment of many inflammatory diseases, including allergic-asthma [20], amyotrophic lateral sclerosis [21], and rheumatoid arthritis [22]. In recent years, studies reveal that celastrol as a triterpenoid is also a promising anti-cancer drug because it is able to inhibit the proliferation of cancer cells, prevent the invasion of cancer cells, block angiogenesis in malignant tissues and also treat sensitive resistant cancer cells [23-25]. In addition, celastrol was also found to protect against obesity and metabolic dysfunction through the activation of a HSF1-PGC1 alpha transcriptional axis [26].
Considering the anti-oxidative and anti-inflammatory activities, we hypothesize that celastrol may attenuate ventilation induced biotrauma to alleviate VILI. This study was undertaken to investigate the lung protective effects of celastrol pretreatment against VILI.
Materials and methods
Animals and interventions
All the experimental procedures related to animals were performed according to the scientific and ethic committees of the Second Military Medical University. Animals were taken care according to the Chinese national regulation for experimental animal care and study. Adult male ICR mice (specific pathogen free) aged 7-9 weeks and weighing 23-28 g (n=40) were purchased from the Experimental Animal Center of the Second Military Medical University. Celastrol was dissolved in DMSO to a storage concentration of 50 mg/ml and was dissolved with PBS (pH 7.0) to a concentration of 1 mg/ml before use [27]. Forty mice were randomly assigned into 4 groups (n=10 per group): control group (Con): mice were intraperitoneally [27] injected with equal volume of DMSO and PBS; control + celastrol group (Con+Ce group): mice were intraperitoneally injected with celastrol at 1 mg/kg for consecutive 3 days; ventilation group (Ven): mice received 3 days of injection of equal volume of DMSO and PBS before the mechanical ventilation on the 4 th day; ventilation + celastrol group (Ven+Ce): mice received 3 days of injection of equal volume of celastrol before the mechanical ventilation on the 4 th day. Mechanical ventilation was performed at 30 ml/kg for 4 h with oxygen concentration 100% after anesthesia with ketamine at 70 mg/kg [28].
Reagents and instruments
Celastrol (Selleckchem, USA), ventilator (Harvard apparatus, USA), enzyme linked immunosorbent assay (ELISA) kits, kits for the detection of measurements of myeloperoxidase (MPO), superoxide dismutase (SOD) and malonaldehyde (MDA) (Nanjing Jiancheng Bioengineering Institute, China), primary antibodies against p-P38, P38, p-JNK 1/2, JNK 1/2 and GAPDH (CST, USA), secondary antibodies and phosphate buffer saline (Beyotime Institute of Biotechnology, China), Chemiluminescence detection kit (Millipore, USA), SYBR Green PCR kit for real time PCR (Thermo, USA), reverse transcription kit (Fermentas, Lithuania) and thermal cycler (ABI, USA) were used in the present study.
Detection of lung wet to dry ratio
After mechanical ventilation, the right lower lobe was collected and weighed as wet weight (W). Then, the lung tissues were dried at 80°C for 48 h and weighed as dry weight (D). The wet to dry ratio (W/D) ratio was calculated to evaluate the lung edema.
Lung HE staining
For pathological examination, as previously reported [28], the left lower lobe was collected, fixed in 4% paraformaldehyde for 24 h, dehydrated in a series of ethanol, transparatized in xylene and embedded in paraffin. Then, lung tissues were cut into 5 μm sections and were subjected to HE staining, followed by observation under a light microscope.
Detection of cytokine contents and MPO, SOD and MDA
After mechanical ventilation, the right upper lobe was collected for the detection of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β and IL-10. In brief, lung tissues were homogenized in normal saline at a ratio of 1:9. After centrifugation, the supernatant was collected. The absorbance was measured at 562 nm after quantification of protein concentration with the BCA method. Detection of MPO and SOD activity, MDA content was performed according to manufacturer’s instructions using the TBA method [29,30].
Western blotting
Western blotting was performed to detect the expression of target proteins according to previous reports [29]. Briefly, lung tissues were homogenized and lyzed in pre-cold buffer. After incubation at 95°C for 5 min, the supernatant was harvested, followed by quantification of protein concentration. Then, 30 μg of protein was collected from each sample and loaded for SDS-PAGE. The proteins were subsequently electronically transferred onto the PVDF membrane. The membrane was blocked in 5% non-fat milk in 0.1% TBST at room temperature for 2 h. After incubation with the primary antibody at 4°C over night, the membrane was rinsed in TBST thrice (5 min for each). After incubation with the secondary antibody (1:2000) at room temperature for 1 h, the membrane was washed thrice in TBST (5 min for each). Visualization was performed with the chemiluminescence kit, and protein bands were photographed.
Real time PCR
The primers used for PCR were as follows: transforming growth factor-β (TGF-β): 5’ CGAGAGGCAGAGATTTATCAG 3’ (forward), 5’ ATGTGAAGATGGGCAAGAC 3’ (reverse); collagen-1: 5’ GCCAAGAAGACATCCCTGAAG 3’ (forward), 5’ TCATTGCATTGCACGTCATC 3’ (reverse); GAPDH: 5’ GTCTTCACCACCATGGAG 3’ (forward), 5’ CCACCCTGTTGCTGTAGC 3’ (reverse). Total RNA was extracted from the lung tissues and then treated with DNase to remove residual DNA. Then, RNA was used for reverse transcription into cDNA. The mixture used for PCR consisted of cDNA template (1 µl), DEPC treated water, 2×SYBR Green Real time PCR Master Mix, Plus solution, 3’-Primer (40 μmol/L) and 5’-Primer (40 μmol/L). After votexing, the experiment was performed at 95°C for 2 min, a total of 40 cycles at 95°C for 15 s, 59°C for 15 s and 72°C for 45 s. The mRNA expression of TGF-β, collagen-1 and GAPDH was detected by analysis of Ct value [31].
Statistical analysis
Statistical analysis was performed with SPSS version 16.0. Data is expressed as mean ± standard deviation. A value of P<0.05was considered statistically significant. Comparisons among groups were done with one way analysis of variance.
Results
Celastrol improves lung pathology and lung edema after VILI
After mechanical ventilation, the lung was collected for pathological examination with HE staining. As shown in Figure 1A, normal alveoli and lung structure were observed in the Con group and the Con+Ce group. However, in the Ven group, there was significant swelling of the alveolar wall and aggregation of neutrophils. In the presence of celastrol pre-treatment, the swelling of the alveolar wall and aggregation of neutrophils were significantly attenuated in the Ven+Ce group. Meanwhile W/D ratio was calculated for the evaluation of lung edema in Figure 1B. In the Ven group, the W/D ratio was significantly higher than in the Con group. However, in the presence of celastrol pretreatment, the lung edema was significantly attenuated, which was characterized by reduction in W/D ratio (all P<0.01).
Figure 1.
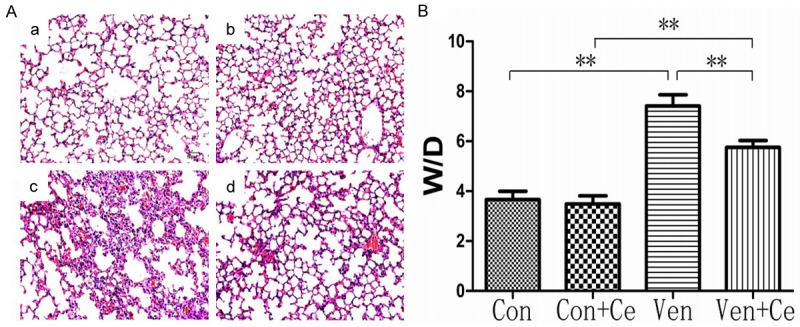
HE staining of the lung in different groups and W/D ratio in different groups. (Celastrol improved the lung pathology and lung edema following VILI). A: In Con group (a) and Con+Ce group (b), the lung structure was normal and only a few inflammatory cells were observed. In Ven group (c), a large number of neutrophil sequestration and infiltration around the pulmonary vessel and airway, distributed in the alveolar and interstitial after ventilation. The Ven+Ce group showed significantly reduced inflammatory cell infiltrations (d). Magnification *200 (a, b, c, d). B: W/D ratio in different groups (Celastrol improve W/D following VILI). In Ven group, the W/D ratio was significantly higher than in Con group (P<0.01). However, in the presence of celastrol pretreatment, the lung edema was significantly attenuated, which was characterized by reduction in W/D ratio (P=0.009<0.01) (**P<0.01).
Celastrol inhibits oxidative stress
Celastrol could significantly reduce MPO activity (Figure 2A) and MDA content (Figure 2B) and increase SOD (Figure 2C) after ventilation. In the Ven group, the MPO activity and MDA content increased markedly as compared to the control group (all P<0.01), but the celastrol pretreatment significantly inhibited MPO activity (P<0.01) and reduced MDA content (P<0.01). In the Ven group, SOD reduced markedly as compared to the Con group (P<0.01), but it significantly increased in the presence of celastrol pretreatment (P<0.05). All data suggest that celastrol could inhibit oxidative stress in mice’s lung.
Figure 2.
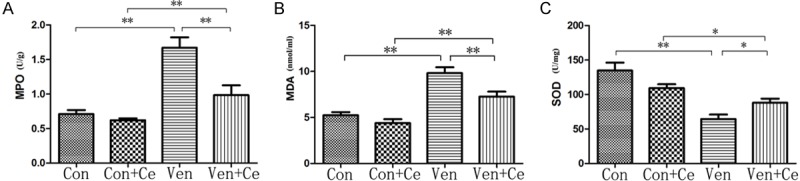
MPO, MDA and SOD in different groups. A: In Ven group, the MPO activity increased markedly as compared to control group (P<0.01), but the celastrol pretreatment significantly inhibited MPO activity (P=0.003<0.01). B: In Ven group, the MDA content increased markedly as compared to control group (P<0.01), but the celastrol pretreatment significantly inhibited increased MDA content (P=0.007<0.01). C: In Ven group, the SOD activity reduced markedly as compared to Con group, but it significantly increased in the presence of celastrol pretreatment (P=0.035<0.05) (*P<0.05 and **P<0.01).
Celastrol inhibits lung inflammation
As shown in Figure 3, mechanical ventilation significantly increased the release of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6) and reduced anti-inflammatory cytokine IL-10 when compared with the Con group (all P<0.01). In the presence of celastrol pre-treatment, the pro-inflammatory cytokines reduced significantly, but IL-10 increased markedly (all P<0.01). The results indicated that celastrol could inhibit the inflammation of the lung.
Figure 3.
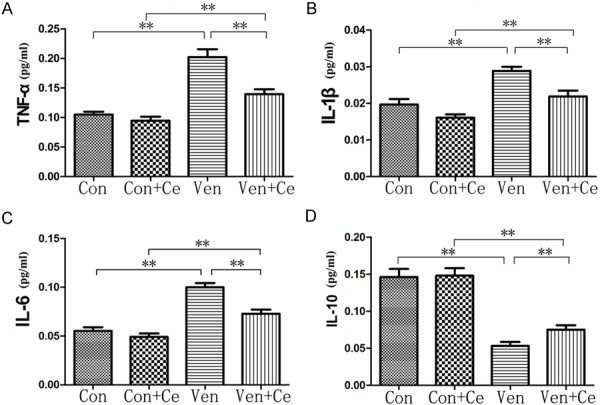
The concentration change of TNF-α (A), IL-1β (B), IL-6 (C) and IL-10 (D). There is no change between group Con and Con+Ce. Mechanical ventilation significantly increased the release of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6) and anti-inflammatory cytokine IL-10 was markedly reduced when compared with Con group (all P<0.01). In the presence of celastrol pre-treatment, the pro-inflammatory cytokines reduced significantly, but IL-10 increased markedly (all P<0.01) (**P<0.01).
Celastrol reduces collagen-1 and TGF-β mRNA expression
VILI mechanism does not only have oxidative and anti-oxidative, inflammatory responses, but also lung fibrosis. The results of real time PCR showed that the mRNA of collagen-1 and TGF-β increased significantly in the lung after mechanical ventilation, and it reduced markedly in the presence of celastrol pre-treatment (all P<0.01) (Figure 4), suggesting that celastrol could ameliorate lung fibrosis.
Figure 4.
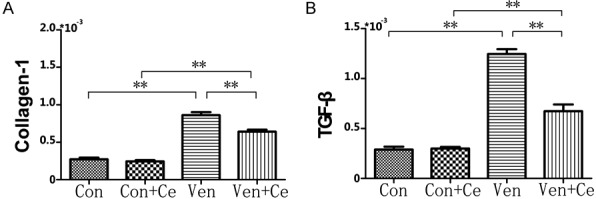
The real time PCR data of collagen-1 (A) and TGF-β (B). The data of real time PCR indicated that the collagen-1 and TGF-β mRNA expression increased significantly in the lung after mechanical ventilation, but it was reduced markedly in the presence of celastrol pre-treatment (all P<0.01) (**P<0.01).
Celastrol reduces the expression of p-P38 and p-JNK 1/2 protein
P-P38 and p-JNK 1/2 protein expression was detected by Western blotting. As shown in Figure 5, the p-P38 and p-JNK 1/2 protein expression increased significantly in the Ven group as compared to the Con group, but the p-P38 and p-JNK 1/2 protein expression reduced markedly in the presence of celastrol pre-treatment. Meanwhile the P38 and JNK 1/2 protein expression did not change in all four groups.
Figure 5.
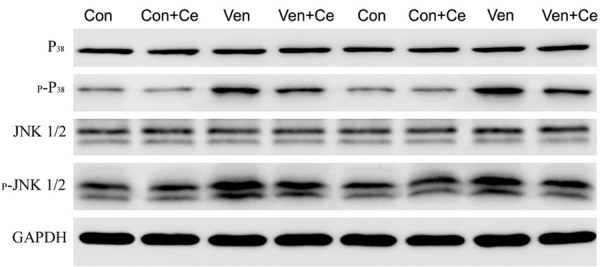
The Western blotting data of p-P38 and p-JNK 1/2 expression. The p-P38 protein and p-JNK 1/2 protein expression increased significantly in Ven group as compared to Con group, but the p-P38 and p-JNK 1/2 protein expression reduced markedly in the presence of celastrol pre-treatment. Meanwhile the P38 and JNK 1/2 protein expression did not change in four groups.
Discussion
VILI is the result of a complex interplay among various mechanical forces acting on lung structures during mechanical ventilation [32]. The onset of VILI has been found to be associated with several possible mechanisms. Although a series of pro-inflammatory and pro-fibrogenetic processes are found to be involved in the pathogenesis of VILI, the cellular and molecular mechanisms underlying lung injury by mechanical stress have not been fully elucidated. In recent years, some studies focus on the role of inflammation and oxidative stress in the pathogenesis of VILI [33,34]. VILI not only aggravates ongoing lung injury, but it may have important systemic consequences via spillover of lung-borne inflammatory mediators into the systemic circulation. However, there are still no effective measures for the prevention of VILI currently.
To date, no study has been undertaken to investigate the protective effects of celastrol on VILI. This study was the first undertaken to investigate the protective effects of celastrol on VILI. Our pathological examination showed the alveolar wall was swelling and a large amount of inflammatory cells infiltrated into the lung after mechanical ventilation, which was suggestive of lung injury. However, 3-day celastrol pretreatment was able to attenuate lung injury which was characterized by alleviation of swelling of the alveolar wall and reduction in infiltrating inflammatory cells, and the detection of W/D ratio also supported the improvement of lung edema, suggesting that celastrol has effects on VILI.
Celastrol can reduce lung injury from the morphology, following from the molecular biology point of view to find its mechanism. Celastrol was also shown to be a potent inhibitor of lipid peroxidation [35,36]. The 50% inhibitory concentration in rat liver mitochondria was about 15 times lower than that of the commonly used anti-peroxidative agent tocopherol. Sassa et al also investigated the structural basis of antiperoxidative activity of the celastrol, and found it had direct radical scavenging activity [37]. The anti-cancer effects of celastrol have been demonstrated to be associated with its anti-oxidative activity [38,39]. In addition, a variety of studies demonstrate the potent anti-inflammatory capability of celastrol in different animal models in vivo and in vitro [40-42]. In this study, the anti-oxidative and anti-inflammatory effects of celastrol pretreatment were also investigated in the VILI mouse model. Our results showed the MPO activity and MDA contents, TNF-α, IL-1β and IL-6 of the lung increased significantly, but SOD and IL-10 reduced markedly in mice after 4-h mechanical ventilation, which confirms the oxidative stress and inflammation following VILI. However, in the presence of celastrol pre-treatment, the MPO activity and MDA contents, TNF-α, IL-1β and IL-6 reduced dramatically, and SOD and IL-10 increased significantly. This indicates that the anti-oxidative and anti-inflammatory capabilities are improved in the case of celastrol pre-treatment.
Mechanical ventilation not only causes inflammation, but also initiates lung fibrosis. Villar et al [43] found that mechanical ventilation for 4 h could significantly activate the fibrosis related pathways (increased collagen-1 and TGF-β expression), leading to the deterioration of lung dysfunction. It has been confirmed that TGF-β is involved in the pathogenesis of lung fibrosis, and inhibition of TGF-β production is able to improve lung fibrosis [31]. Did celastrol also attenuate lung fibrosis? A recent study reveals that celastrol is able to improve myocardial fibrosis [44]. Our data proved that celastrol markedly reduced collagen-1, and TGF-β mRNA expression increases after mechanical ventilation.
Furthermore, which signal pathway takes part in the mechanism? Uhlig et al [45] found that the p-P38 protein expression follows mechanical ventilation. Jung et al [46] also found that celastrol was able to exert anti-oxidative and anti-inflammatory effects via inhibition of the phosphorylation of MAPK in BV-2 cells. Chen et al [47] found that MAPK pathway could interact with the TGF-β pathway, and by reducing the TGF-β expression, p-P38 and p-JNK expression was also reduced. Fortunately in this study, the results showed that VILI up-regulates p-P38 and p-JNK 1/2 expression in the lung, which however is significantly reduced in the presence of celastrol pre-treatment.
Taken together, this study indicates that celastrol is able to inhibit oxidative stress, lung inflammation and fibrosis to attenuate lung edema and lung injury secondary to mechanical ventilation, which may be, at least partially related to the inhibition of MAPK p-P38 and p-JNK 1/2 pathway. Our findings may provide evidence for the clinical treatment of VILI with celastrol.
Acknowledgements
We thank the Changlin Ye in the Shanghai Institute of Physical Education for his technical support. This study was funded by the Xinhua Hospital Foundation School of Medicine of Shanghai Jiaotong University (grant number 13YJ12) and the National Natural Science Foundation (grant number 81372100).
Disclosure of conflict of interest
None.
References
- 1.Adams AB, Simonson DA, Dries DJ. Ventilatorinduced lung injury. Respir Care Clin N Am. 2003;9:343–362. doi: 10.1016/s1078-5337(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 2.Halbertsma FJ, Vaneker M, Scheffer GJ. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med. 2005;63:382–392. [PubMed] [Google Scholar]
- 3.Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, Slutsky AS. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology. 2014;121:189–198. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slutsky AS. History of mechanical ventilation. From vesalius to ventilator-induced lung injury. Am J Respir Crit Care Med. 2015;191:1106–1115. doi: 10.1164/rccm.201503-0421PP. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116(Suppl):9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss D, Ricard JD, Saumon G. On the physiologic and clinical relevance of lung-borne cytokines during ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1467–1471. doi: 10.1164/rccm.200206-611CP. [DOI] [PubMed] [Google Scholar]
- 8.Marini JJ, Hotchkiss JR, Broccard AF. Bench-tobedside review: microvascular and airspace linkage in ventilator-induced lung injury. Crit Care. 2003;7:435–444. doi: 10.1186/cc2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 11.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 12.Chiumello D, Carlesso E, Brioni M, Cressoni M. Airway driving pressure and lung stress in ARDS patients. Crit Care. 2016;20:276. doi: 10.1186/s13054-016-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan C, Qin YZ, Zhang NX, Zhang NX, Wang SP. Clinical study of mechanical ventilation in acute cardiogenic pulmonary edema patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:350–354. [PubMed] [Google Scholar]
- 14.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 15.Peng B, Gu YJ, Wang Y, Cao FF, Zhang X, Zhang DH, Hou J. Mutations Y493G and K546D in human HSP90 disrupt binding of celastrol and reduce interaction with Cdc37. FEBS Open Bio. 2016;6:729–734. doi: 10.1002/2211-5463.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog Dis. 2016;74 doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao L, Zhou Z, Cai Y, Dakhov O, Shi P, Bai Y, Ji H, Shen W, Wang J. Celastrol suppresses tumor cell growth through targeting an AR-ERGNF-kappaB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One. 2013;8:e58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y, Meng G, Xie L, Wang J, Xiao Y, Shan L, Zhou S, Wei L, Ferro A, Ji Y. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One. 2013;8:e65477. doi: 10.1371/journal.pone.0065477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Liu D, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y. Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kappaB-mediated matrix metalloproteinase-9 expression. PLoS One. 2013;8:e68905. doi: 10.1371/journal.pone.0068905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DY, Park JW, Jeoung D, Ro JY. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612:98–105. doi: 10.1016/j.ejphar.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–484. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine”, is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Straub A, Tian Z, Bassler N, Cheng J, Peter K. Celastrol, a triterpene extracted from Tripterygium wilfordii Hook F, inhibits platelet activation. J Cardiovasc Pharmacol. 2009;54:240–245. doi: 10.1097/FJC.0b013e3181b21472. [DOI] [PubMed] [Google Scholar]
- 25.Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNFinduced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, Liu J, Finkel T, Mueller E. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1alpha transcriptional axis. Cell Metab. 2015;22:695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Zhou Y, Fan Y, Zhou D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008;264:101–106. doi: 10.1016/j.canlet.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Dong WW, Liu YJ, Lv Z, Mao YF, Wang YW, Zhu XY, Jiang L. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Radic Biol Med. 2015;88:404–416. doi: 10.1016/j.freeradbiomed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YQ, Liu YJ, Mao YF, Dong WW, Zhu XY, Jiang L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clin Nutr. 2015;34:752–760. doi: 10.1016/j.clnu.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HX, Duan GL, Wang CN, Zhang YQ, Zhu XY, Liu YJ. Protective effect of resveratrol against endotoxemia-induced lung injury involves the reduction of oxidative/nitrative stress. Pulm Pharmacol Ther. 2014;27:150–155. doi: 10.1016/j.pupt.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Luo F, Zhuang Y, Sides MD, Sanchez CG, Shan B, White ES, Lasky JA. Arsenic trioxide inhibits transforming growth factor-beta1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res. 2014;15:51. doi: 10.1186/1465-9921-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Caro L, Lorente JA, Marin-Corral J, Sánchez-Rodríguez C, Sánchez-Ferrer A, Nin N, Ferruelo A, de Paula M, Fernández-Segoviano P, Barreiro E, Esteban A. Role of free radicals in vascular dysfunction induced by high tidal volume ventilation. Intensive Care Med. 2009;35:1110–1119. doi: 10.1007/s00134-009-1469-5. [DOI] [PubMed] [Google Scholar]
- 34.Maretta M, Toth S, Jonecova Z, Kruzliak P, Kubatka P, Pingorova S, Vesela J. Immunohistochemical expression of MPO, CD163 and VEGF in inflammatory cells in acute respiratory distress syndrome: a case report. Int J Clin Exp Pathol. 2014;7:4539–4544. [PMC free article] [PubMed] [Google Scholar]
- 35.Sassa H, Takaishi Y, Terada H. The triterpene celastrol as a very potent inhibitor of lipid peroxidation in mitochondria. Biochem Biophys Res Commun. 1990;172:890–897. doi: 10.1016/0006-291x(90)90759-g. [DOI] [PubMed] [Google Scholar]
- 36.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 37.Sassa H, Kogure K, Takaishi Y, Terada H. Structural basis of potent antiperoxidation activity of the triterpene celastrol in mitochondria: effect of negative membrane surface charge on lipid peroxidation. Free Radic Biol Med. 1994;17:201–207. doi: 10.1016/0891-5849(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Zhang X, Zhao M, Wang Y, Cheng X, Wang D, Xu Y, Du Z, Yu X. Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer. 2011;11:170. doi: 10.1186/1471-2407-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrivastava S, Jeengar MK, Reddy VS, Reddy GB, Naidu VG. Anticancer effect of celastrol on human triple negative breast cancer: possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol Pathol. 2015;98:313–327. doi: 10.1016/j.yexmp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of Thunder God Vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 42.Zanphorlin LM, Alves FR, Ramos CH. The effect of celastrol, a triterpene with antitumorigenic activity, on conformational and functional aspects of the human 90 kDa heat shock protein Hsp90alpha, a chaperone implicated in the stabilization of the tumor phenotype. Biochim Biophys Acta. 2014;1840:3145–3152. doi: 10.1016/j.bbagen.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodríguez N, Kacmarek RM, Slutsky AS. Activation of the Wnt/beta-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One. 2011;6:e23914. doi: 10.1371/journal.pone.0023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang L, Zhang C. Celastrol-induced suppression of the MiR-21/ERK signalling pathway attenuates cardiac fibrosis and dysfunction. Cell Physiol Biochem. 2016;38:1928–1938. doi: 10.1159/000445554. [DOI] [PubMed] [Google Scholar]
- 45.Uhlig U, Haitsma JJ, Goldmann T, Poelma DL, Lachmann B, Uhlig S. Ventilation-induced activation of the mitogen-activated protein kinase pathway. Eur Respir J. 2002;20:946–956. doi: 10.1183/09031936.02.01612001. [DOI] [PubMed] [Google Scholar]
- 46.Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- 47.Chen KH, Hsu HH, Yang HY, Tian YC, Ko YC, Yang CW, Hung CC. Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway. Int J Biochem Cell Biol. 2016;74:135–144. doi: 10.1016/j.biocel.2016.03.001. [DOI] [PubMed] [Google Scholar]


