Abstract
Dickkopf-1 (DKK1) has been reported as a key regulator in the progression of esophageal carcinoma (EC). Aldehyde dehydrogenase 1A1 (ALDH1A1) possesses stem-like properties and predicts patient outcome in several cancers. However, whether DKK1 regulates cancer stem-like properties of EC cells through modulating ALDH1A1 activity remains unclear. In this study, we found that DKK1 knockdown significantly reduced cell proliferation, colony formation and CK18 expression. Additionally, knockdown of DKK1 also decreased the expression of ALDH1A1 involved in a c-Jun-dependent manner through a pathway consisting of p38/JNK/c-Jun pathway. Furthermore, the downregulation of ALDH1A1 gene expression in Eca109 resulted in decreased expression of cancer stem cell-associated markers SOX2, Bmi1 and vimentin. Therefore, our results demonstrated that DKK1 maintained cancer stem-like properties of EC cells via ALDH1A1/SOX2 axis. DKK1 may act as a therapeutic target for the treatment of EC.
Keywords: DKK1, esophageal carcinoma, c-Jun, ALDH1A1, SOX2
Introduction
Esophageal carcinoma (EC) is one of the most lethal malignancies of the digestive tract, and at the time of diagnosis, most of the patients are at advanced stages [1]. In spite of the use of modern surgical techniques combined with various treatment modalities, such as radiotherapy and chemotherapy, the overall 5-year survival rate of EC still remains at 40% to 60% [2]. Tumor proliferation is the main characteristic features in the patients of EC [3-5], so a novel molecular target for therapeutic approaches preventing tumor proliferation is highlighted for EC patients.
DKK1 encodes a secreted glycoprotein, which is known as a negative regulator of the Wnt signaling pathway in tumor cells [6-8]. Importantly, someone have shown DKK1 was a novel serologic and prognostic biomarker for esophageal carcinomas [9]. In spite of these studies, whether DKK1 regulating cancer stem-like properties of esophageal cancer cells and its mechanism needs to be further studied.
Aldehyde dehydrogenase 1A1 (ALDH1A1) is a cytosolic enzyme, which is the main subtypes of Aldol dehydrogenase (ALDH), can catalyze intracellular oxidation of acetaldehyde to acetic acid. ALDH1A1 is a cancer stem-like cell-associated protein in various malignant tumors and its level correlates with the patient outcome [10-12]. Therefore, we speculate that whether there is a possibility that DKK1 maintained cancer stem-like properties of esophageal carcinoma cells via modulating ALDH1A1 expression.
In this study, we found that knockdown of DKK1 inhibited the proliferation, colony formation and CK18 expression in Eca109 cells. Moreover, we discovered that DKK1 promoted the expression of ALDH1A1, SOX2, Bmi1, and vimentin through p38 and JNK signal pathway.
Materials and methods
Cell culture
Esophageal carcinoma cell line Eca109 was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. The cells were cultured in RPMI-1640 (GIBCO, USA) medium containing 10% fetal bovine serum (GIBCO, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin maintained at 37°C in humidified air containing 5% CO2.
DKK1 RNA interference
Firstly, the experiment was divided into two groups: (1) Scramble group: transfected with negative control scramble; (2) siDKK1 group: transfected with siRNA-DKK1. DKK1 siRNA sequence: AUAGCGUUGGAAUUGAGAACCGAGU (Shanghai GenePharma, China). Esophageal carcinoma cell line Eca109 were seeded at a density of 2 × 105 per well in a 6-well culture plate containing complete medium 24 h prior to transfection. When transfected with siDKK1 cells, 10 μl of siDKK1, 5 μl of Lipofectamine 2000 (Invitrogen, USA), 500 μl of Opti-MEMI and 1500 μl of DMEM/F-12 were added to each well. Negative control cells were transfected with siDKK1 at the same dose as the negative control scramble and Lipofectamine 2000. The efficacy of DKK1 knockdown was assessed by Western blot using an anti-human DKK1 rabbit polyclonal antibody (sc-25516, Santa Cruz, USA).
Western blotting
Cells were lysed in RIPA lysis buffer at 4°C for 30 min and centrifuged at 12,000 rpm for 15 min. The protein concentrations were quantified using the BCA protein assay (Pierce, Rockford). Equal quantities of protein were loaded and electrophoresed in a 10% SDS-PAGE gel, which was then transferred to PVDF membrane. The membrane was incubated for 60 min in 5% skimmed milk to block. This was followed by an incubation at 4°C with anti-human DKK1 antibody (sc-25516, Santa Cruz), p38 antibody (8203S, CST), p-P38 antibody (9913S, CST), JNK antibody (9252S, CST), p-JNK antibody (9251S, CST), c-Jun antibody (9165S, CST), p-c-Jun antibody (9164S, CST), ALDH1A1 antibody (12035S, CST), Sox2 antibody (9092S, CST), vimentin antibody (5741S, CST), β-actin antibody (12262S, CST). The membranes were washed three times for 10 min 0.1% Tween-20 and then incubated for 1 h with HRP-conjugated secondary antibody (Boster Biological Technology, China) at room temperature. The membranes were then detected using ECL substrate following manufacturer’s recommendation. β-actin was used as an endogenous protein for normalization.
RT-PCR
Total RNA was extracted from cell lines by using of Trizol reagent (Invitrogen, USA) according to the manufacture’s instruction. Real-time PCR was performed using an instrument of ABI 7000 PCR (Applied Biosystems, USA). The ALDH1A1 primers sequence: Forward 5’-GCACGCCAGACTTACCTGTC-3’, Reverse 5’-CCTCCTCAGTTGCAGGATTAAAG-3’; The Sox2 primers sequence: Forward 5’-CTCGTGCAGTTCTACTCGTCG-3’, Reverse 5’-AGCTCTCGGTCAGGTCCTTT-3’; The Bmi1 primers sequence: Forward 5’-GCTGCCAATGGCTCTAATGAA-3’, Reverse 5’-TGCTGGGCATCGTAAGTATCTT-3’; the β-actin primers sequence: Forward 5’-GGGAAATCGTGCGTGACA-3’, Reverse 5’-TCAGGAGGAGCAATGATC-3’. The relative amount of mRNA was calculated using 2-ΔCt method. Gene expression was normalized by β-actin. All data were obtained from three individual experiments.
Cell proliferation assay
Cell proliferation was performed with Cell Counting Kit-8 (CCK-8) (Dojindo, Tokyo, Japan). According to the instructions. Cell Counting Kit-8 reagent was added at 0, 24, 48, and 72 h respectively after seeding 4 × 103 cells per well in into 96-well plates and transfected with mimics or control, and incubated at 37°C for 2 h. The OD (optical density) 450 nm value was detected by using a microplate reader (Bio-Rad, USA).
Colony formation assay
Cells were seeded in 6-well culture plates containing 2 × 105 cells/well and transfected with siDKK1 and negative control scramble. After 24 hours of incubation, cells were washed twice with PBS and stained with Giemsa solution (Biyuntian, China). The number of colonies was counted under a microscope. The colony formation efficiency was calculated as: efficiency = (number of colonies/number of cells inoculated) × 100%. Each experiment was performed in triplicates.
Immunocytochemical analysis
The expression of stem cell marker CK18 in cells was examined by immunofluorescence. Cells on slides were fixed in 4% paraformaldehyde and washed three times with PBS, then incubated with PBS containing 0.1% Triton X-100 and 1% normal serum for 30 min at room temperature. The primary and secondary antibodies were diluted as follows: rabbit anti-CK18 (1:500), FITC-conjugated goat anti-rabbit IgG (1:400). Nuclear DNA was dyed with DAPI. For all immunochemistry experiments, negative staining controls were carried out by omitting the primary antibody.
Nude mice model
For subcutaneous xenografts, well-cultured Eca109 cells (1 × 104) in 100 μl PBS were injected subcutaneously into the left flank of 5-week-old male nude mice (CLEA Japan). All animal experiments were performed under the approval of the Animal Research Committee of Nanjing University.
Statistical analysis
Data are presented as means ± SD of three independent experiments. Differences between groups were analysed by GraphPad Prism 5 software (GraphPad Software, CA, USA) with Student’s t-test. Differences were considered statistically significant at P < 0.05.
Results
Knockdown of DKK1 inhibited proliferation, colony formation, and CK18 expression in EC cells
The Eca109 cells were transfected with siRNA-DKK1 and negative control. The levels of DKK1 protein was significantly decreased in DKK1 siRNA treated cells compared with scramble-treated cells (Figure 1A). In addition, the proliferation and colony formation of Eca109 cells were obviously inhibited following the treatment with DKK1 siRNA (Figure 1B and 1C). For further characterization, the immunocytochemical staining against CK18, which is known as the cancer stem-like biomarkers, was applied on Eca109 cells. Knockdown of DKK1 significantly decreased CK18 expression compared with scramble group (Figure 1D). Taken together, our findings verified that Knockdown of DKK1 inhibited proliferation, colony formation, and CK18 expression in EC cells.
Figure 1.
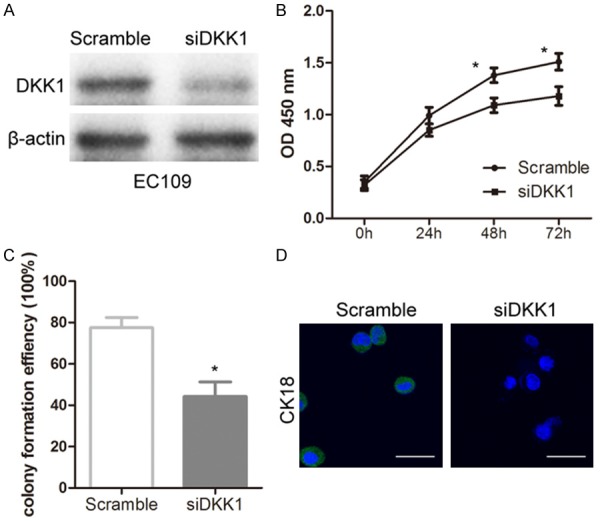
Knockdown of DKK1 inhibited the proliferation, colony formation, and CK18 expression in Eca109 EC cells. A: Western blotting was used to analyze DKK1 expression after transfection with DKK1-siRNA and control for 24 hours in Eca109 cells. B: CCK-8 assay was used to evaluated proliferation changes after the treatment of DKK1-siRNA and control for 24, 48, and 72 hours in Eca109 cells. C: Colony formation efficiency was obviously decreased after knockdown of DKK1 for 24 hours. D: Immunohistochemistry was performed on Eca109 cells stained with antibody directed against CK18 (green) and DAPI (blue). Statistically significant differences are indicated: *P < 0.05; Student’s t test. The experiment was repeated at least three times.
Ablation of DKK1 decreased ALDH1A1 expression through inactivation of P38/JNK/c-Jun signal pathway
RT-PCR and Western-Blot analysis showed that the levels of ALDH1A1 mRNA and protein were remarkably decreased in DKK1 siRNA treated cells compared with scramble-treated cells (Figure 2A-C). Due to ALDH1A1 expression modulated by AP-1 complexes on the ALDH1A1 promoter [13], we detected whether DKK1 siRNA influenced the activation of c-Jun through MAPK pathway. We found that p-p38 and p-JNK levels were downregulated by the treatment with DKK1 siRNA. In addition, the level of p-c-Jun was also decreased due to the inactivation of p38 and JNK (Figure 2B and 2D), thus resulted in inhibiting the expression of ALDH1A1.
Figure 2.
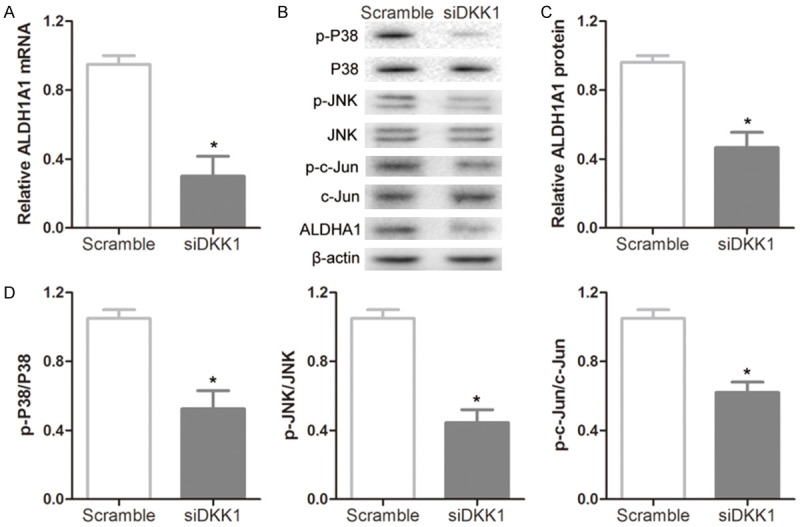
Knockdown of DKK1 resulted in decreased ALDH1A1 expression by inactivating p38/JNK/c-Jun signal pathway. A: Knockdown of DKK1 resulted in downregulation of ALDH1A1 mRNA levels. B-D: Representative immunoblots and densitometry analysis of ALDH1A1, phosphorylated p38 (p-p38), p-JNK and p-c-Jun. All samples were collected from Eca109 cells after 24 hours of transfection with DKK1-siRNA and control. Statistically significant differences are indicated: *P < 0.05; Student’s t test. The experiment was repeated at least three times.
Knockdown of DKK1 inhibited cancer stem cell-associated genes expression of EC cells
To further determine whether knockdown of DKK1 might affect cancer stem-like properties in EC cells, the expression levels of some stem cell-associated genes were evaluated in DKK1 siRNA treated cells. Real-time PCR analysis indicated that the expression levels of Sox2, Bmi1 and vimentin mRNA in DKK1 siRNA treated cells were significantly lower than scramble-treated cells (Figure 3A). Western blotting and densitometry analysis also revealed that the expression levels of Sox2 and vimentin were down-regulated in DKK1 siRNA treated cells than scramble-treated cells (Figure 3B). Together, these data indicated that DKK1 acted as a promoter of cancer stem-like properties via modulating ALDH1A1 expression involved in a c-Jun-dependent manner through a pathway consisting of p38 and JNK (Figure 3C).
Figure 3.
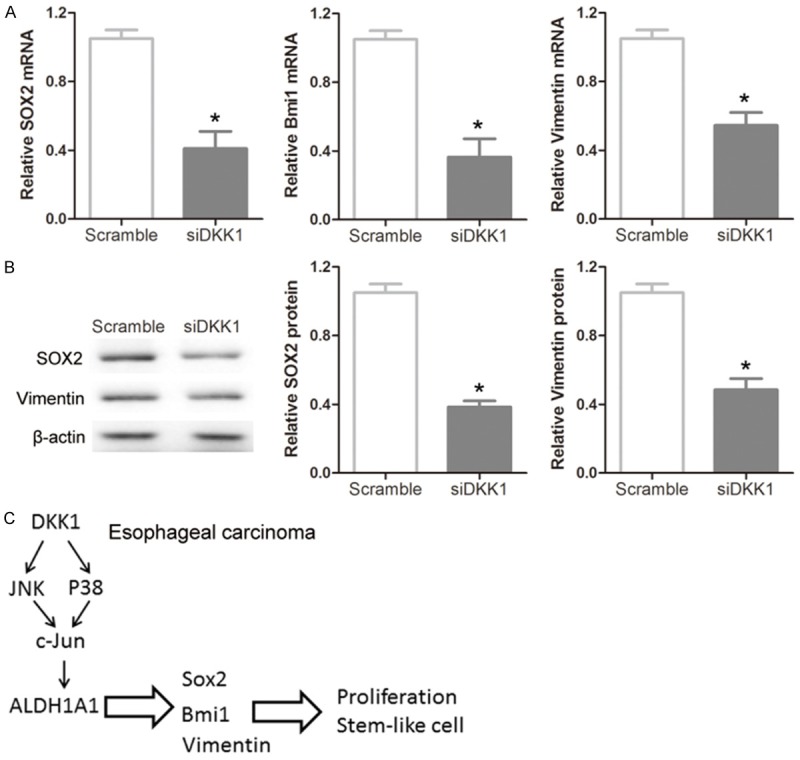
Involvement of the ALDH1A1 downstream pathway. A: mRNA levels of Sox2, Bmi1 and vimentin were downregulated in Eca109 cells when measured by RT-PCR after knockdown of DKK1 for 24 hours. B: The levels of Sox2 and vimentin proteins were also downregulated by representative immunoblots and densitometry analysis in Eca109 EC cells. C: The signal pathway suggesting that ALDH1A1 is activated by p38 and JNK-mediated modulation of c-Jun. Statistically significant differences are indicated: *P < 0.05; Student’s t test. The experiment was repeated at least three times.
Pivotal effect of DKK1 on tumor-initiating capacity of EC cells
Having demonstrated the role of the DKK1 in the control of the cancer stem-like properties of EC cells, we next wished to ask whether DKK1 also plays a role in the control of tumor-initiating capacity of EC cells as well as of their self-renew. As the results showed that knockdown of DKK1 with siRNA compared with scramble significantly extended survival of mice in the subcutaneous xenograft model (Figure 4). Thus, the results suggest that DKK1-dependent activation of ALDH1A1 is essential for the gain of tumor-initiating capacity in EC cells.
Figure 4.
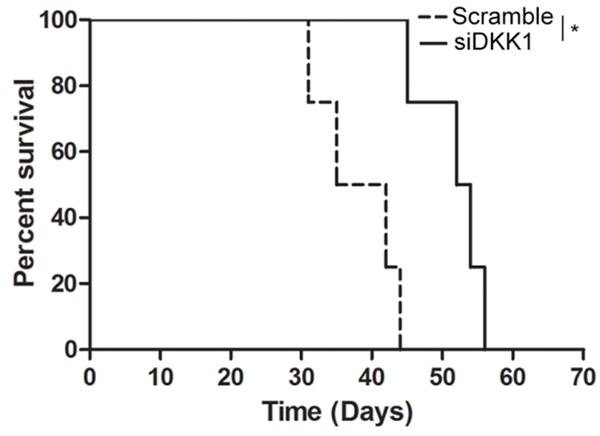
Pivotal effect of DKK1 on tumor-initiating capacity of EC cells. Eca109 cells were tranfected with scramble or DKK1 siRNA as indicated in the figure for 48 h, and the cells (1 × 104) were then implanted subcutaneously into nude mice. Kaplan-Meier survival curves of the mice (5 mice per group) are shown. *P < 0.05.
Discussion
Previous studies have shown that DKK1 overexpressed in many malignant cancers including breast cancer [14], hepatocellular carcinoma [15], lung cancer and esophageal carcinoma [9,16], indicating a potential oncogenic function of DKK1. Herein, we explored the effects and mechanism of DKK1 on the cancer stem-like properties of EC cell line Eca109. Our results showed that knockdown of DKK1 inhibited the proliferation, colony formation, and CK18 expression in Eca109 cells. However, extra work is required to explore the role of DKK1 in EC stem-like properties.
ALDH1A1 defines cancer stem-like cells and predicts poor prognosis in patients with prostate cancer, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, ovarian cancer, liver cancer, breast cancer [17-22]. DKK1 prevent osteolytic bone proliferation by activation of JNK and transcriptional activation of ALDH1 through c-Jun-responsive promoter elements [23]. Moreover, ALDH1A1high cancer stem-like cells contribute to the invasion, metastasis and poor outcome of human esophageal carcinoma [18]. ALDH1A1 might be used to predicting poor prognosis in patients with EC. Besides, DKK1 regulated ALDH1A1 expression involved in detoxification of chemotherapeutic agents. Our observations are similar in that we confirm that ablation of DKK1 resulted in the decrease of p-p38, p-JNK and p-c-Jun phosphorylation, which in turn caused transcriptional inactivation of ALDH1A1.
Sox2 is a transcription factor with a high-mobility group DNA-binding domain that functions as a master regulator during embryogenesis and organogenesis [24]. Additionally, Sox2 is an amplified lineage-survival oncogene in esophageal squamous cell carcinomas [25]. The oncoprotein and stem cell renewal factor Bmi1 associates with poor clinical outcome in oesophageal cancer [26]. Furthermore, we found that knockdown of DKK1 inhibited the expression of stem cell-associated genes Sox2, Bmi1 and vimentin. Moreover, DKK1-dependent activation of ALDH1A1 is essential for the gain of tumor-initiating capacity in EC cells. These findings suggested that DKK1 maintained cancer stem-like properties of EC cells via ALDH1A1/SOX2 axis. DKK1 may act as a therapeutic target for the treatment of EC.
Acknowledgements
This work was supported by grants from the Project of Medical Science and Technology Development Program of Nanjing (YKK14182), and Project of Science and Technology Development Program of Nanjing Medical University (2014NJMU167).
Disclosure of conflict of interest
None.
References
- 1.Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Gunji Y, Kobayashi S, Hayashi H, Ochiai T. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 2003;5:486–494. doi: 10.1067/msy.2003.139. [DOI] [PubMed] [Google Scholar]
- 2.Tamoto E, Tada M, Murakawa K, Takada M, Shindo G, Teramoto K, Matsunaga A, Komuro K, Kanai M, Kawakami A, Fujiwara Y, Kobayashi N, Shirata K, Nishimura N, Okushiba S, Kondo S, Hamada J, Yoshiki T, Moriuchi T, Katoh H. Gene-expression profile changes correlated with tumor progression and lymph node metastasis in esophageal cancer. Clin Cancer Res. 2004;11:3629–3638. doi: 10.1158/1078-0432.CCR-04-0048. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Ding F, Cao W, Liu Z, Liu W, Yu Z, Wu Y, Li W, Li Y. Stomatin-like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;5:1639–1646. doi: 10.1158/1078-0432.CCR-05-1858. [DOI] [PubMed] [Google Scholar]
- 4.Lee KH, Goan YG, Hsiao M, Lee CH, Jian SH, Lin JT, Chen YL, Lu PJ. MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res. 2009;15:2529–2538. doi: 10.1016/j.yexcr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, Zeng M, Li J, Song L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;5:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 6.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;52:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 7.Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;1:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 8.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;13:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, Fujita M, Hosokawa M, Tsuchiya E, Kohno N, Kondo S, Nakamura Y, Daigo Y. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;6:2517–2525. doi: 10.1158/0008-5472.CAN-06-3369. [DOI] [PubMed] [Google Scholar]
- 10.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;5:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CP, Tsai MF, Chang TH, Tang WC, Chen SY, Lai HH, Lin TY, Yang JC, Yang PC, Shih JY, Lin SB. ALDH-positive lung cancer stem cells confer resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Lett. 2013;1:144–151. doi: 10.1016/j.canlet.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Fleischman AG. ALDH marks leukemia stem cell. Blood. 2012;15:3376–3377. doi: 10.1182/blood-2012-02-406751. [DOI] [PubMed] [Google Scholar]
- 13.Makia NL, Amunom I, Falkner KC, Conklin DJ, Surapureddi S, Goldstein JA, Prough RA. Activator protein-1 regulation of murine aldehyde dehydrogenase 1a1. Mol Pharmacol. 2012;4:601–613. doi: 10.1124/mol.112.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;4:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST, Ho C, Li R, Fan ST, Cohen SN, Chen X, So S. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005;23:3737–3747. doi: 10.1038/sj.onc.1208479. [DOI] [PubMed] [Google Scholar]
- 16.Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H, Takiguchi S, Fujiwara Y, Matsuura N, Mori M, Doki Y. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;7:2058–2064. doi: 10.1245/s10434-009-0476-7. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, Stass SA, Jiang F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest. 2010;2:234–244. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao HL, Wang WG, Xu SL, Yang J, Cui W, Liu Q, Wang Z, Guo W, Xiong G, Yang K, Qian C, Zhang X, Zhang P, Cui YH, Bian XW. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod Pathol. 2014;5:775–783. doi: 10.1038/modpathol.2013.189. [DOI] [PubMed] [Google Scholar]
- 19.Qian X, Wagner S, Ma C, Coordes A, Gekeler J, Klussmann JP, Hummel M, Kaufmann AM, Albers AE. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2014;7:1151–1158. doi: 10.1007/s00432-014-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng E, Mitra A, Tripathi K, Finan MA, Scalici J, McClellan S, Madeira da Silva L, Reed E, Shevde LA, Palle K, Rocconi RP. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS One. 2014;9:e107142. doi: 10.1371/journal.pone.0107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;1:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 22.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;7:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 23.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;3:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 24.Gen Y, Yasui K, Zen Y, Zen K, Dohi O, Endo M, Tsuji K, Wakabayashi N, Itoh Y, Naito Y, Taniwaki M, Nakanuma Y, Okanoue T, Yoshikawa T. SOX2 identified as a target gene for the amplification at 3q26 that is frequently detected in esophageal squamous cell carcinoma. Cancer Genet Cytogenet. 2010;2:82–93. doi: 10.1016/j.cancergencyto.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Ribeiro U Jr, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;11:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa R, Tsujimura T, Tao L, Kamikonya N, Fujiwara Y. The oncoprotein and stem cell renewal factor BMI1 associates with poor clinical outcome in oesophageal cancer patients undergoing preoperative chemoradiotherapy. BMC Cancer. 2012;12:461. doi: 10.1186/1471-2407-12-461. [DOI] [PMC free article] [PubMed] [Google Scholar]


