Abstract
Several studies have demonstrated extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs) could promote neural regeneration following central nervous system injury. However, the therapeutic effects of MSC-EVs in peripheral nerve regeneration remain poorly understood. This study was aimed to investigate the therapeutic effects of local application of rat bone marrow mesenchymal stem cell (BMSCs) derived EVs in rat sciatic nerve crush injury model. EVs were isolated from the culture supernatants of BMSCs under serum-free conditioned medium, and identified by transmission electron microscopy (TEM) and scanning electron microscope (SEM). Then, the crush-injured segment of rat was treated by BMSC-EVs or PBS, and assessed the nerve regeneration. Results showed that BMSC-EVs size ranged from 40 to 300 nm and had a typical membrane structure under TEM and SEM. BMSC-EVs treatment promote the function recovery by sciatic function Index, improve the histomorphometric repair in nerve regeneration, and increase the expression of GAP-43, a marker for axon regeneration. The present study demonstrated that BMSC-EVs could promote the functional recovery and nerve regeneration of crush-injured sciatic nerves in rat. BMSC-EVs provide a novel cell-free therapeutic approach to peripheral nerve therapy.
Keywords: Bone marrow mesenchymal stem cell, extracellular vesicles, sciatic nerve crush injury
Introduction
Peripheral nerve injury is a serious complication that can result in complete functional loss or permanent impairment. Despite the continuous improvement of techniques in the repair of peripheral nerves, the outcomes after peripheral nerve injury have been disappointing. In order to recover the injured peripheral nerve to the pre-injury level, investigators have done a lot of research such as allografts [1,2]. While functional outcomes from reconstructive transplantation are limited by the immunological consequences of allotransplantation such as rejection [3], which is a major challenge in the process of nerve regeneration. Recently, cell-based therapy, particularly mesenchymal stem cells (MSCs), has emerged as a promising approach for enhancing nerve regeneration [4]. Intravenous injection of MSCs has been shown to modulate the local environment with the down-regulation of inflammation and the promotion of axonal regeneration in the mouse model of sciatic crush [5]. In addition, local injection of MSCs has been shown to increase the number of newborn nerve fibers [6], neurovascular [7] and restore the peripheral nerve function of the injury, with detectable of expression of nerve growth related factors in the supernatant of MSCs [8]. Furthermore, after the transplantation of MSCs, the expression level of nerve growth related factors was significantly increased at the point of sciatic nerve injury [7]. The above study provides good evidence supporting the paracrine effect of stem cell-based therapy. In these contexts, MSCs may deliver critical signals to nerve damage point that contribute to functional recovery and then change the cell behavior or transfer certain cytokines to relieve injury [9-11].
Extracellular vesicles (EVs), as a new mechanism of cell-to-cell communication [12], are nano-sized circular membrane fragments that released by various cell types, including stem cells [13] and progenitors [14] and serve as shuttles for the selective pattern of ligands, receptors, enzymes, cytokines, transcription factors, mRNA and microRNA into target cells [15]. The therapeutic effects of EVs have been actively investigated in animal models of various diseases, such as kidney damage, myocardial injury and liver damage [16]. Recently, Intravenous administration of MSC-EVs has been shown to improve functional recovery and enhance neurite remodeling, neurogenesis, and angiogenesis in an ischemic stroke model [17]. However, the role of MSC-EVs on sciatic nerve crush injury has yet to be examined.
In this study, we constructed the sciatic nerve crush injury model in rat, and assessed the therapy effect of EVs from rat bone marrow MSCs (BMSC). Data show that rat BMSC-EVs can protect rat from severe sciatic nerve injury. MSC-derived EVs has great value in implications for a potentially new therapy for the treatment of peripheral nerve injury.
Materials and methods
Cell cultures
BMSCs were isolated and cultured according to previously described protocol [18]. The cells from 80-100 g Sprague Dawley (SD) rats were collected by flushing the femurs and tibiae with phosphate-buffered saline (PBS), and then the cells were cultured in 25 cm2 flasks (EuroClone) in low glucose Dulbecco’s modified Eagle’s medium (L-DMEM; GIBCO-BRL, Carlsbad, CA, USA), with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 U/ml) at 37°C in 5% CO2 atmosphere. The experimental procedure was approved by the Institutional Animal Care Committee of Jiangsu University. After 4 days, nonadherent cells were removed, and the medium was changed every three days. When 70%-80% adherent cells were confluent, they were trypsinized with 0.25% trypsin-EDTA (Invitrogen), harvested, and expanded in larger flasks. A homogenous cell population was usually obtained at passage 3-5 for the experiments.
BMSCs were characterized by the expression of CD105 (endoglin), CD73, and lack of hematopoietic (CD34) and B lymphocyte (CD19) markers was assessed as previously described [19]. Surface markers expressed by BMSCs were detected by FCM using PE anti-CD19, PE anti-CD34, PE anti-CD73, FITC anti-CD105 mAb (Becton-Dickinson, San Jose, CA, USA). Staining was performed according to the manufacturer’s protocol, and at least 10,000 events were analyzed by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA) using Cell Quest software.
Isolation of EVs
EVs were obtained from the conditioned culture medium of BMSCs, according to a previously described protocol with slight modifications [20]. Briefly, BMSCs were cultured in without serum medium for 48 h, collected the culture supernatant, then centrifuged at 2,000 g for 20 minutes to remove debris. The cell-free supernatants were centrifuged at 100,000 g for 1 h at 4°C (Beckman Coulter Optima L-90K ultracentrifuge). The resultant pellet was washed in PBS, and then was centrifuged again at 100,000 g for 1 hour to pellet the EVs. The pellets were resuspended in 100 μL of PBS and aliquots were stored at -80°C until further use. The protein concentration of the EVs preparations was quantified by the BCA method (Beyottime Biotechnology, China).
Electron microscopy
For TEM analysis, purified EVs were resuspended in PBS and loaded on a formvar-coated copper grid. Excess fluid was removed with filter paper, and the samples were stained with 1% uranyl acetate for 30 s and then washed, dried and stained with 2% lead citrate for another 30 s. The grids were examined under a transmission electron microscope (Philips, Tecnai12, Netherland) at 80 kV, and electromicrographs were taken and subjected to analyses. For SEM analysis, EVs were fixed in Karnovsky fixative, dehydrated in alcohol, dried on glass surface and then coated with gold by sputter coating. The samples were observed on a scanning electron microscope (Hitachi, S4800-II, Japan). Images were allowed via secondary electron at a working distance of 15 to 25 mm and an accelerating voltage of 20 to 25 kV.
Experimental animals and surgical procedures
Adult male SD rats (200-230 g, n=12) were purchased and maintained in the animal experimental center of Jiangsu University in China. They were kept in individual cages and allowed free access to tap water and standard rat chow in a temperature-controlled room (22-24°C) and relative humidity of 55-70% with 12 h light/dark cycle. This work was carried out in accordance with the ethics Committee of Jiangsu University for the use of laboratory animals (Permit Number: JSU 14-212).
For sciatic nerve crush, the rats were anesthetized with intraperitoneal injections of ketamine (75 mg/kg) and xylazine (10 mg/kg) during all surgical procedures. The right hindquarter was shaved, and then a 10 mm incision was made on the posterior and parallel to the femur. Between biceps femoris and vastus lateralis were bluntly dissected to expose the sciatic nerve. The crush injury was performed 10 mm proximal to its trifurcation using a 3 mm-wide hemostat for 30 s [21]. After creation of the 3 mm-wide crush injury, EVs group rats were immediately injected at a concentration of 45 μg/30 μL of PBS without Ca2+ and Mg2+ into the lesion using a microinjector (Shanghai Medical Laser Instrument, China), while the control rats received 30 μL of PBS. At the end of the experiment, the animals (6 animals per group per time point) were sacrificed by anesthesia as mentioned before at the fourth week after surgery.
Functional assessment
Functional evaluation of sciatic nerve was expressed by the sciatic function index (SFI) at 1, 2, 3 and 4 weeks after surgery as previously described [22]. Briefly, the rats hind paws were dipped with ink, and then the rats were allowed to walk through a tunnel so that the footprints could be displayed on a strip of white paper loaded onto the bottom of the tunnel. The footprints of normal (N) and experimental (E) sides were collected and measured. At least four footprints per animal were taken from each side. Footprints were measured for the following parameters: between the third toe ant heels (PL), first and fifth toe (TS), and second and fourth toe (ITS), and the contra lateral normal side (NPL, NTS, and NITS, respectively). The SFI was calculated according to the following mathematical formula [22]:
SFI=-38.3 × (EPL-NPL)/NPL + 109.5 × (ETS-NTS)/NTS + 13.3 × (EITS-NITS)/NITS-8.8.
Calculated indices from this formula ranged between a score from 0 to 100. Around 0 corresponds to normal function, and -100 corresponds to a total dysfunction.
Morphological assessment
Nerve tissue were harvested and immersed in 4% neutral-buffered formaldehyde at 4°C for 48 hours. The tissue were embedded in paraplast paraffin under light microscopy, and then divided into sections (3 mm thick) and stained with H&E for general histological analysis. Pictures were capture by Nikon Ti-S microscope (Ti-S, Nikon, Japan).
Immunohistochemical analysis
GAP-43 was used as marker for axon regeneration. Nerve tissue (3 mm thick) were deparaffinized and rehydrated. Briefly, after non-specific immunoreactions were blocked with 1% BSA for 30 min, sections were incubated primary antibody solution (1:200, immunoWay, USA) for 1 h at room temperature, followed by incubation with HRP-conjugated secondary antibodies. Pictures were capture by Nikon Ti-S microscope (Ti-S, Nikon, Japan).
Statistical analysis
Statistical analysis was performed using the Student’s t-test with the GraphPad Prism V 5.0 software program (GraphPad, San Diego, CA, USA). P value <0.05 was considered statistically significant.
Results
Characterization of EVs
To confirmed that the isolated pellets were genuine EVs, the harvested pellet was captured by transmission electron microscope (TEM) and Scanning electron microscope (SEM). By TEM, EVs displayed to be mainly round and oval in shape, ranged in size from 40-300 nm, aggregated in clusters (Figure 1A). As shown in Figure 1B, in situ EVs revealed the unique membrane structure characteristics by SEM, and their morphology and dimensions were similar to Figure 1A.
Figure 1.
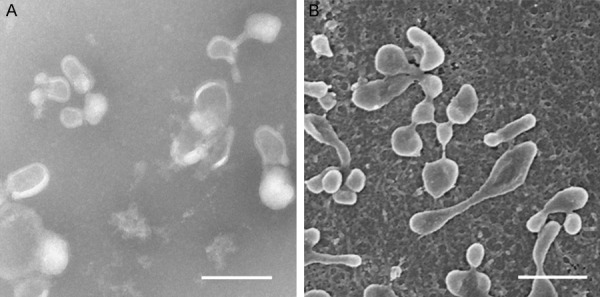
Rat BMSC-EVs characterized by transmission electron microscopy and Scanning electron microscope. A. EVs viewed under transmission electron microscope. Scale bar: 0.2 µm. B. EVs viewed under Scanning electron microscope. Scale bar: 0.2 µm.
Rat sciatic nerve injury and nerve regeneration assessment
To examine the effects of MSC-EVs in rat nerve injury repair, we constructed a rat sciatic nerve injury model by surgery. As shown in Figure 2A and 2B, sciatic nerve 3 mm crush injury point and EVs injection point. SFI values are showed in Figure 3. The SFI score in all groups is near zero before surgery. After sciatic nerve crush, the mean SFI decreased to -100 as a result of the complete loss of sciatic nerve function in all rats. In one week after the injury, no significant differences were observed between EVs group and control group (P>0.05). From the second week to the fourth week, SFI improved significantly in the EVs groups compared with that of the control group (P<0.05) (Figure 2C). These results indicate that MSC-EVs can protect rat from severe sciatic nerve injury.
Figure 2.
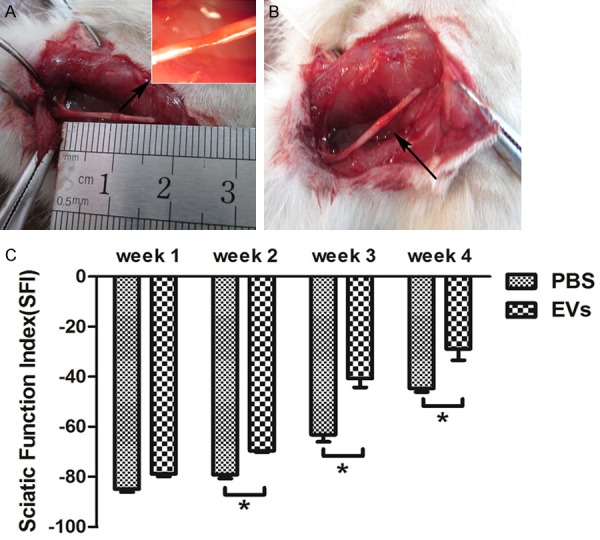
Rat BMSC-EVs treatment of sciatic nerve lesion by crush induction. A. Sciatic nerve lesion induction (blank arrow: injure point). B. Rat BMSC-EVs injection followed by the damage point (blank arrow: injection point. C. Rat BMSC-EVs improved the sciatic nerve function index (SFI). SFI for each group at each time point is shown. Bars represent the mean ± SEM (n=6 per group) of 12 mice from 2 independent experiments. Significance analyzed by an independent Student’s test. *P<0.05.
Figure 3.
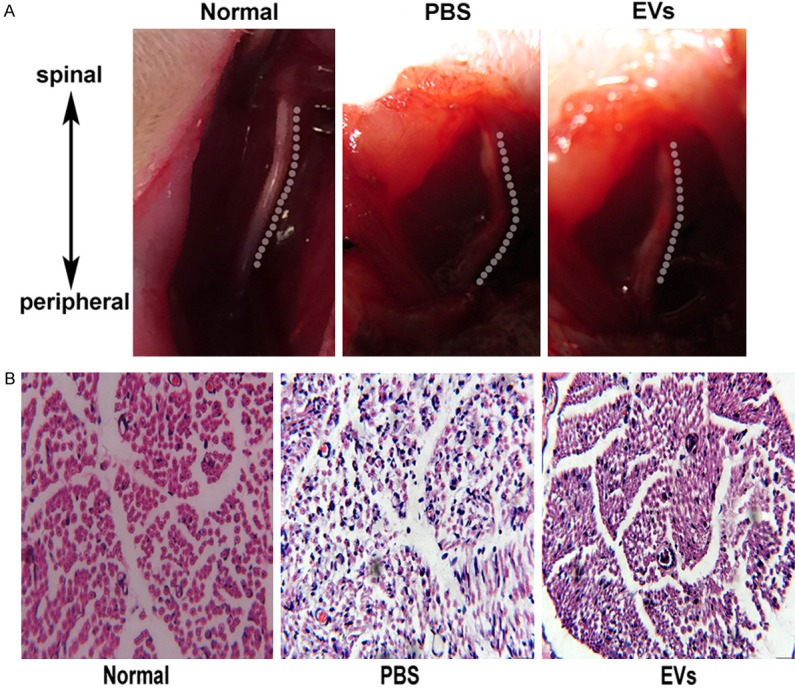
At week 4 after surgery, the rats were killed and their injured nerves were analyzed for histology. A. The anatomical morphology of the injured nerves for each group is shown. B. The histology (hematoxylin-eosin staining) for each group is shown (magnification, 400 ×).
Morphologic analysis of nerve regeneration
To evaluate the effect of EVs, neurological morphology after sciatic nerve crush in fourth week was observed. As shown in Figure 3A, the anatomical morphology of the injured nerves treated with EVs is closely resembled to normal rat, while PBS-treated rat still remain injury morphology. By H&E staining, injection of EVs regenerated nerve myelin, which showed incomplete shape and thickness, but was very similar to normal nerves myelin. However, the regenerated nerves myelin from PBS-injected rat was irregular and thin (Figure 3B). The results suggest that MSC-EVs can really repair sciatic nerve injury.
Immunohistochemical analysis
GAP-43 is the major protein kinase C component of growth cones and developing nerve terminals, which is considered a good marker for evaluating the extent of axonal regeneration [23]. Immunostaining for the GAP-43 protein was measured in the cross-sections of crushed sciatic nerves from rats treated with EVs or PBS at fourth week after the surgery. As shown in Figure 4, EVs treatment increased the brown of GAP-43 expression compared with PBS-treated rat. The results indicate that MSC-EVs may promote axonal regeneration, which was involved in nerve injury repair.
Figure 4.
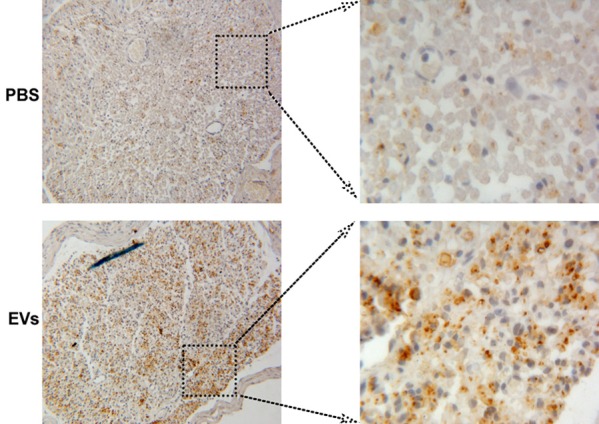
Rat BMSC-EVs increased GAP-43 expression. GAP-43 expression of the injured nerves for each group is shown at week 4 after surgery. The brown particles used as positive staining of the GAP-43 (magnification, 400 ×).
Discussion
After peripheral nerve injury, the peripheral nervous system is endowed with an intrinsic regeneration potential, which can lead to an almost complete recovery. However, nerve regeneration often fails in cases of proximal nerve injury, diffuse axonal loss, or chronic insults [21]. Clinically, surgical repair is usually the primary treatment for peripheral nerve injury, but patients can’t restore normal motor control and fine sensory function [24]. Mesenchymal stromal cells represent a promising therapeutic approach for organ repair, and their biological characteristics have been recognized, such as repair, immunological, transmission, secretion properties and so on [25]. Several studies demonstrated that the administration of bone marrow-derived MSCs in peripheral nerve injury could improve nerve regeneration, with axonal regrowth and myelin formation [26-30]. At present, no strong evidence basis is displayed that transplanted MSCs truly trans-differentiate into cell populations specific to the injured organ. Therefore, the paracrine mechanism has been proposed to be one of the underlying mechanisms of transplanted stem cells with therapeutic effect [31].
EVs are small vesicles released by diverse cell types, including platelets, dendritic cells, endothelial cells, and MSCs. EVs exert their effects on fundamental biological processes in a pleiotropic manner, such as directly activating cell surface receptors via protein and bioactive lipid ligands, merging their membrane contents into the recipient cell plasma membrane, delivering proteins, mRNA, miRNA, bioactive lipids and even whole organelles into target cells [15,32-35]. A recent study showed that the administration of BMSC-EVs can promote functional recovery and neurovascular plasticity in a stroke model [17]. Later, the research group further confirmed that the protective mechanism of BMSC-EVs was involved in transferring microRNA-133b to astrocytes and neurons [36]. Additionally, BMSC-EVs have been shown to protect optic nerve against injury through promoting survival of retinal ganglion cells (RGC) and regeneration of their axons, while partially preventing RGC axonal loss and RGC dysfunction [37].
In this study, rat BMSC-EVs have been successfully extracted and displayed a spherical shape of the membrane structure under TEM and SEM, their size and shape are consistent with the previous publication [38]. According to recent advances for extracellular vesicles (EVs), the terms ‘exosome’ and ‘microvesicle’ have been used interchangeably in many published studies. Exosomes and microvesicle are two major types of extracellular vesicles that can be differentiated by their size and content [39]. However, there are studies found that microvesicle exhibit CD63, a typical marker of exosomes, as determined by NTA [40]. Because of an as yet incomplete understanding of extracellular vesicle biogenesis, inconsistencies in extracellular vesicle purification protocols and a lack of thorough vesicle characterization, therefore, we use the term ‘extracellular vesicle’ to refer to both of these vesicle types [41]. In this study, we found that rat BMSC-EVs promote the function recovery of sciatic nerve damage and myelination. Rat BMSC-EVs treatment significantly improved motor performance, repaired the anatomical morphology of the injured nerves, and promoted the myelin and axon regeneration by H&E staining and GAP-43 expression. These data are supported with a previous study showing that the local implantation of BMSCs can promote nerve regeneration and the recovery of nerve function in sciatic nerve crush injury model [42]. Consistent with our data, Raisi et al. [43] demonstrated that omental adipose MSC-EVs drastically improved the process of myelination and the structural recovery of regenerated nerve fibers in a rat sciatic nerve transection model.
Conclusions
The results of the present study have demonstrated that BMSC-EVs could restore nerve function and promote nerve regeneration in sciatic nerve crush injury model. However, the detailed repair mechanism of BMSC-EVs needs further research.
Acknowledgements
The current study was supported by grants from Social Development Projects of Jiangsu Province (Jiangsu, China; no. BE2017697), the Natural Science Foundation of Jiangsu Province (Jiangsu, China; no. BK20141295), the clinical science and technology development foundation of Jiangsu University (Jiangsu, China; no. JLY20160081), Social Development Projects of Zhenjiang (Zhenjiang, China; no. SH2015033), Key Medical Personnel of Zhenjiang, “LiuGeYi” Projects of Jiangsu Province (Jiangsu, China; no. LGY2016055), and the Affiliated Hospital of Jiangsu University (Zhenjiang, China; no. jdfyRC2015010).
Disclosure of conflict of interest
None.
References
- 1.Olson L. Regeneration in the adult central nervous system: experimental repair strategies. Nat Med. 1997;3:1329–1335. doi: 10.1038/nm1297-1329. [DOI] [PubMed] [Google Scholar]
- 2.Ignatiadis IA, Tsiampa VA, Yiannakopoulos CK, Xeinis SF, Papalois AE, Xenakis TH, Beris AE, Soucacos PN. A new technique of autogenous conduits for bridging short nerve defects. An experimental study in the rabbit. Acta Neurochir Suppl. 2007;100:73–76. doi: 10.1007/978-3-211-72958-8_16. [DOI] [PubMed] [Google Scholar]
- 3.Sönmez E, Siemionow ME. Nerve allograft transplantation. London: Springer; 2015. pp. 551–559. [Google Scholar]
- 4.Liu Y, Sun GW. Key issues of stem cell therapy for peripheral nerve injury. Neural Regeneration Research. 2015:10. [Google Scholar]
- 5.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adiposederived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–1272. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Guo L, Ahn HS, Kim MH, Kim SW. Amniotic mesenchymal stem cells display neurovascular tropism and aid in the recovery of injured peripheral nerves. J Cell Mol Med. 2014;18:1028–34. doi: 10.1111/jcmm.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee EJ, Xu L, Kim GH, Kang SK, Lee SW, Park SH, Kim S, Choi TH, Kim HS. Regeneration of peripheral nerves by transplanted sphere of human mesenchymal stem cells derived from embryonic stem cells. Biomaterials. 2012;33:7039. doi: 10.1016/j.biomaterials.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Rubina BS, Michiel PD, Nicola B. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel FB. Stem cell secretome and paracrine activity. Springer International Publishing. 2016 [Google Scholar]
- 12.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 15.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabin K, Kikyo N. Microvesicles as mediators of tissue regeneration. Transl Res. 2014;163:286–295. doi: 10.1016/j.trsl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–5. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu F, Li T, Ding Y, Sun L, Tu T, Zhou F, Qi W, Jiang X, Fang J, Hu J, Zhu W, Sun X. Cytotoxic effects of 4-methylimidazole on bone marrow mesenchymal stem cells in vitro. Am J Transl Res. 2015;7:1736–46. [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Ge S, Zhou D, Zhang H, Sun L, Wang X, Su J. [Rat bone marrow mesenchymal stem cells promote apoptosis and inhibit proliferation and migration of RSC96 cells] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:190–195. [PubMed] [Google Scholar]
- 20.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adiposederived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–72. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]
- 22.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz LI, Perrone-Bizzozero NI. The relationship of GAP-43 to the development and plasticity of synaptic connections. Ann N Y Acad Sci. 1991;627:58–74. doi: 10.1111/j.1749-6632.1991.tb25914.x. [DOI] [PubMed] [Google Scholar]
- 24.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98:16. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci Rep. 2016;6:31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–453. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Gao XP, Guo ZY, Sun X. Effects of intravenous transplanted stem cells on nerve crush injury. Orthopedic Journal of China. 2014 [Google Scholar]
- 29.Zheng L, Cui HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med. 2012;23:2291–302. doi: 10.1007/s10856-012-4694-3. [DOI] [PubMed] [Google Scholar]
- 30.Shang XC, Jia-Xiang GU, Zhang NC. The experimental research of peripheral nerve regeneration with rat bone marrow mesenchymal stem cells induced by GDNF gene. Chinese Journal of Laboratory Diagnosis. 2014 [Google Scholar]
- 31.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 33.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 34.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 35.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 36.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6:1273–1285. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, Xu R, Liu Y, Watabe K, Vemuri MC, Pochampally R. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953–67. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould SJ. Exosomes and microvesicles. Encyclopedia of Biological Chemistry. 2013:262–264. [Google Scholar]
- 40.Gerceltaylor C, Atay S, Tullis RH, Kesimer M, Taylor DD. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal Biochem. 2012;428:44. doi: 10.1016/j.ab.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 41.El AS, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 42.Yang G, Yin WT, Xue JW, Li CY, Fan DY. Repairing sciatic nerve crush injury by transplantation of bone marrow mesenchymal stem cells. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;12:4948–4951. [Google Scholar]
- 43.Raisi A, Azizi S, Delirezh N, Heshmatian B, Farshid AA, Amini K. The mesenchymal stem cell-derived microvesicles enhance sciatic nerve regeneration in rat: a novel approach in peripheral nerve cell therapy. J Trauma Acute Care Surg. 2014;76:991. doi: 10.1097/TA.0000000000000186. [DOI] [PubMed] [Google Scholar]


