Abstract
Obesity is a complex metabolic disease, which causes serious impairment to the health of people. This study aimed to determine the influence of different-doses of soy isoflavones (SIF) on testicular morphology, testosterone levels and the expression of genes and proteins related to testosterone synthesis in diet-induced obesity (DIO) male rats. We used high-fat diet (HFD) to establish a DIO male rat model, then obese rats orally received SIF at doses of 0, 50, 250 and 500 mg/kg per day for four weeks. Results revealed that the body weight was obviously increased, and seminiferous tubules were significantly deformed in obese rats compared with that in normal diet fed controls. After SIF treatment, DIO male rats exhibited decreased body weight in a dose-dependent manner, accompanied with significantly alleviated testicular damages, as well as increased testosterone levels and steroidogenic acute regulatory (StAR), cytochrome P450 11A1 (CYP11A1), cytochrome P450 17A1 (CYP17), hydroxysteroid dehydrogenase-3β (HSD3β), and hydroxysteroid dehydrogenase-17β (HSD17β) protein and mRNA levels. In conclusion, SIF could alleviate testicular damages, increase testosterone levels, and upregulate the expression of proteins and genes related to testosterone synthesis in DIO male rats, which would be important for obesity male reproduction treatment.
Keywords: Soy isoflavones, obesity, testis, testosterone, synthesis
Introduction
The prevalence of overweight and obesity continue to rise in the world, which result from the imbalance between energy intake and energy output, endocrine, genetic factors, as well as other factors [1]. One of the more significant factors that influence obesity is the change in food consumption, such as consuming high-fat Westernized fast food or local fast food [2]. In addition, obesity can lead to further morbidity caused by diabetes [3], atherosclerosis [4], hypertension [5], and cardiovascular diseases [6]. At the same time, many adverse effects of obesity on female reproduction have been reported, such as menstrual disorder, anovulation, polycystic ovarian syndrome, increased risk of miscarriage, and reduced conception rate [7,8]. Moreover, obesity may also impair male reproduction [9-12]. Obesity has been reported to reduce semen quality and impact fertility by affecting spermatogenesis [13]. A high incidence of infertility in association with metabolic disturbances and hormonal dysregulation was confirmed in obese men [14].
On the other hand, due to the lower frequency of obesity and related diseases in Asian countries, attention has been turned toward to the Asian diet, which consists highly of soy and soy-based products [15]. SIF, a phytoestrogen, has a structure similar to estrogen 17β-estradiol that enables them to bind the estrogen receptor [16]. Genistein and daidzein are present at high concentrations in soybeans [17]. Evidence indicated that genistein and daidzein acted as estrogen receptors alpha and beta agonists and/or antagonists, and had the capacity to regulate cell proliferation, growth, and function [18]. Many effects and functions of SIF have been reported. For example, supplied soy protein to young women for one month was found to affect endocrine profiles and cause alterations in menstrual cycles [19]. Furthermore, premenopausal women fed with dietary soy protein for 14 days exhibited mammary gland tissue proliferation associated with increased expression of the progesterone receptor [20]. At present, literatures involving SIF and its effects are controversial. For example, a recent multigenerational study linked genistein to mammary hyperplasia in male rats, but found no adverse effects on androgen-sensitive end-points [21]. However, other studies found that serum testosterone levels were elevated in groups of rats exposed to dietary isoflavones in the perinatal period, while soy and soy products interferes with reproductive development and causes anomalies of the male reproductive tract [22-24].
Therefore, we established a DIO male rat model by HFD and feeding different-doses of SIF to investigate the change in male testicular morphology and testosterone levels, as well as the expression of proteins and genes related to testosterone synthesis.
Materials and methods
Animals and treatments
Seventy-five male Sprague Dawley (SD) rats (five weeks old; Dashuo, China) were randomly divided into two groups: control group (n=15), fed with a normal diet (54% corn, 14% wheat bran, 13% alfalfa meal, 10% cotton meal, 6% fish meal, 1.5% vitamin and mineral, 1% limestone, 0.3% sodium chloride, and 0.2% dicalcium phosphate); and obesity group (n=70), fed with HFD (69.5% normal diet, 15% pork fat, 15% sucrose and 0.5% pig bile) for nine weeks. The criterion for DIO rat is that the body weight of rat in the HFD group is 1.4 times more than that in the control group [25]. The DIO rats were further randomly divided into four groups (n=8/group), and were fed with HFD with 0, 50, 250 and 500 mg/kg doses of SIF (NF-20140806, North China Pharmaceutical Co., China) for four weeks. The compounds of the SIF extracts were quantified by high-performance liquid chromatography (HPLC), and are shown in Table 1. These rats were decapitated for subsequent experiments. The use of rats, as well as all experimental procedures that involve animals, were approved by the Sichuan Agricultural University Animal Care and Use Committee.
Table 1.
Composition of soy isoflavone extracts
| Compounds | Content |
|---|---|
| Daidzin | 50.98% |
| Glycitin | 30.36% |
| Genistein | 8.80% |
| Daidzein | 1.20% |
| Glycitein | 0.24% |
| Genistin | 0.06% |
| Total isoflavones | 91.64% |
Body weight and plasma measurements
Body weight was measured weekly. The blood sample was collected from the lateral tail vein. A 1- to 2-mm section was cut from the tip of the tail with a sterile scalpel blade. Then, blood was milked from the base of the tail to the tip until a sufficient volume of blood was collected for blood biochemical analysis (Beckman CX4, USA). Rat plasma lipids including triglycerides (TG), total cholesterol (TC), high density lipoprotein (HDL) and low density lipoprotein (LDL) were measured at the last week of feeding with HFD (week 9), and after additional feeding with SIF at the second and fourth week (week 11 and 13).
Histopathologic evaluation
Part of the testicle tissues were fixed in 4% paraformaldehyde. Then, hematoxylin and eosin (H&E) staining and Oil-Red-O staining were performed according to manufacturer’s instructions (Beyotime, China). Photomicrographs were obtained using a digital microscope camera system (Nikon DS-Ri1, Japan). The morphological structure of the seminiferous tubules were tested by Image Pro Plus software (Media Cybernetics, USA). 40 fields (five fields per rat, ×400 magnification) in eight rats were randomly selected to count total cells in each tube and calculate the mean diameter of seminiferous tubules in one group. The lipid droplets in the testis were determined by integrated optical density (IOD). Briefly, the image were analyzed using Image Pro Plus software. By selecting the “colour-chosen target” in the option bar of the morphologic analysis system, all lipid droplets in the field were marked in color. Then, “calculating” in the option bar was selected to automatically calculate the IOD value.
Immunohistochemistry
Testicle paraffin sections were dewaxed in xylene, rehydrated through a graded series of ethanol solutions, washed in distilled water and PBS, and endogenous peroxidase activity was blocked by incubation with 3% H2O2 in methanol for 20 minutes. The sections were saturated with normal 10% goat sera and 0.3% triton for 30 minutes in order to eliminate non-specific irrelevant proteins staining, and were incubated with the primary (rabbit) antibodies (Table 2) for 17 hours at 4°C (working dilution: 1:200). After washing in PBS, the slices were exposed to 1% biotinylated goat anti-rabbit IgG secondary antibody (10J26C, Boster, China) for one hour at 37°C, and incubated with streptavidin-biotin complex (SABC; 10J26C, Boster, China) for 30 minutes at 37°C. In order to visualize the immunoreaction, the sections were immersed in diaminobenzidine hydrochloride (DAB; AR1000, Boster, China); then, the slices were monitored microscopically, and was stopped by immersion in distilled water as soon as blue staining was visible.
Table 2.
Antibodies used in immunohistochemistry
| Name | Company | Cat# | Source | Dilution |
|---|---|---|---|---|
| StAR | Bioss, China | bs-3570R | Rabbit | 1:200 |
| CYP11A1 | Bioss, China | bs-10099R | Rabbit | 1:200 |
| CYP17 | Bioss, China | bs-3905R | Rabbit | 1:200 |
| HSD3β | Bioss, China | bs-16552R | Rabbit | 1:200 |
| HSD17β | Boster, China | BA2814-1 | Rabbit | 1:200 |
In this study, protein expression levels of the testis regulatory molecules were determined by integrated optical density (IOD). Briefly, photographs of the testicle were taken using a digital microscope camera system (Nikon DS-Ri1, Japan). For each section, five fields of 0.064 mm2 from each area of the image were analyzed using Image Pro Plus software (Media Cybernetics, USA). By selecting the “colour-chosen target” in the option bar of the morphologic analysis system, all positive immunoreactive cells in the field were marked in color. Then, “calculating” in the option bar was selected to automatically calculate the IOD value.
Quantitative real-time PCR
Total RNA was extracted from tissues using RNAiso Plus (9108/9109, Takara, Japan). RNA was subjected to reverse transcription with reverse transcriptase according to manufacturer’s instructions (RR047A, Takara, Japan). Reverse transcription reactions were stored at -80°C. Quantitative real-time PCR was performed using the Bio-Rad iQ5 system. 2 μl cDNA template, 1 μl of forward/reverse primers, 8.5 μl of RNase-free water and 12.5 μl of SYBR Premix Ex TaqTM II system (DRR820A, Takara, Japan) were added in each reaction. Reactions were incubated at 95°C for 3 min, followed by 40 cycles of denaturation (95°C, 10 s), annealing (55-56°C depending on the primer sets, 30 s) and dissociation (95°C, 10 s; 65°C to 95°C, 5 min). Chicken β-actin expression was used as an internal reference housekeeping gene. Oligonucleotide primers were designed using Primer 5 software and synthesized at Takara (Dalian, China; Table 3).
Table 3.
Real-time fluorescence quantitative PCR primer sequence
| Gene | Primer | Sequences (5’-3’) | Product size (bp) | Tm (°C) |
|---|---|---|---|---|
| StAR | F | TTCCGACTGGAGGTGCTGCTA | 109 | 55 |
| R | CCTTGATTTCCTTGACATTTGGGT | |||
| CYP11A1 | F | CGATGACCTATTCCGCTTTGC | 131 | 56 |
| R | TGTGGAACATCTGGTAGACGGC | |||
| CYP17 | F | CAATCTCTGGGCACTGCATCAC | 119 | 56 |
| R | GCAAGTAACTCTGCGTGGGTGTA | |||
| HSD3β | F | GAGTGCCAGCCTTCGTCTACA | 151 | 55 |
| R | ACTACCTTCTCGGCCATCCTTAT | |||
| HSD17β | F | TGACCAAGACCGCCGATGA | 149 | 55 |
| R | GTACCACTGGCATTGTGATG | |||
| β-actin | F | ATCTTCATGGTGCTAGGAGC | 135 | 56 |
| R | TCCCAGCACCCTTCATAGCAT |
Enzyme linked immunosorbent assay (ELISA)
For testosterone extracts and assay, fresh testicles (0.1 g) were grounded in liquid nitrogen, and suspended in a 0.9 ml solution containing 10 mM of PBS (pH 7.4), 2 seconds/time, 10 seconds at a time by Ultrasonic crusher (Scientz JY 92-IIN, China) last for 30 minutes gained the homogenate, then centrifuged at 4°C for 10 minutes at 2,500 rpm, and the resulting supernatant was collected to determine testosterone level activities, according to manufacturer’s instructions (ml002868, Mlbio, China).
Statistical analysis
Comparisons between the control group and obesity group were conducted using t-test, and one-way ANOVA test with LSD correction was used compare with different groups. Data were expressed as mean ± standard deviation (X ± SD). Analyses were performed using the SPSS 20.0 software (IBM Corp, USA) for windows. P<0.05 was considered statistically significant.
Results
Effect of SIF on body weight in DIO male rats
After nine weeks of feeding, DIO male rats gained more body weight than rats in the control (Ctr) group (Figure 1A). After fed with different-doses of SIF, the body weight of the DIO male rats was significantly reduced (Figure 1C), accompanied with a remarkable reduction in TC and LDL concentrations (Figure 1F and 1H). These data indicate that SIF could mitigate the body weight of DIO male rats in a dose-dependent manner, which was consistent with our previous work [25].
Figure 1.
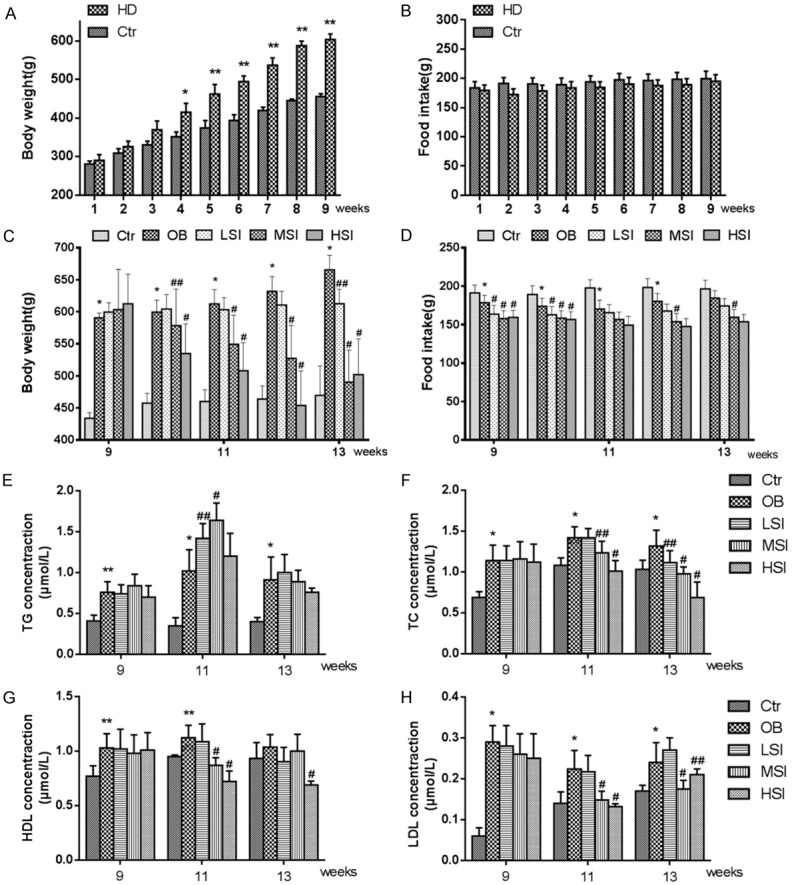
Different doses of SIF can mitigate the body weight of DIO male rats, as well as plasma TC and LDL concentrations. A. Quantification shows the body weight of rats fed with normal diets and HFD. B. Quantification shows no difference in food intake of rats feed with normal diets and HFD. C. Quantification shows the body weight trend of control rats fed with normal diets and DIO rats fed with HFD with the addition of different doses of SIF. D. Quantification shows the food intake trend of DIO rats feed with HFD and the addition with different doses of soy isoflavones. E-H. Quantification shows the TG, TC, HDL and LDL concentration in control rats fed with normal diets and DIO rats fed with HFD with the addition of different doses of SIF. The values are presented as means ± standard deviation. *P<0.05, **P<0.01 vs. Control group; #P<0.05, P<0.01 vs. Obesity group.
Pathological observation of testicles
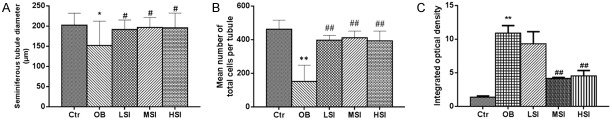
H&E staining revealed that the seminiferous tubules were deformed, seminiferous epithelia were significantly atrophic and necrotic, and cell adhesions between spermatogenic cells and sertoli cells were loosely arranged in OB (Figure 2). However, the pathological lesions of testis were significantly alleviated compared with those in obesity group (Figure 2). Oil-Red-O staining indicated that a small number of red lipid droplets were found in the Ctr group (Figure 2). However, these droplets were found in large numbers at the edge of seminiferous tubules, which were scattered manner inside the cytoplasm of cells in OB. And the addition of SIF to the diet could significantly improve this phenotype, especially in MSI (P<0.01) and HSI (P<0.01) group, the lipid droplets levels significantly decreased (Figure 3C).
Figure 2.
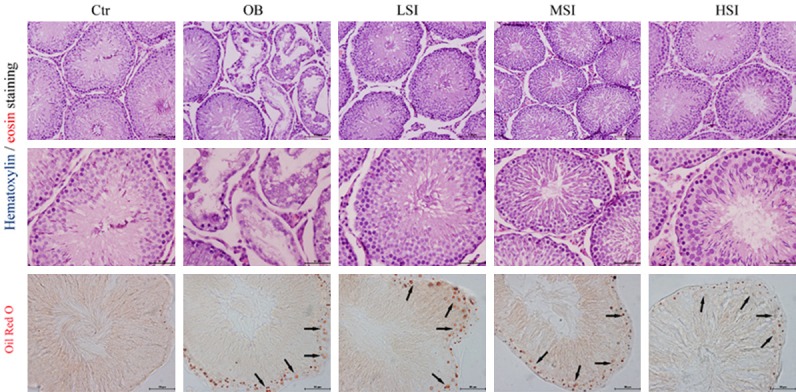
Representative images of H&E staining and Oil-Red-O show the seminiferous tubule in the control and obesity groups, as well as different doses of SIF groups (scale bar: 100 μm and 50 μm).
Figure 3.
The influence of different doses of SIF on testicular morphology and lipid levels. A. Quantification shows the diameter of seminiferous tubules of control rats and DIO rats fed with HFD with the addition of different doses of SIF. B. Quantification shows the mean number of the total cells per tubule in control rats and DIO rats fed with HFD with the addition of different doses of SIF. C. The influence of different doses of SIF on lipid droplets levels in the seminiferous tubule. The values are presented as means ± standard deviation. *P<0.05, **P<0.01 vs. Control group; #P<0.05, P<0.01 vs. Obesity group.
Seminiferous tubule diameter and the mean number of total cells per tubule
The diameter of seminiferous tubules and total cells per tubule in the OB group were significantly lower than Ctr group (P<0.05 or P<0.01) and SIF groups (P<0.05 or P<0.01) (Figure 3A and 3B).
Expression of proteins related to testosterone synthesis by immunohistochemistry
Stained in blue-purple, immunochemical localization of testosterone synthesis proteins were expressed in the Leydig cell of testis (Figure 4). These protein expression levels were highest in Ctr group. Compared with the OB group, these protein expression levels significantly increased in the SIF groups, as shown in Figure 5A.
Figure 4.
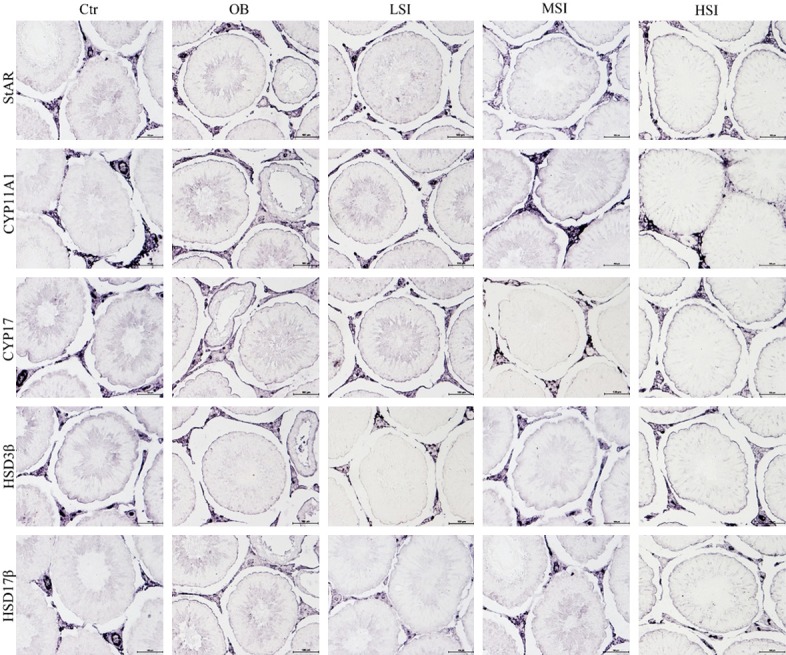
Expression of StAR, CYP11A1, CYP17, HSD3β and HSD17β protein in the testis (Immunohistochemistry, scale bar: 100 μm).
Figure 5.
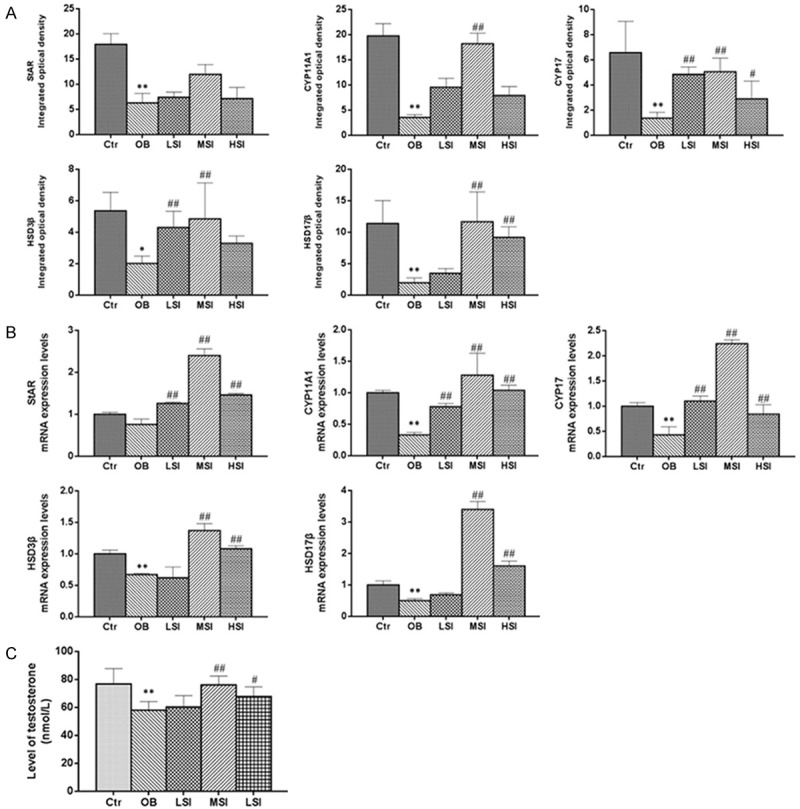
The influence of different doses of SIF on testosterone levels and the expression of proteins and genes related to testosterone synthesis in DIO male rats. A. Integrated optical densities (IODs) of StAR, CYP11A1, CYP17, HSD3β and HSD17β protein expression in the testis. B. StAR, CYP11A1, CYP17, HSD3β and HSD17β mRNA expression levels in the testis. C. The levels of testosterone in the testis. The values are presented as means ± standard deviation. *P<0.05, **P<0.01 vs. Control group; #P<0.05, P<0.01 vs. Obesity group.
Testosterone synthesis regulatory mRNA expression by RT-PCR
The expression of testosterone synthesis regulatory mRNA are shown in Figure 5B. Compared with the Ctr group, mRNA expression levels of CYP11A1, CYP17, HSD3β and HSD17β significantly decreased in the OB (P<0.01). Compared with OB group, the mRNA expression levels of StAR, CYP11A1, and CYP17 markedly increased in the LSI group (P<0.01), and the mRNA expression level of StAR, CYP11A1, CYP17, HSD3β and HSD17β were significantly upregulated in the MSI and HSI group (P<0.01).
Testosterone levels by ELISA
The levels of testosterone are shown in Figure 5C. The testosterone levels were highest in Ctr group and lowest in the OB. Compared with the OB, testosterone levels were significantly elevated in the MSI (P<0.01) and HIS groups (P<0.05).
Discussion
The incidence of obesity continuous to increase in the world, and threatens the health of several people. In order to study the effect of obesity on male testis, we used HFD to establish a DIO male rat model. This was conducted with the hope of mimicking the pathophysiology induced by obesity in humans [26]. The present study shows that HFD can promote rat obesity development due to the increase in plasma lipid levels, along with TG, TC and LDL, which was accompanied by significant weight gain. The low frequency of obesity and related metabolic disorders in Asian populations has drawn attention towards soy, which is a characteristic component in Asiatic diets. SIF, which is a phytoestrogen, has been reported to affect adiposity either directly by modulating lipogenesis, lipolysis and adipogenesis, or indirectly by modulating the appetite or energy expenditure [27]. Clinical studies have also suggested that soy protein or isoflacones may improve metabolic parameters. For instance, many reports demonstrated the significant reduction in plasma concentrations of TC and LDL in humans exposed to soy proteins [28-31]. In this study, when we fed obesity rats with SIF, we found that SIF could reduce the body weight of DIO male rats, especially in rats in the MSI and HSI group. This was accompanied by a significant decrease in TC and LDL concentration, which suggest that SIF can mitigate abnormalities in HFD-induced obese male rats. Furthermore, the effects on adiposity are dose-dependent, since the magnitude of adipose weight reduction correlates with increasing doses of soy-derived phytoestrogens [32]. This result is consistent with previous literatures [33-35].
The prevalence of infertility is approximately 15%, with male factors accounting for 30 to 50% of this rate [36]. Environmental effects, metabolic dysfunction, and genetic polymorphisms apparently associated with a decline in male reproductive ability [37], while only obesity has been shown conclusively to be involved in this phenomenon [13,14,38,39]. In an attempt to further uncover how obesity affects the male testis, we examined the morphological structure of rat testis through H&E staining and Oil-Red-O staining, since it has been reported that the most susceptible part of the male genital organ is the germinal epithelium of seminiferous tubules [40]. In OB rats, obvious pathological changes in the testicles were characterized by atrophic, necrotic, and distorted seminiferous tubules. After SIF supplement, testicular damages were significantly alleviated. SIF treatment protected against the HFD-induced falling mean number of total cells per tubule and seminiferous tubule diameter. Besides, middle and high doses of SIF have less lipid accumulation in testicular tissue, which indicates that SIF may inhibit adipose tissue deposition and promote the clearance of lipid droplets in obese male rats. These results are consistent with previous studies, showing that SIF could inhibit adipose tissue deposition and promote lipolysis [27,32,34]. Although a previous study revealed atrophy of testis in the epididymis of Beagle dogs [41], as well as in mice [42] treated with high doses of purified isoflavone, we found that HSI exposure did not induce an adverse effect on testicular morphology in obese rats.
Clinical studies on obesity revealed that fatty tissue accumulation was closely associated with the decreases in plasma levels of testosterone [43,44], which concurs with our results. SIF, which is a plant-derived estrogen, may exert both anti-estrogenic and estrogenic effects on metabolism, depending on several factors including its concentration, the concentrations of endogenous estrogens, and individual characteristics [34]. In this present study, results revealed that SIF may exert anti-estrogenic effects in obesity rats, which would weaken the estrogen negative effect. This would lead to the increase in both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion, which in turn would increase testosterones in the SIF groups. Although serum LH and FSH levels were not assayed in the present study, many studies has reported that increased LH and FSH levels has been attributed to increased cholesterol availability and utilization [45-48]. Besides, SIF can enhance circulating leptin levels in HFD rats by elevating the efficiency of translating leptin mRNA into leptin protein [49] which stimulates the secretion of GnRH and LH [50,51]. In mammalian species, Leydig cell development is regulated by the pituitary gonadotropin LH and steroid hormones [52]. LH can stimulate the phosphorylation of StAR protein, which is critical for the translocation of cytosolic cholesterol into the mitochondrial [53]. Then, the cholesterol is converted into pregnenolone by CYP11A1, and pregnenolone moves out of the mitochondria and into the smooth endoplasmic reticulum where it is acted upon, in succession, by CYP17, HSD3β and HSD17β, in order to form progesterone, androstenedione and testosterone, respectively [54]. Therefore, the amount and activity of these enzymes are associated with the amount of testosterone produced.
Conclusion
This study showed that SIF exposure in obese rats could decrease the body weight with a dose dependent manner. In addition, SIF played a protective role in testis and testicular damage induced by obesity, accompanied with increased testosterone levels and the upregulation of testosterone synthesis proteins and mRNAs. Therefore, SIF could be considered as a promising therapeutic approach for the treatment of testicular dysfunction in obese males. According to previous studies and our results, the testosterone synthesis mechanisms of SIF were summarized in Figure 6.
Figure 6.
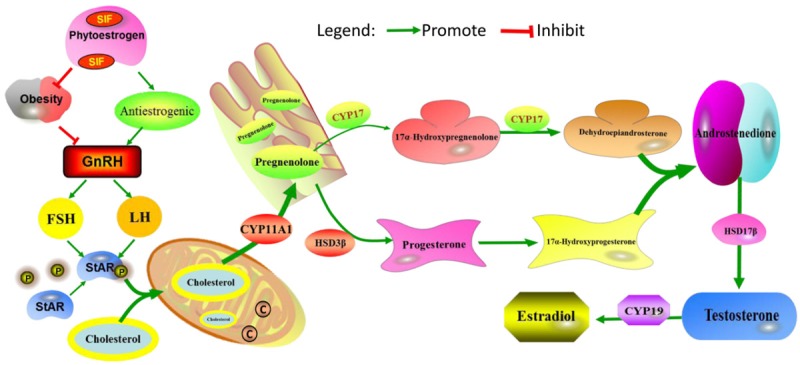
Schematic diagram of the intervention of SIF in testosterone synthesis. SIF may exert anti-estrogenic effects in obesity rats, and increase in both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion, which in turn would increase testosterones in the SIF groups. LH can stimulate the phosphorylation of StAR protein, then, the cholesterol is converted into pregnenolone by CYP11A1, and pregnenolone moves out of the mitochondria and into the smooth endoplasmic reticulum where it is acted upon, in succession, by CYP17, HSD3β and HSD17β, in order to form progesterone, androstenedione and testosterone, respectively.
Acknowledgements
This work was supported by grants from National Key Technology Support Program (2014BAI03B01) and National Key Scientific Instrument and Equipment Development Project of China (2013YQ49085906).
Disclosure of conflict of interest
None.
References
- 1.Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. 2016;7:165–174. doi: 10.4239/wjd.v7.i8.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musaiger AO, Souza R. Nutritional profile of local and western fast foods consumed in Bahrain. Ecology of Food and Nutrition. 2007;46:143–161. [Google Scholar]
- 3.Rohde U, Hedbäck N, Gluud LL, Vilsbøll T, Knop FK. Effect of the endobarrier gastrointestinal liner on obesity and type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2016;18:300–305. doi: 10.1111/dom.12603. [DOI] [PubMed] [Google Scholar]
- 4.Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 5.Commodore MY, Samuel LJ, Dennison-Himmelfarb CR, Agyemang C. Hypertension and overweight/obesity in Ghanaians and Nigerians living in West Africa and industrialized countries: a systematic review. J Hypertens. 2014;32:464–472. doi: 10.1097/HJH.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laura RA, Aldo RE, María DPD, Alicia N, Sonia EM. Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev. 2013;71:168–179. doi: 10.1111/j.1753-4887.2012.00533.x. [DOI] [PubMed] [Google Scholar]
- 7.Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Women’s Health. 2008;4:183–194. doi: 10.2217/17455057.4.2.183. [DOI] [PubMed] [Google Scholar]
- 8.Dionne CG, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y, Liu Y, Xue K, Gu G, Fan W, Xu Y, Ding Z. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10:e0120775. doi: 10.1371/journal.pone.0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27:2365–2372. doi: 10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- 11.Hofny ER, Ali ME, Abdel-Hafez HZ, Kamal EE, Mohamed EE, Abd El-Azeem HG, Mostafa T. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril. 2010;94:581–584. doi: 10.1016/j.fertnstert.2009.03.085. [DOI] [PubMed] [Google Scholar]
- 12.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkeboek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgård C, Lassen TH, Jørgensen N. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 14.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Placido L, Celestino G, José FI, Ana A, Fernando D, Ignacio A, Javier FB. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine. 2011;18:245–250. doi: 10.1016/j.phymed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 17.Kurzer MS, Xia X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–81. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 18.Ren MQ, Kuhn G, Wegner J, Chen J. Isoflavones, substances with multi-biological and clinical properties. Eur J Nutr. 2001;40:135–146. doi: 10.1007/pl00007388. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–373. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- 20.McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, Potten CS, Bundred NJ. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431–1435. doi: 10.1093/ajcn/68.6.1431S. [DOI] [PubMed] [Google Scholar]
- 21.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos K. Barry2 Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28:342–353. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, He X, Supko JG. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology. 2007;148:4475–4488. doi: 10.1210/en.2007-0327. [DOI] [PubMed] [Google Scholar]
- 23.Richard MS, Bronwen M, Keith M, Irene G, Chris MK, Alan SM, Marion W. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17:1692. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol. 2003;169:1582–1586. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Pang DJ, Luo QH, Chen XL, Gao Q, Shi LQ, Liu WT, Zou YF, Li LX, Chen ZL. Soy isoflavones regulate lipid metabolism through an AKT/mTORC1 pathway in diet-induced obesity (DIO) MALE RATs. Molecules. 2016;21:586. doi: 10.3390/molecules21050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortoriello DV, McMinn J, Chua SC. Dietaryinduced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–447. doi: 10.1210/en.2003-1406. [DOI] [PubMed] [Google Scholar]
- 27.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229:1127–1135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 28.Gardner CD, Newell KA, Cherin R, Haskell WL. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:728–35. doi: 10.1093/ajcn/73.4.728. [DOI] [PubMed] [Google Scholar]
- 29.Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol. Lowering effect 12 of soy in postmenopausal women. J Nutr. 2004;134:3277–3283. doi: 10.1093/jn/134.12.3277. [DOI] [PubMed] [Google Scholar]
- 30.Jayagopal V, Albertazzi P, Hepburn DA, Atkin SL, Kilpatrick ES, Howarth EM, Jennings PE. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 31.Allison DB, Gadbury G, Schwartz LG, Murugesan R, Kraker JL, Heshka S, Fontaine KR, Heymsfield SB. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. Eur J Clin Nutr. 2003;57:514–522. doi: 10.1038/sj.ejcn.1601587. [DOI] [PubMed] [Google Scholar]
- 32.Lephart ED, Setchell KD, Handa RJ, Lund TD. Behavioral effects of endocrine-disrupting substances: phytoestrogens. ILAR J. 2004;45:443–454. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- 33.Chen JR, Zhang J, Lazarenko OP, Cao JJ, Blackburn ML, Badger TM, Ronis MJ. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013;27:3514–3523. doi: 10.1096/fj.12-226464. [DOI] [PubMed] [Google Scholar]
- 34.Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol. 2009;304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YB, Zhang Y, Li LN, Zhao XY, Na XL. Soy isoflavone and its effect to regulate hypothalamus and peripheral orexigenic gene expression in ovariectomized rats fed on a high-fat diet. Biomed Environ Sci. 2010;23:68–75. doi: 10.1016/S0895-3988(10)60034-7. [DOI] [PubMed] [Google Scholar]
- 36.Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98:3532–42. doi: 10.1210/jc.2012-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper TG, Handelsman DJ. Falling sperm counts and global oestrogenic pollution: postscript. Asian J Androl. 2013;15:208–211. doi: 10.1038/aja.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, Clément P, Briot P, Berthaut I, Levy V, Cedrin-Durnerin I, Benzacken B, Chavatte-Palmer P, Levy R. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15:622–625. doi: 10.1038/aja.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramlau-Hansen CH, Hansen M, Jensen CR, Olsen J, Bonde JP, Thulstrup AM. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: two decades of follow-up. Fertil Steril. 2010;94:610–618. doi: 10.1016/j.fertnstert.2009.01.142. [DOI] [PubMed] [Google Scholar]
- 40.Ali KH, Rasoul S, Abbas A, Hasan M, Ehsan S. Crocin prevention of anemia-induced changes in structural and functional parameters of mice testes. Journal of Applied Biomedicine. 2015;13:213–223. [Google Scholar]
- 41.McClain RM, Wolz E, Davidovich A, Pfannkuch F, Bausch J. Subchronic and chronic safety studies with genistein in dogs. Food Chem Toxicol. 2005;43:1461–1482. doi: 10.1016/j.fct.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Strauss L, Makela S, Joshi S, Huhtaniemi I, Santti R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol. 1998;144:83–93. doi: 10.1016/s0303-7207(98)00152-x. [DOI] [PubMed] [Google Scholar]
- 43.Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Comment. J Urol. 2009;181:2669–2670. doi: 10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- 44.Pauli EM, Legro RS, Demers LM, Kunselman AR, Dodson WC, Lee PA. Diminished paternity and gonadal function with increasing obesity in men editorial comment. J Urol. 2009;181:1830–1831. doi: 10.1016/j.fertnstert.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitarygonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ER alpha but not ER beta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 46.Napier ID, Simon L, Perry D, Cooke PS, Stocco DM, Sepehr E, Doerge DR, Kemppainen BW, Morrison EE, Akingbemi BT. Testicular development in male rats is sensitive to a soy-based diet in the neonatal period. Biol Reprod. 2014;90:40. doi: 10.1095/biolreprod.113.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shayu D, Rao AJ. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol Cell Endocrinol. 2006;249:40–50. doi: 10.1016/j.mce.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Han XF, Cao XH, Tang J, Du XG, Zeng XY. Active immunization against GnRH reduces the synthesis of GnRH in male rats. Theriogenology. 2013;80:1109–1116. doi: 10.1016/j.theriogenology.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Zhang HM, Chen SW, Zhang LS, Feng XF. The effects of soy isoflavone on insulin sensitivity and adipocytokines in insulin resistant rats administered with high-fat diet. Nat Prod Res. 2008;22:1637–1649. doi: 10.1080/14786410701869598. [DOI] [PubMed] [Google Scholar]
- 50.Smith GD, Jackson LM, Foster DL. ATL>Leptin regulation of reproductive function and fertility. Theriogenology. 2002;51:73–86. doi: 10.1016/s0093-691x(01)00658-6. [DOI] [PubMed] [Google Scholar]
- 51.Yu WH, Kimura M. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997;94:1023–1223. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abney TO. The potential roles of estrogens in regulating Leydig cell development and function: a review-an oestradiol-mediated process? Steroids. 1999;64:610–617. doi: 10.1016/s0039-128x(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 53.Jessica DS, Morgan S, John D, Mahmoud M, Barbara WK, Frank FB, Edward EM, Benson TA. Developmental exposures of male rats to soy isoflavones impact Leydig cell differentiation. Biol Reprod. 2010;83:488–501. doi: 10.1095/biolreprod.109.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]