Abstract
Background and Aims: MicroRNA-34a (miR-34a) has been shown to be a potential diagnostic and prognostic marker for several cancers. In addition, miR-34a has been reported to suppress osteosarcoma cell proliferation in vitro. However, the clinical value of miR-34a is still unknown. In the present study, we evaluated serum miR-34a level in osteosarcoma patients to explore its diagnostic and prognostic value for this particular malignancy. Methods: Serum from 120 patients with primary osteosarcoma, and 87 sex- and age-matched healthy individuals were obtained. Serum miR-34a level was measured with by a real-time quantitative reverse transcription-polymerase chain reaction assay (qRT-PCR) and correlation with clinicopathological characteristics was further analyzed using t test. Receiver operating curves (ROC), Kaplan-Meier curves, and log-rank analyses as well as Cox proportional hazard models were used to evaluate diagnostic and prognostic significance. Results: The serum miR-34a levels was significantly decreased in the serum of osteosarcoma patients compared to healthy controls (P < 0.001). Low miR-34a had significant association with clinical stage (P = 0.006), distant metastasis (P = 0.002), tumor grade (P = 0.038) and response to chemotherapy (P = 0.017). The Kaplan-Meier curve showed that patients with high miR-34a level survived significantly longer than patients with low miR-34a levels (P = 0.036). Multivariate analysis demonstrated that miR-34a level (P = 0.001) was an independent prognostic biomarker for overall survival. To distinguish osteosarcoma patients from healthy controls, ROC/AUC analysis indicated an AUC of 0.83 (sensitivity 0.68; specificity 0.92). Conclusions: Decreased miR-34a might be related to the metastasis of osteosarcoma and might be a novel diagnostic and prognostic biomarker in osteosarcoma.
Keywords: Osteosarcoma, serum, marker, microRNA-34a
Introduction
Osteosarcoma (OS) is the most common malignancy of bone and among the deadliest cancers in adolescents [1,2]. OS patients are commonly treated with multiagent neoadjuvant chemotherapy, combined with surgery to remove the primary tumor mass and subsequent adjuvant chemotherapy. Introduction of chemotherapy has increased the mean 5-year survival rates of patients with localized disease from 20% in the early 1970s to above 60% at present [3]. However, there has been no impressive progress in improving the survival rate of those with recurrence or metastasis over the last three decades [4]. Unfortunately, most of the current strategies have limited efficacy in the treatment of metastatic and recurrent OS, which remains a major challenge in bone cancer fields. Therefore, there is an urgent need to develop novel early molecular markers of diagnostic and therapeutic targets for osteosarcoma. Detection of cancer biomarkers in human serum is a novel method in diagnosis and prognosis of cancer. The serum level of bone-specific ALP reflects the cellular activity of osteoblasts, and elevated serum level of ALP has been found during bone formation or increased bone turnover [5]. However, there is still no effective serum indicator for diagnosis and prognosis of patients with OS.
MicroRNAs (miRs), a class of small non-coding RNA molecules, play critical roles in a variety of biological events, including development, cell proliferation and cell differentiation [6,7]. MiRNAs negatively regulate gene expression by binding to the 3’-untranslated regions (UTRs) of the corresponding target mRNAs of protein-coding genes, thereby leading to mRNA degradation or translation inhibition [8-10]. Multiple miRs are involved in the invasion and metastasis of different types of cancers, including gastric cancer, breast cancer, hepatocellular carcinoma, colorectal cancer and OS [11-17].
Serum miR-34a is potential biomarker of pancreatic ductal adenocarcinoma [18]. Xiang et al. has reported that reduced serum and intratumoral miR-34a expression levels were independent risk factors for developing BM. Migration and invasion experiments indicated that a reverse correlation existed between miR-34a and HCC tumor migration and invasion [19]. Tian et al. has reported that reduced expression of miR-34a was associated with vascular invasion, and advanced TNM stage. Kaplan-Meier revealed that reduced expression of miR-34a was associated with poor overall survival [20]. Recently, Wu et al. demonstrated that miR-34a was significantly downregulated in osteosarcoma tissues and cell lines, and overexpression of miR-34a inhibited the proliferation of MG-63 and Saos-2 cells. Furthermore, xenograft nude mice model showed that miR-34a inhibited osteosarcoma growth in vivo [21]. Zou et al. demonstrated that miR-34a has a negative regulatory effect on osteosarcoma cell proliferation, migration and invasion [22]. However, the role of serum miR-34a levels in the diagnosis and prognosis of OS has not been reported.
In the present study, we detected the expression levels of miR-34a in serum samples of osteosarcoma patients and healthy individuals. Then, the correlations between serum miR-34a level and clinicopathological factors or overall survival of osteosarcoma patients were evaluated. The prognostic value of miR-34a expression level was demonstrated for overall survival of patients with osteosarcoma.
Materials and methods
Patients and specimens
120 patients diagnosed with osteosarcoma and 87 sex- and age-matched healthy individuals from the affiliated hospital of Qingdao University between March 2007 and July 2012 were recruited in this study. Neither chemotherapy nor radiotherapy had been used in all of the patients before surgery treatment. The clinical stage of the osteosarcoma patients was classified according to the Tumor Node Metastasis (TNM) Classification of Malignant Tumors (Sixth edition) from the Union for International Cancer Control (UICC). Clinical information of patients was obtained from medical records and pathology reports. All of the osteosarcoma patients received regular followed-up. Overall survival time was defined as the time interval from primary surgery to the date of death or last follow-up. Prior patients’ written consent and approval were obtained from the affiliated hospital of Qingdao University according to institutional regulations. We have obtained consent to publish from the participant to report individual patient data.
Total RNA isolation
For each subject, 3 mL venous blood was collected from 207 cases and placed in a test tube. The whole blood samples were incubated at 37°C for 1 h and then centrifuged immediately at 1500 g for 15 min at 4°C. The supernatant serum was stored at -20°C until analysis. Total RNA was isolated from 400 μl serum sample by using mirVana miRNA isolation kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The concentration and quality of total RNA were monitored by NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and Agilent’s 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA was reversely transcribed into cDNA (20 µl solution), and qRT-PCR was conducted for each sample using NCode™ EXPRESS SYBR® GreenER™ miRNA qRT-PCR Kit Universal (Invitrogen Corporation, USA) and Light Cycler 96® Real-Time PCR System (Roche, Germany) in a final 20 µl reaction volume according to the protocol of manufacturer. At the end of PCR cycles, melting curve analyses were performed to validate the specific generation of the expected PCR products. The Cq uniformity (SD < 0.2) of Real-Time PCR System had been detected by measuring the samples in triplicates. In order to avoid test errors that were caused by pollution we set up two negative controls per test. miR-34a primers were purchased from GENEWIZ (Suzhou, China). The relative expression levels of miR-34a for qRT-PCR were normalized by the mean Cq value of U6 snRNA, and were calculated utilizing the 2-ΔΔCt method.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Associations between serum levels of miR-34a and clinicopathological characteristics or survival of osteosarcoma patients were evaluated using t test. Nonparametric received operating characteristic (ROC) curves were generated to assess diagnostic efficiency. Disease-free survival time was measured as the time from the surgery day until the date of cancer reoccurrence or patient death or the day of the last live follow-up to represent disease progression. Survival probabilities were estimated with a Kaplan-Meier analysis, and significant differences were analyzed with a log-rank test. Multivariate analysis of prognostic factors was performed using a COX regression analysis. All P values were two-sided and P < 0.05 was considered statistically significant. The statistical analyses of all experimental data were conducted using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
In this study, it was shown that the demographic and clinicopathological data of the cases in Table 1. Our study comprised of 120 osteosarcoma patients. The mean age was 28.9 ± 10.4 years. Of the 87 healthy controls, male:female was 49:38; the age ≤ 20:> 20 were 51:36. All patients featured osteosarcoma with I-III Enneking stage and received the same curative resection treatment. During the follow-up period, 49 patients (40.8%) died of disease. Distant metastases developed in 37 patients after the original diagnosis. Of these patients, 7 had bone metastases and 30 had lung metastases (2 patients had both bone and lung metastases). The median overall and disease-free survival of patients was 32 months (95% confidence interval [CI], 31.3-45.3 months) and 26 months (95% CI: 24.6-37.1 months), respectively.
Table 1.
Osteosarcoma patient characteristics
| Characteristic | Osteosarcoma (n = 120) | miR-34a level | p-value |
|---|---|---|---|
| Age (Years) | 0.142 | ||
| ≤ 20 | 72 | 4.08 ± 0.31 | |
| > 20 | 48 | 4.21 ± 0.36 | |
| Gender (n, %) | 0.263 | ||
| Male | 69 | 4.17 ± 0.36 | |
| Female | 51 | 4.13 ± 0.32 | |
| Tumor location | 0.562 | ||
| Femur | 73 | 4.18 ± 0.36 | |
| Tibia | 47 | 4.12 ± 0.33 | |
| Tumor size (cm) | 0.094 | ||
| < 6 | 78 | 4.68 ± 0.42 | |
| ≥ 6 | 42 | 3.87 ± 0.29 | |
| Clinical stage | 0.006 | ||
| IIA | 28 | 5.12 ± 0.64 | |
| IIB | 69 | 3.94 ± 0.25 | |
| III | 23 | 2.83 ± 0.16 | |
| Tumor grade | 0.038 | ||
| Low | 52 | 3.43 ± 0.31 | |
| High | 68 | 5.04 ± 0.38 | |
| Distant Metastasis | 0.004 | ||
| Yes | 37 | 2.34 ± 0.13 | |
| No | 83 | 5.79 ± 0.68 | |
| Response to chemotherapy | 0.017 | ||
| Poor | 89 | 3.66 ± 0.24 | |
| Good | 31 | 5.37 ± 0.32 |
Serum miR-34a in osteosarcoma patients
qRT-PCR was conducted to determine the levels of serum miR-34 in 120 patients with osteosarcoma and 87 sex- and age-matched healthy controls. The relative serum miR-34a level was 0.72 ± 0.13 patients with osteosarcoma, which was significantly decreased in comparison with those of healthy controls (4.16 ± 0.34, P < 0.001).
Serum miR-34a and association with clinicopathologic features
The correlations between serum miR-34a and clinicopathologic characteristics of tumors are shown in Table 2. Serum miR-34a level was significantly lower in stage III, distant metastases, low stage and poor response to chemotherapy than those with IIA or IIB stages, no metastases, high stage and good response to chemotherapy. However, no significant difference was observed between the serum miR-34a and patient gender, age, tumor location, tumor size and histological grade.
Table 2.
Multivariate survival analysis of overall survival in 120 osteosarcoma patients
| Variables | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age | 0.58 | 0.42-1.17 | 0.286 |
| Gender | 1.29 | 0.93-2.77 | 0.573 |
| Tumor location | 0.87 | 0.64-1.54 | 0.742 |
| Tumor size | 1.23 | 0.89-2.67 | 0.193 |
| Clinical stage | 2.84 | 2.16-7.45 | 0.026 |
| Tumor grade | 1.47 | 1.03-2.48 | 0.12 |
| Distant Metastasis | 4.72 | 3.86-10.15 | 0.017 |
| Response to chemotherapy | 0.93 | 0.67-2.34 | 0.163 |
| miR-34a level | 3.18 | 2.87-9.56 | 0.001 |
Decreased expression of miR-34a in serum level associates with poor prognosis in osteosarcoma patients
We used the 4.16 as the cutoff value. Serum miR-34a value > 4.16 was the high levels and serum miR-34a value ≤ 4.16 was the low levels. The overall survival (P = 0.036, Figure 1A) and disease-free survival (P = 0.013, Figure 1B) of the patients with osteosarcoma was assessed using Kaplan-Meier survival analysis. The Kaplan-Meier curves for overall survival showed that osteosarcoma patients with high serum miR-34a level survived significantly longer than those with low miR-34a levels. Multivariate Cox proportional hazards model analysis suggested that expression level of serum miR-34a was a significant independent prognostic factor of overall survival for patients with osteosarcoma (P = 0.001, shown in Table 2).
Figure 1.
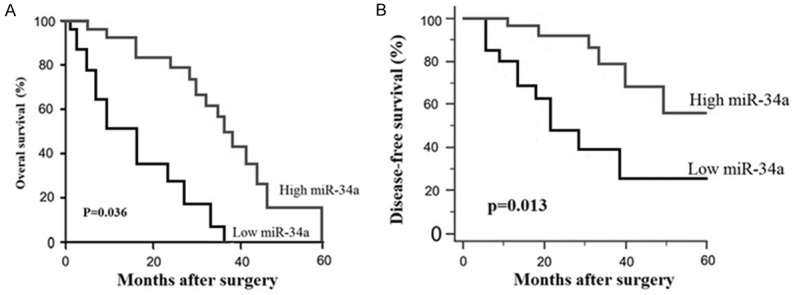
Kaplan-Meier survival curves for osteosarcoma patients with high or low expression of miR-34a. The overall survival curves and disease free survival curves for two groups of osteosarcoma patients with low and high expression of serum miR-34a. A. The overall survival rate of osteosarcoma patients with high miR-34a expression were significantly higher than those with low miR-34a; B. The disease-free survival rate of osteosarcoma patients with high miR-34a level were significantly higher than those with low miR-34a level.
ROC analysis of serum miR-34a level in osteosarcoma patients
ROC/AUC analysis revealed sensitivity and specificity of different serum miR-34a levels. ROC curve analysis illustrated that serum miR-34a level was a potential biomarker for screening OS patients form controls (AUC of 0.83 (sensitivity 0.68; specificity 0.92) (Figure 2A). To assess the potential of miR-34a as a diagnostic biomarker to distinguish advanced cancer patients (IIB/III) from early clinical stages (IIA), we got an AUC of 0.79 (95% CI 0.73-0.95) with a sensitivity of 0.83 and a specificity of 0.70 (Figure 2B).
Figure 2.
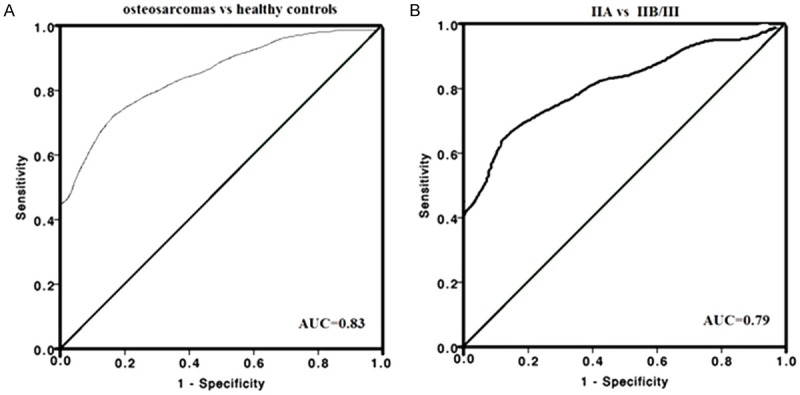
ROC analysis for serum miR-34a for distinguishing osteosarcoma patients from healthy controls. A. ROC for serum miR-34a differentiating osteosarcoma patients from controls; B. Distinguishing osteosarcoma patients with IIB/III stages from those with IIA stages.
Discussion
Osteosarcoma derives from primitive bone-forming mesenchymal cells and is the most common type of primary bone malignancy [23]. Over the past few decades, the introduction of combinatorial chemotherapy has improved the 5-year overall survival rate of patients with osteosarcoma to approximately 50-60% [24]. However, osteosarcoma-related morbidity remains high due to the difficulty of early diagnosis and the lack of efficient therapeutic approaches for osteosarcoma. Therefore, it is necessary to identify highly sensitive and specific diagnostic and prognostic biomarkers to diagnose osteosarcoma at an early stage and initiate aggressive therapy.
Overexpression of miR-34a reportedly suppresses tumor progression and leads to improved prognoses, whereas reduced miR-34a expression is associated with poor overall survival in several cancers [25-27]. Gao et al. found that lower miR-34a expression was correlated with reduced progression-free survival and overall survival in glioma patients [28], but Genovese et al. found that lower miR-34a expression led to improved overall survival in glioblastoma patients [29]. However, the role of miR-34a as a serum diagnostic and prognostic biomarker has not been previously explored in osteosarcoma patients. In the present study, we quantified the serum expression levels of miR-34a in osteosarcoma patients and healthy controls and then assessed the potential value of miR-34a as a serum diagnostic and prognostic marker in osteosarcoma patients.
The results from our study show for the first time that the expression of miR-34a was remarkably decreased in the serum of osteosarcoma patients. Furthermore, we showed that the expression of miR-34a could be used to discriminate osteosarcoma from healthy controls, with a specificity of 68% and sensitivity 92%. Notably, decreased serum miR-34a levels were found to be significantly associated with distant cancer metastasis and advanced clinical stage in osteosarcoma patients, which suggests that miR-34a might act as a tumor suppressor in the development of osteosarcoma. In addition, we proved that low levels of miR-34a significantly correlated with poor prognosis in OS patients. Therefore, we suggested that serum miR-34a levels could be a diagnostic biomarker and prognostic factor in the progression of OS.
Chemotherapy is an important treatment modality for osteosarcoma. However, it often fails because of chemoresistance. The underlying mechanisms of chemoresistance are still poorly understood. In recent years, growing evidence demonstrate miR-34a has a key role in tumor cell responses to chemotherapeutic agents and may serve as an effective antitumor therapeutic target [30-34]. Li et al. has reported that increased expression of a panel of tumor suppressive microRNAs (miRNAs), including miR-34a, miR-143, miR-145, and miR-200b/c that were typically lost in osteosarcoma, was observed during diallyl trisulphide treatment, which suggested that miRNAs may be involved in the chemotherapeutic response of osteosarcoma [35]. In the present study, we found that serum miR-34a was higher in OS patients with good response to chemotherapy than those in patients with poor response to chemotherapy, suggesting that miR-34a could be as a marker to predict chemosensitivity in OS patients.
In summary, our findings provide the first hints that serum miR-34a level may be a useful diagnostic and prognostic biomarker that could be used for risk stratification and selection of osteosarcoma patients. In addition, the potential role of miR-34a in osteosarcoma warrants further investigation.
Acknowledgements
Shandong Province College Science and Technology Project (J15LL10, ZR2015050013) and Shandong Natural Research Foundation (zr2016hm31) were received in support of this work.
Disclosure of conflict of interest
None.
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. In: Institute NC, editor. NIH Pub No 06-5767. Bethesda, MD: SEER Cancer Statistics Review; 2013. pp. 1975–2010. [Google Scholar]
- 3.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65:904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 6.Sun K, Lai EC. Adult-specific functions of animal microRNAs. Nat Rev Genet. 2013;14:535–548. doi: 10.1038/nrg3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–488. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng B, Dong TT, Wang LL, Zhou HM, Zhao HC, Dong F, Zheng MH. Colorectal cancer migration and invasion initiated by microRNA-106a. PLoS One. 2012;7:e43452. doi: 10.1371/journal.pone.0043452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberger N, Walker RC, Kim CH, Winter S, Hunter KW. Inherited variation in miR-290 expression suppresses breast cancer progression by targeting the metastasis susceptibility gene Arid4b. Cancer Res. 2013;73:2671–2681. doi: 10.1158/0008-5472.CAN-12-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Fang F, Chang R, Yang L. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor beta receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology. 2013;58:205–217. doi: 10.1002/hep.26315. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J, Ye Y, Wang Q, Long Z, Zhou Y, Du C, He X, Shi Y. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 15.Nakka M, Allen-Rhoades W, Li Y, Kelly AJ, Shen J, Taylor AM, Barkauskas DA, Yustein JT, Andrulis IL, Wunder JS, Gorlick R, Meltzer PS, Lau CC, Man TK. Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget. 2017 doi: 10.18632/oncotarget.18236. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiwara T, Uotani K, Yoshida A, Morita T, Nezu Y, Kobayashi E, Yoshida A, Uehara T, Omori T, Sugiu K, Komatsubara T, Takeda K, Kunisada T, Kawamura M, Kawai A, Ochiya T, Ozaki T. Clinical significance of circulating miR-25-3p as a novel diagnostic and prognostic biomarker inosteosarcoma. Oncotarget. 2017;8:33375–33392. doi: 10.18632/oncotarget.16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu J, Sun Y, Guo Q, Niu D, Liu B. Serum miR-95-3p is a diagnostic and prognostic marker for osteosarcoma. Springerplus. 2016;5:1947. doi: 10.1186/s40064-016-3640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alemar B, Izetti P, Gregório C, Macedo GS, Castro MA, Osvaldt AB, Matte U, Ashton-Prolla P. miRNA-21 and miRNA-34a are potential minimally invasive biomarkers for the diagnosis of pancreatic ductal adenocarcinoma. Pancreas. 2016;45:84–92. doi: 10.1097/MPA.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 19.Xiang ZL, Zhao XM, Zhang L, Yang P, Fan J, Tang ZY, Zeng ZC. MicroRNA-34a expression levels in serum and intratumoral tissue can predict bone metastasisin patients with hepatocellular carcinoma. Oncotarget. 2016;7:87246–87256. doi: 10.18632/oncotarget.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian YW, Shen Q, Jiang QF, Wang YX, Li K, Xue HZ. Decreased levels of miR-34a and miR-217 act as predictor biomarkers of aggressive progression and poor prognosis in hepatocellular carcinoma. Minerva Med. 2017;108:108–113. doi: 10.23736/S0026-4806.16.04616-4. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Zhong D, Gao Q, Zhai W, Ding Z, Wu J. MicroRNA-34a inhibits human osteosarcoma proliferation by downregulating ether à go-go 1 expression. Int J Med Sci. 2013;10:676–82. doi: 10.7150/ijms.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y, Huang Y, Yang J, Wu J, Luo C. miR-34a is downregulated in human osteosarcoma stemlike cells and promotes invasion, tumorigenic ability and self-renewal capacity. Mol Med Rep. 2017;15:1631–1637. doi: 10.3892/mmr.2017.6187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16:543–556. doi: 10.1586/14737140.2016.1168697. [DOI] [PubMed] [Google Scholar]
- 24.Abarrategi A, Tornin J, Martinez-Cruzado L, Hamilton A, Martinez-Campos E, Rodrigo JP, González MV, Baldini N, Garcia-Castro J, Rodriguez R. Osteosarcoma: cells-of-origin, cancer stem cells, and targeted therapies. Stem Cells Int. 2016;2016:3631764. doi: 10.1155/2016/3631764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marino MT, Grilli A, Baricordi C, Manara MC, Ventura S, Pinca RS, Bellenghi M, Calvaruso M, Mattia G, Donati D, Tripodo C, Picci P, Ferrari S, Scotlandi K. Prognostic significance of miR-34a in Ewing sarcoma is associated with cyclin D1 and ki-67 expression. Ann Oncol. 2014;25:2080–2086. doi: 10.1093/annonc/mdu249. [DOI] [PubMed] [Google Scholar]
- 26.Peurala H, Greco D, Heikkinen T, Kaur S, Bartkova J, Jamshidi M, Aittomäki K, Heikkilä P, Bartek J, Blomqvist C, Bützow R, Nevanlinna H. MiR-34a expression has an effect for lower risk of metastasis and associates with expression patterns predicting clinical outcome in breast cancer. PLoS One. 2011;6:e26122. doi: 10.1371/journal.pone.0026122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamieson NB, Morran DC, Morton JP, Ali A, Dickson EJ, Carter CR, Sansom OJ, Evans TR, McKay CJ, Oien KA. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clin Cancer Res. 2012;18:534–545. doi: 10.1158/1078-0432.CCR-11-0679. [DOI] [PubMed] [Google Scholar]
- 28.Gao H, Zhao H, Xiang W. Expression level of human miR-34a correlates with glioma grade and prognosis. J Neurooncol. 2013;113:221–228. doi: 10.1007/s11060-013-1119-1. [DOI] [PubMed] [Google Scholar]
- 29.Genovese G, Ergun A, Shukla SA, Campos B, Hanna J, Ghosh P, Quayle SN, Rai K, Colla S, Ying H, Wu CJ, Sarkar S, Xiao Y, Zhang J, Zhang H, Kwong L, Dunn K, Wiedemeyer WR, Brennan C, Zheng H, Rimm DL, Collins JJ, Chin L. MicroRNA regulatory network inference identifies miR-34a as a novel regulator of TGF-β signaling in glioblastoma. Cancer Discov. 2012;2:736–749. doi: 10.1158/2159-8290.CD-12-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 31.Zenz T, Mohr J, Eldering E, Kater AP, Bühler A, Kienle D, Winkler D, Dürig J, van Oers MH, Mertens D, Döhner H, Stilgenbauer S. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–3808. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 32.Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rokhlin OW, Scheinker VS, Taghiyev AF, Bumcrot D, Glover RA, Cohen MB. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol Ther. 2008;7:1288–1296. doi: 10.4161/cbt.7.8.6284. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Dong K, Gao P, Long M, Lin F, Weng Y, Ouyang Y, Ren J, Zhang H. microRNA-34a sensitizes lung cancer cell lines to DDP treatment independent of p53 status. Cancer Biother Radiopharm. 2013;28:45–50. doi: 10.1089/cbr.2012.1218. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhang J, Zhang L, Si M, Yin H, Li J. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis. 2013;34:1601–1610. doi: 10.1093/carcin/bgt065. [DOI] [PubMed] [Google Scholar]


