Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that promote the progression of cancer by negatively regulating gene expression. Down-regulation of miR-483-5p was reported in a number of cancers. However, the biological functions of miR-483-5p in esophageal squamous cell carcinomas are not fully understood. In this study, the expression levels of miRNAs in the immortalized human esophageal epithelial cell line SHEE and the malignantly transformed esophageal carcinoma cell line SHEEC were examined by miRNA microarray chip. The expression level of miR-483-5p was verified by a quantitative reverse transcription-polymerase chain reaction. Growth, apoptosis, and colony formation ability were also examined in SHEEC cells after transfection with inhibitors targeting miR-483-5p. And the target genes of miR-483-5p were predicted using bioinformatics approaches and the expression profile of SHEEC cells transfected with the miRNA inhibitors. protein levels of the target gene in SHEEC cells with a control or miRNA inhibitors were measured using Western blotting. The expression of miR-483-5p was elevated in SHEEC cells as compared to the SHEE cells. Silencing of miR-483-5p expression in SHEEC cells inhibited both the proliferation and formation of colonies and increased apoptosis. We also identified hepatocyte nuclear factor 4α (HNF4A) as a target of miR-483-5p in SHEEC cells. Knockdown of HNF4A recapitulated the effects of miR-483-5p. Our data showed that the miR-483-5p/HNF4A axis affected the malignant transformation of immortalized human esophageal epithelial cells and is a potential therapeutic target for ESCC.
Keywords: MiR-483-5p, SHEE/SHEEC, malignant transformation, HNF4A
Introduction
Esophageal cancer is the eighth most common malignancy and the sixth most common cause of cancer-related death worldwide. In East Asia, esophageal squamous cell carcinoma (ESCC) is the leading type of esophageal cancer [1-3]. A number of epidemiological investigations determined that esophageal carcinogenesis and the malignant development of esophageal cancers are complex processes that involve multiple etiologic factors, including genetic background, environmental stimuli, nutritional conditions and cultural habits [4-6]. Despite these epidemiological observations, the biological mechanisms involved in ESCC occurrence and progression are not fully understood [7,8].
MicroRNAs (miRNAs) are small, non-coding RNAs that negatively regulate gene expression by binding to the 3’-untranslated region of target mRNAs, and cause translational repression or degradation. Past studies found that the deregulation of miRNAs was associated with human malignancies [9,10], because they function as oncogenes or tumor suppressors and promote tumor initiation and progression. Therefore, the discovery of miRNAs provides new opportunities to explore the molecular mechanisms of cancer. In our study, we investigated the role of miRNAs in ESCC carcinogenesis in vitro by using the immortalized human esophageal epithelial cell line (SHEE) [11,12], which had a high metabolic capacity from its expression of several biotransformation enzymes. We also used the malignantly transformed esophageal carcinoma cell line (SHEEC) [13], which was produced by exposing SHEE cells to the chemical carcinogen TPA (12-O-tet-radeanoy-l phorbol-13-acetate).
We found that the expression of miR-483-5p was significantly up-regulated (>2-fold) in SHEEC cells compared to those in SHEE cells. Subsequent experiments of cellular functions demonstrated that miR-483-5p acted as an oncogene by affecting cell viability, cell apoptosis, and colony formation of ESCC. Furthermore, the hepatocyte nuclear factor 4 alpha (HNF4A) was identified as a direct functional target of miR-483-5p in ESCC.
Materials and methods
Cell cultures
The SHEE and SHEEC cell lines were purchased from the Central Laboratory of the Tumor Hospital (Medical College of Shantou University, China). The cell lines were routinely cultivated in culture medium 199 (Gibco-Life Technologies, Carlsbad, CA) with 10% bovine serum and 100 U penicillin/streptomycin in a humidified atmosphere of 5% CO2 and 95% air. The cell shape and size, anchorage-dependent growth, and contact-inhibited growth were measured by phase-contrast microscopy.
The MiRNA microarray assay
The microarray assay was performed using a service provider (LC Sciences, Houston, TX). Briefly, the SHEE and SHEEC cells were washed with precooled phosphate-buffered saline and the total RNA was harvested using a TRIzol (Invitrogen, Carlsbad, CA) and the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands) according to manufacturer protocol. The assay began with 4-8 μg of total RNA that was 3’-extended with a poly(A) tail using poly(A) polymerase. An oligonucleotide tag was then ligated to the poly(A) tail for later fluorescent dye staining. Hybridization was performed overnight on a μParaflo microfluidic chip using a micro-circulation pump (Atactic Technologies, Houston, TX) [14,15]. On the microfluidic chip, each detection probe consisted of a chemically modified nucleotide coding segment complementary to target microRNA (from the miRBase, http://www.mirbase.org/) or other RNA (control or customer defined sequences) and a spacer segment of polyethylene glycol to extend the coding segment away from the substrate. The detection probes were made by in situ synthesis using photo-generated reagent chemistry. The hybridization melting temperatures were balanced by chemical modifications of the detection probes. Hybridization was performed in a 100 μL 6X SSPE buffer (0.90 M NaCl, 60 mM Na2HPO4, 6 mM EDTA, pH 6.8) containing 25% formamide at 34°C. After RNA hybridization, tag-conjugating Cy3 dye was circulated through the microfluidic chip for dye staining. Fluorescent images were collected using the GenePix 4000B laser scanner (Molecular Devices, Sunnyvale, CA) and digitized using Array-Pro image analysis software (Media Cybernetics, Rockville, MD). The data were analyzed by first subtracting the background and then normalizing the signals using a LOWESS filter (Locally-weighted Regression) [15,16].
Quantitative reverse-transcription-polymerase chain reaction
Based on the microarray results, the expression levels of miRNAs significantly different between the SHEE and SHEEC cells were examined using a quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Briefly, cDNA from both cell lines was reverse transcribed with a miScript Reverse Transcription Kit (Qiagen), and then qRT-PCR was determined by a miScript SYBR Green PCR Kit (Qiagen). An amplification reaction protocol was implemented for 40 cycles at 95°C for 15 seconds and 60°C for 30 seconds. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence passed through the fixed threshold. All miRNA expression levels were normalized to that of U6 by the 2-ΔΔCt method. All samples were processed in triplicate.
Analysis of cell viability and apoptosis
For the cell viability assay, SHEEC cells were plated in 96-well plates (3×103 cells/well). After 24 hours they were transfected with miR-483-5p inhibitor or the negative control group (NC) for 24, 48 and 72 hours. The absorbance at 450 nm was determined by a Synergy 2 microplate reader (BioTek, Winooski, VT) to measure the transfection rate. Cell viability was evaluated with the Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo), and was expressed as the percentage according to the following formula: (ODtest - ODblank)/(ODcontrol - ODblank), where ODtest was the OD of the transfected cells, ODcontrol was the OD of SHEEC cells, and ODblank was the OD of the wells without SHEEC cells. For the apoptosis assay, SHEEC cells were plated in six-well plates (5×104 cells/well), cultured for 24 hours, and then transfected with a miR-483-5p inhibitor or NC. After another 48 hours, the cells were harvested using 0.25% trypsin, washed twice with ice-cold PBS, and resuspended in a 500 µL binding buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). The cells were stained with 5 µL annexin-V-fluoresein-5-isothiocyanate (FITC) and 5 µL propidium iodide (PI) for 15 minutes in the dark at room temperature, and analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ). The results were expressed as the percentage of apoptotic cells among the total number of cells counted.
The colony formation assay
A colony formation assay was used to identify the colony forming ability of SHEEC cells and transfected with miR-483-5p inhibitor cells. Cells were trypsinized, counted and seeded for the colony formation assay in 6-cell plates at 1000 cells per well. During colony growth, the culture medium was replaced every 3 days. The colony was counted only if it contained more than 50 cells, and the final number of colonies was counted on the 15th day after seeding. The colony formation rate was calculated using the following formula: Colony formation rate = (number of colonies/number of seeded cells) ×100‰. Each treatment was performed in triplicate.
Western blotting
Whole cell extracts were prepared with a cell lysis reagent (Sigma-Aldrich, St. Louis, MO) according to the manual, and the protein was quantified by a BCA assay (Pierce, Rockford, IL). The protein samples then were separated by SDS-PAGE (10%) and detected by Western blotting using polyclonal (rabbit) anti-HNF4A (Santa Cruz Bio-technology, Santa Cruz, CA). Goat anti-rabbit IgG (Pierce, Rockford, IL) secondary antibodies conjugated to horseradish peroxidase and ECL detection systems (Super Signal West Femto., Pierce) were used for detection.
The luciferase reporter assay
The 3’-UTR sequence of HNF4A predicted to interact with the miR-483-5p or a mutated sequence within the target sites was synthesized and inserted into the XbaI and FseI sites of the pGL3 control vector (Promega, Madison, WI). These constructs were called pGL3-HNF4A-3’UTR-wt or pGL3-HNF4A-3’UTR-mut. For the reporter assay, SHEEC cells were plated onto 24-well plates and transfected with the above constructs and P-miR-483-5p or P-miR-control vectors using the GenJet Plus DNA in vitro transfection reagent (SignaGen, MD). A Renilla luciferase vector pRL-SV50 (Promega, Madison, WI) was also co-transfected to normalize the differences in transfection efficiency. After 48 hours, the cells were harvested and assayed using the dual-luciferase reporter assay system (Promega, Madison, WI) according to manufacturer protocol. The experiment was performed in duplicate in three independent experiments.
Statistical analysis
All statistical analysis was determined by SPSS 17.0 software (SPSS, Chicago, IL). Values were expressed as mean ± SD. The two-tailed Student’s test was used to compare both groups. Multiple-group comparisons were assessed using a one-way analysis of variance, and Tukey’s post hoc test was used to determine which groups differed from each other. Differences were considered significant at P<0.05.
Result
Microarray identification of differentially expressed miRNAs between SHEE and SHEEC cell lines
In this study, a miR microarray was used to profile the changes in miRNA expression levels between two cell lines: the immortalized human esophageal cell line SHEE and the malignantly transformed esophageal carcinoma cell line SHEEC. A cluster analysis and a volcano plot were used to identify miRNAs with the most significant changes in expression. We then identified 35 miRNA genes (13 upregulated and 21 downregulated) with different expressions (P<0.05) in SHEEC cells when compared with those in SHEE cells (Figure 1). Seven of these miRNA genes that were differently expressed had a p value of <0.01. The miRNAs with different expressions with at least a two-fold change had signals ≥2000 as shown in Table 1.
Figure 1.
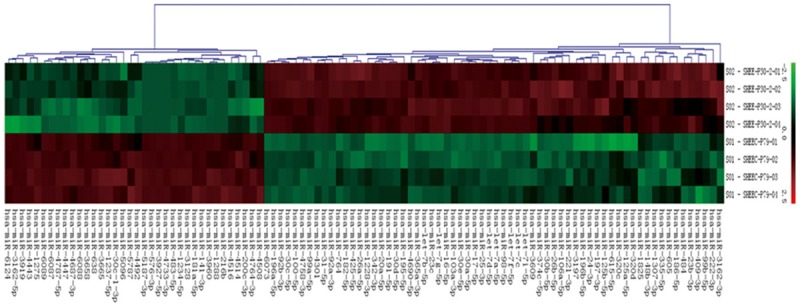
Hierarchical cluster analysis of miRs expression. The heat map diagram shows the result of the two-way hierarchical clustering of genes and samples. Each column represents a miRs and each row represents a sample. The miRs clustering tree is shown on the bottom, and the sample clustering tree is shown at the right. The color scale at the top illustrates the relative expression level of a miRs.
Table 1.
MiRs differentially expressed in SHEE cells and S
| Upregulation of miRs | Fold (>2) | P-value | Downregulation of miRs | Fold (>1) | P-value |
|---|---|---|---|---|---|
| hsa-miR-483-5p | 3.59 | 0.000 | hsa-miR-25-3p | 1.81 | 0.000 |
| hsa-miR-181a-5p | 1.85 | 0.000 | hsa-miR-92b-3p | 2.30 | 0.000 |
| hsa-miR-4787-5p | 2.43 | 0.000 | hsa-miR-92a-3p | 1.69 | 0.001 |
| hsa-miR-98-5p | 1.70 | 0.000 |
The expression of miR-483-5p increased in SHEEC cells
Based on the miRNA microarray analysis, we focused on the miRNAs that were the most significantly up-regulated in SHEEC in comparison to SHEE. We considered their possible regulatory actions in carcinogenesis and examined their profiles using real-time RT-PCR. Only the miR-483-5p expression levels were substantially increased in SHEEC cells when compared to those in SHEE cells (Table 2).
Table 2.
Identification of differentially regulated miRs by qRT-PCR
| miRs | Sequence 5→3’ | qRT-PCR (SHEEC/SHEE) | P-value |
|---|---|---|---|
| Upregulation of miRs | |||
| hsa-miR-483-5p | CUCUAG UAGU GCCGGUCGGAGA | 5.25 | 0.000 |
| hsa-miR-181a-5p | AACAUUCAACGCUGUCGGUGAGU | 2.31 | 0.000 |
| hsa-miR-4787-5p | GCGGGGGUGGCGGCGGCAUCCC | 3.23 | 0.000 |
| Downregulation of miRs | |||
| hsa-miR-25-3p | CGGGACUGGCCAGUGUUGAG | 1.59 | 0.000 |
| hsa-miR-92b-3p | UAUUGCACUCGUCCCGGCCUCC | 1.06 | 0.070 |
| hsa-miR-92a-3p | UAUUGCACUUGUCCCGGCCUGU | 4.53 | 0.000 |
| hsa-miR-98-5p | UGAGGUAGUAAGUUGUAUUGUU | 2.24 | 0.000 |
Knockdown of miR-483-5p inhibited proliferation in SHEEC cells and induced apoptosis
To determine the role of miR-483-5p in the SHEEC cell growth and apoptosis, SHEEC cells were transfected with miR-483-5p inhibitors. Knock-down of miR-483-5p significantly suppressed cell viability (Figure 2A). In contrast, the rate of apoptosis in the miR-483-5p inhibitors-transfected group was significantly increased when compared to the NC-transfected group (19.63 ± 2.89% vs. 4.41 ± 1.67%; Figure 2B). Therefore, the suppression of cell growth observed after the knockdown of miR-483-5p in SHEEC cells may have been caused by apoptosis.
Figure 2.
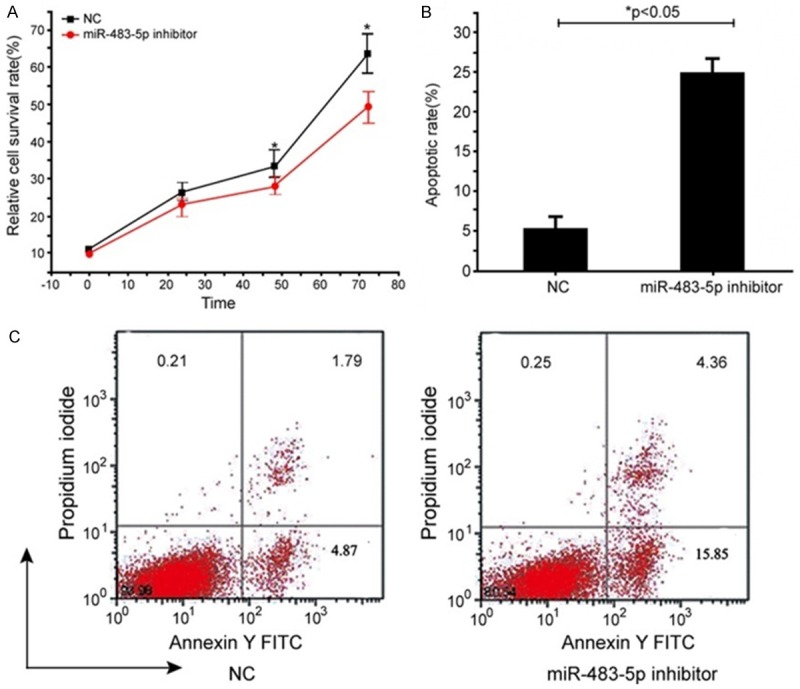
Changes in cell viability and apoptosis after knockdown of miR-483-5p. SHEEC cells were transfected with inhibitors and negative control (NC). At 48 hours, post-transfection and cell proliferation were measured by the CCK-8 assay. Relative cell survival rates in the inhibitors group decreased significantly compared with the transfectedSHEEC cells group (NT) (*P<0.05) (A). Apoptosis was determined by a flow cytometry analysis. Knockdown of miR-483-5p led to a significant increase in apoptosis of SHEEC cells at 48 hours post-transfection, compared with the NT groups (*P<0.05) (B). Representative pictures of the apoptosis assay are shown in (C).
Knockdown of miR-483-5P inhibited colony growth
Next, we examined whether knockdown of miR-483-5p affected the degree of cell malignancy by performing colony formation assays. The results showed that anchorage-independent colony formations in the miR-483-5p inhibitors-transfected group were markedly lower than those in the NC-transfected group (24.4 ± 4.49‰ vs. 52.27 ± 3.57‰; Figure 3). Therefore, the expression of miR-483-5P supported anchorage-independent colony growth of SHEEC cells and could function as an oncogene.
Figure 3.
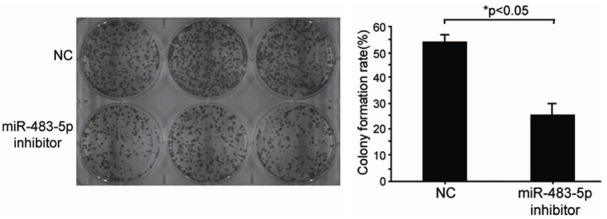
Changes of anchorage-independent growth by knockdown of miR-483-5p. SHEEC cells were properly injected with inhibitors. The cells were harvested by trypsinization and seeded in six-well plates. The colony forming experiments were performed in soft agarose. Knockdown of miR-483-5p (inhibitors) significant reduced colony formation in SHEEC cells, compared with the NC and SHEEC cells (NT) groups (*P<0.05). The data are the mean ± SD of three replicate experiments.
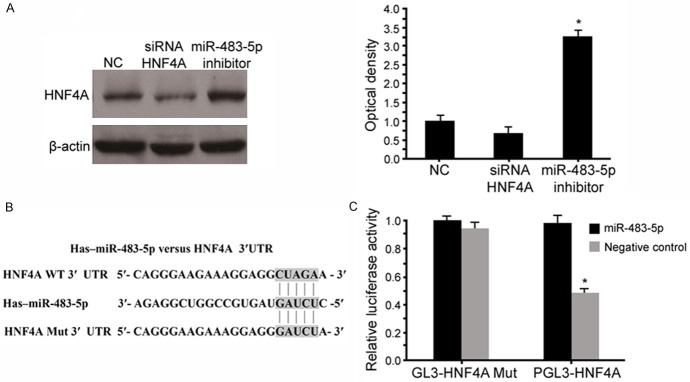
miR-483-5p directly targeted HNF4A
To better understand the underlying molecular mechanisms, we performed a bioinformatics analysis using www.mirco-RNA.org, miRNA-PicTar, and TargetScan to predict the possible target gene of miR-483-5p. We found that HNF4A contained theoretical miR-483-5p binding sites in its 3’UTR. To verify these results, we constructed luciferase reporter vectors containing the wild-type (Wt) or mutant (Mut) miR-483-5p target sequences of the HNF4A 3’-UTR (Figure 4A). Overexpression of miR-483-5p significantly inhibited the luciferase activity of the Wt HNF4A 3’-UTR reporter gene, but not the Mut reporter gene (Figure 4C). Next, we transfected HNF4A siRNAs in SHEEC cells. A Western blot analysis revealed that HNF4A expression was significantly decreased by HNF4-A siRNA when compared to the negative control group (scramble control siRNA) (P<0.05). In addition, the inhibition of miR-483-5p markedly increased the expression of HNF4A (Figure 4B), but the silenced HNF4A did not affect the miR-483-5p expression. As such, HNF4A was a direct target of miR-483-5p in SHEEC cells.
Figure 4.
HNF4A is a direct target of miR-483-5p. miR-483-5p binding sites in the HNF4A 3’UTR region. A Western blot analysis demonstrated that the transfection of miR-483-5p reduced HNF4A protein expression (A). HNF4A mutation indicates the HNF4A 3’UTR with a mutation in miR-483-5p binding sites (B). Relative luciferase assay comparing the pGL3-HNF4A and pGL3-HNF4A mutation vectors in SHEEC cells. Firefy luciferase activity was normalized to Renilla luciferase activity (C). Values are expressed as the mean ± standard deviation. *P<0.05 vs. control.
Discussion
MiR is one of the most important post-transcriptional regulators in the initiation and development of a variety of tumors, including those in ESCC [17-22]. Strong evidence verified the important role of miR in common types of cancer. Among the miRNAs associated with ESCC, oncogenic miR-25 was proven to enhance the motility of ESCC cells by redistributing adherens junctions and activating betacatenin signaling [23]. The tumor suppressor miR-21 inhibited ESCC cell growth and its invasive abilities and also promoted apoptosis by targeting FASL, TIMP3, and RECK [24]. However, only recently were studies conducted regarding the role of miR in chemically induced carcinogenesis of ESCC.
In this study, we used SHEEC cells, generated by treatment of SHEE cells with TPA to investigate the role of miRNAs in chemical carcinogenesis. We found that the expression of miR-483-5p was elevated in SHEEC cells when compared with those in the parental SHEE cells. To further explore the function of miR-483-5p in ESCC, we decreased its expression with inhibitors. After knockdown of miR-483-5p in SHEEC cells, we observed significant inhibition of proliferation concomitant with increased apoptosis. In addition, we found that knockdown of miR-483-5p effectively inhibited colony growth of SHEEC cells. Therefore, miR-483-5p was involved in carcinogenesis through inhibition of apoptosis.
The first identification of miR-483 was in human fetal liver. Recently, miR-483 was shown to be dysregulated and associated with lower disease-specific survival rates in some cancers [25-27]. Despite the oncogenic role of miR-483 as determined by previous studies, the role of miR-483 in tumorigenesis and molecular mechanisms through which miR-483 regulates carcinogenesis remains unknown. Here, we demonstrated that miR-483-5p expression levels were higher in SHEEC cells than in SHEE cells. According to the miRNA target prediction website www.mirco-RNA.org, miRNA-PicTar and TargetScan identified HNF4A as a possible target of miR-483-5p. Our results showed that miR-483-5p directly targeted the 3’UTR of HNF4A, as its overexpression was associated with suppression of luciferase activity in a reporter plasmid driven by the HNF4A-3’UTR.
In addition, we observed significant upregulation of HNF4A protein levels following miR-483-5p knockdown, which indicated the post-transcriptional regulation of HNF4A by targeting its 3’UTR. HNF4A is considered the master regulator of hepatocyte differentiation. Recent studies found a novel role for HNF4A in the regulation of cell proliferation within multiple tissues, including the liver, pancreas, and kidney [28-31]. The loss of HNF4A resulted from the induction of EMT genes and oncogenic transformation [32,33]. Our results showed that the overexpression of miR-483-5p was a possible mechanism for the loss of HNF4A expression in ESCC.
Conclusion
Our study demonstrated that miR-483-5p was significantly increased in SHEEC cells when compared with those in SHEE cells. Ectopic miR-483-5p resulted in the promotion and proliferation of the colony formation abilities of SHEEC cells by directly targeting HNF4A. Our study provides a promising therapeutic role for miR-483-5p in ESCC, which appears to act in part by mimicking the pharmacological inhibitors of HNF4A.
Acknowledgements
This work was supported by the National Natural Science Fund (Grant No. U1304809) and the key scientific research projects of Henan Provincial Department of Education.
Disclosure of conflict of interest
None.
References
- 1.Tang WR, Chen ZJ, Lin K, Su M, Au WW. Development of esophageal cancer in Chaoshan region, China: association with environmental, genetic and cultural factors. Int J Hyg Environ Health. 2015;218:12–18. doi: 10.1016/j.ijheh.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Wang AH, Liu Y, Wang B, He YX, Fang YX, Yan YP. Epidemiological studies of esophageal cancer in the era of genome-wide association studies. World J Gastrointest Pathophysiol. 2014;5:335–343. doi: 10.4291/wjgp.v5.i3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung CS, Lee YC, Wu MS. Prevention strategies for esophageal cancer: perspectives of the east vs. west. Best Pract Res Clin Gastroenterol. 2015;29:869–883. doi: 10.1016/j.bpg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Xu W, Liu Z, Bao Q, Qian Z. Viruses, other pathogenic microorganisms and esophageal cancer. Gastrointest Tumors. 2015;2:2–13. doi: 10.1159/000380897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludmir EB, Stephens SJ, Palta M, Willett CG, Czito BG. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol. 2015;6:287–295. doi: 10.3978/j.issn.2078-6891.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yingying L, Zhiguang L, Li W, Yuanyuan Y, Ping L, Linsong W. Serum miRNAs as new biomarkers for esophageal squamous cell carcinoma. Yi Chuan. 2015;37:315–320. doi: 10.16288/j.yczz.14-441. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700–1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 8.Andrici J, Hu SX, Eslick GD. Facial flushing response to alcohol and the risk of esophageal squamous cell carcinoma: a comprehensive systematic review and meta-analysis. Cancer Epidemiol. 2016;40:31–38. doi: 10.1016/j.canep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Piletic K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. 2016;90:2405–2419. doi: 10.1007/s00204-016-1815-7. [DOI] [PubMed] [Google Scholar]
- 10.Flatmark K, Hoye E, Fromm B. microRNAs as cancer biomarkers. Scand J Clin Lab Invest Suppl. 2016;245:S80–S83. doi: 10.1080/00365513.2016.1210330. [DOI] [PubMed] [Google Scholar]
- 11.Gao SG, Wang LD, Feng XS, Qu ZF, Shan TY, Xie XH. Absorption and elimination of photofrin-II in human immortalization esophageal epithelial cell line SHEE and its malignant transformation cell line SHEEC. Ai Zheng. 2009;28:1248–1254. doi: 10.5732/cjc.008.10585. [DOI] [PubMed] [Google Scholar]
- 12.Shen ZY, Xu LY, Chen MH, Cai WJ, Shen J, Chen JY, Zeng Y. Cytogenetic and molecular genetic changes in malignant transformation of immortalized esophageal epithelial cells. Int J Mol Med. 2003;12:219–224. [PubMed] [Google Scholar]
- 13.Shen ZY, Xu LY, Li EM, Cai WJ, Shen J, Chen MH, Cen S, Tsao SW, Zeng Y. The multistage process of carcinogenesis in human esophageal epithelial cells induced by human papillomavirus. Oncol Rep. 2004;11:647–654. [PubMed] [Google Scholar]
- 14.Gao X, Gulari E, Zhou X. In situ synthesis of oligonucleotide microarrays. Biopolymers. 2004;73:579–596. doi: 10.1002/bip.20005. [DOI] [PubMed] [Google Scholar]
- 15.van Midwoud PM, Groothuis GM, Merema MT, Verpoorte E. Microfluidic biochip for the perifusion of precision-cut rat liver slices for metabolism and toxicology studies. Biotechnol Bioeng. 2010;105:184–194. doi: 10.1002/bit.22516. [DOI] [PubMed] [Google Scholar]
- 16.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Du X. Noncoding RNAs in gastric cancer: research progress and prospects. World J Gastroenterol. 2016;22:6610–6618. doi: 10.3748/wjg.v22.i29.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter JV, Galbraith NJ, Yang D, Burton JF, Walker SP, Galandiuk S. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Br J Cancer. 2017;116:762–774. doi: 10.1038/bjc.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong S, Yin H, Dong C, Sun K, Lv P, Meng W, Ming L, He F. Predictive value of plasma microRNA-216a/b in the diagnosis of esophageal squamous cell carcinoma. Dis Markers. 2016;2016:1857067. doi: 10.1155/2016/1857067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Y, Li L, Liu J, Wang L, Zhou Y. MiR-495 inhibits esophageal squamous cell carcinoma progression by targeting Akt1. Oncotarget. 2016;7:51223–51236. doi: 10.18632/oncotarget.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin HD, Liao XY, Chen YB, Huang SY, Xue WQ, Li FF, Ge XS, Liu DQ, Cai Q, Long J, Li XZ, Hu YZ, Zhang SD, Zhang LJ, Lehrman B, Scott AF, Lin D, Zeng YX, Shugart YY, Jia WH. Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. Am J Hum Genet. 2016;98:709–727. doi: 10.1016/j.ajhg.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SP, Su HX, Zhao D, Guan QL. Plasma miRNA-506 as a prognostic biomarker for esophageal squamous cell carcinoma. Med Sci Monit. 2016;22:2195–2201. doi: 10.12659/MSM.899377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Li M, Hu C, Duan H. Clinical significance of serum miR-223, miR-25 and miR-375 in patients with esophageal squamous cell carcinoma. Mol Biol Rep. 2014;41:1257–1266. doi: 10.1007/s11033-013-2970-z. [DOI] [PubMed] [Google Scholar]
- 24.Kimura S, Naganuma S, Susuki D, Hirono Y, Yamaguchi A, Fujieda S, Sano K, Itoh H. Expression of microRNAs in squamous cell carcinoma of human head and neck and the esophagus: miR-205 and miR-21 are specific markers for HNSCC and ESCC. Oncol Rep. 2010;23:1625–1633. doi: 10.3892/or_00000804. [DOI] [PubMed] [Google Scholar]
- 25.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, Sidhu SB. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15:7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 26.Qiao Y, Ma N, Wang X, Hui Y, Li F, Xiang Y, Zhou J, Zou C, Jin J, Lv G, Jin H, Gao X. MiR-483-5p controls angiogenesis in vitro and targets serum response factor. Febs Lett. 2011;585:3095–3100. doi: 10.1016/j.febslet.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Ma N, Li F, Li D, Hui Y, Wang X, Qiao Y, Zhang Y, Xiang Y, Zhou J, Zhou L, Zheng X, Gao X. Igf2-derived intronic miR-483 promotes mouse hepatocellular carcinoma cell proliferation. Mol Cell Biochem. 2012;361:337–343. doi: 10.1007/s11010-011-1121-x. [DOI] [PubMed] [Google Scholar]
- 28.Chang HR, Nam S, Kook MC, Kim KT, Liu X, Yao H, Jung HR, Lemos R Jr, Seo HH, Park HS, Gim Y, Hong D, Huh I, Kim YW, Tan D, Liu CG, Powis G, Park T, Liang H, Kim YH. HNF4alpha is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut. 2016;65:19–32. doi: 10.1136/gutjnl-2014-307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, Davies SR, Wang S, Wang P, Kinsinger CR, Rivers RC, Rodriguez H, Townsend RR, Ellis MJ, Carr SA, Tabb DL, Coffey RJ, Slebos RJ, Liebler DC, Nci C. Proteogenomic characterization of human colon and rectal cancer. Nature. 2014;513:382–387. doi: 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuong LM, Chellappa K, Dhahbi JM, Deans JR, Fang B, Bolotin E, Titova NV, Hoverter NP, Spindler SR, Waterman ML, Sladek FM. Differential effects of hepatocyte nuclear factor 4alpha isoforms on tumor growth and T-Cell factor 4/AP-1 interactions in human colorectal cancer cells. Mol Cell Biol. 2015;35:3471–3490. doi: 10.1128/MCB.00030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz B, Algamas-Dimantov A, Hertz R, Nataf J, Kerman A, Peri I, Bar-Tana J. Inhibition of colorectal cancer by targeting hepatocyte nuclear factor-4alpha. Int J Cancer. 2009;124:1081–1089. doi: 10.1002/ijc.24041. [DOI] [PubMed] [Google Scholar]
- 32.Osanai M, Chiba H, Kojima T, Fujibe M, Kuwahara K, Kimura H, Satoh M, Sawada N. Hepatocyte nuclear factor (HNF)-4alpha induces expression of endothelial Fas ligand (FasL) to prevent cancer cell transmigration: a novel defense mechanism of endothelium against cancer metastasis. Jpn J Cancer Res. 2002;93:532–541. doi: 10.1111/j.1349-7006.2002.tb01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N. Corrigendum: mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2015;528:152. doi: 10.1038/nature16136. [DOI] [PubMed] [Google Scholar]