Abstract
This study aimed to explore the influence of microRNA-27a on immune response to mycobacterium tuberculosis (Mtb) and the molecular mechanism. MiRNA-27a was screened one of the downregulated miRNAs in Mtb infected macrophages. The concentrations of IFN-γ, IL-β, IL-6, and TNF-α in THP-1 macrophages after infection with Mtb and simultaneous transfection with miR-27a mimics or inhibitor were determined by ELISA. Colony-forming unit (CFU) assay was used to determine the survival situation of THP-1 infected with Mtb after transfection with miR-27a mimics or inhibitor. We used luciferase reporter assay and western blotting to study the relationship between miR-27a and IRAK4. MiR-27a was found differential expressed in Mtb infected macrophages, and the expressions of miR-27a in Mtb-infected THP-1 were remarkably downregulated with the increase of time and dose. Compared with the control, the levels of IFN-γ, IL-β, IL-6, and TNF-α were enhanced after macrophages infected with Mtb, while further transfection of miR-27a mimics abolished the increase. IRAK4 was found the target gene of miR-27a and transfection of miR-27a mimics decreased the relative level of IRAK4. The concentration of IFN-γ, IL-β, IL-6, and TNF-α in Mtb infected macrophages were reduced significantly after transfection of miR-27a mimics, while simultaneous transfection of pcDNA-IRAK4 abolished the decrease, which is the upstream molecular of NF-κB. In conclusion, miR-27a plays a key role in immune response to Mtb infection and intervening on miR-27a may be an effective way to treat TB.
Keywords: Mtb, MiR-27a, immune response, NF-κB
Introduction
Tuberculosis (TB) is disease infected with mycobacterium tuberculosis (Mtb) in respiratory [1]. About one-third TB was caused by Mtb, which was approximately 1.7 million annually [2]. Macrophages play a pivotal role in suppressing the growth and replication of Mtb by initiating innate immune responses [3]. MicroRNA (miRNA) was reported to affect immune function in macrophages from TB patients [4]. Indeed, in silico analysis predicted that human miRNA might target mycobacterial genes and potentially suppress the expression of genes involved in the development and survival of the bacillus [5].
MiRNAs is found can regulate the expression of genes and proteins [6]. Some studies showed miR-29a and miR-144 were differential expressed in macrophages of TB patients, and were speculated influence immune function [7]. Manipulation of host miRNA expression may be another mechanism by which Mtb is able to subvert immune detection and persist intracellularly within macrophages. In activated macrophages induced TLR2, miR-27a was found downregulated and prevent overly exuberant inflammatory responses via reducing cytokine expression [8]. Immune responses are under specific and intensive regulation by miRNAs in papillary thyroid carcinoma (PTC), including miR-27a-5p [9]. In lungs of septic mice, miR-27a is critical in regulating inflammatory response, and knocking down of miR-27a down regulates expression levels of TNF-α and IL-6 significantly by decreasing the phosphorylation level of NF-κB p65 [10]. NF-κB plays an important role in regulating many biological responses, and inflammation [11]. In epithelial cells infected with enteroinvasive bacteria, NF-κB can regulate innate immune response [12]. Transfection of bacteria can induce immune response and NF-κB can affect the transcription of host genes [12]. However, the research about the regulation of miRNA on the immune response in TB and the molecule mechanism is needed.
To study the role of miRNAs in regulating immune response, we used microRNA microarray and qRT-PCR to screen the differential expressed microRNA. We screened miR-27a and explored whether miR-27a play a role in the regulation of regulation of the innate immune response induced by Mtb. Our results suggest that abnormal microRNA-27a expression is associated with the immune response activated by mycobacterium via targeting IRAK4.
Material and methods
Cell culture
We obtained human THP-1 macrophages in the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were kept in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) in humidified air. The air has 5% CO2 and the temperature was 37°C. Dulbecco’s Modified Eagle’s Medium contained 10% FBS (Hyclone, USA) and 1% penicillin/streptomycin.
Infection of mycobacterium tuberculosis
Mtb H37Rv was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown at 37°C in Difco Middlebrook 7H9 Medium. As H37Rv was at logarithmic phase, we adjusted the concentration to 1.0 g/L [8]. The normal THP-1 cells (uninfected) were the control.
Cell transfection
We obtained oligonucleotides containing miR-27a-mimic, miR-27a-mimic negative control (mimic-NC), miR-27a-inhibitor and miR-27a-inhibitor negative control (inhibitor-NC) from GenePharma Co. (Shanghai, China). THP-1 cells were transfected with the oligonucleotides by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
qRT-PCR
We applied miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) to isolate miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) based on the protocol of manufacturer. We used Nanodrop 2000 (Thermo Fisher Scientific, San Jose, CA, USA) to determine RNA purity and concentration. The RNA samples were immediately stored at -80°C for next cDNA conversion. Genes were amplified by specific oligonucleotide primer, and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control. The detection and quantification contained the following steps: first, reverse transcription was performed at 55°C for 30 min, initial activation for 15 min at 95°C, next 40 cycles of denaturation were conducted at 94°C for 15 s, then annealing for 30 s at 55°C, extension for 30 s at 72°C. The expression level was normalized using U6 small nuclear RNA by the 2-ΔCt method.
Western blotting
Cells were inoculated to 6-well plate with each plate of 5×105 cells and cultivated for 24 h. The culture solution was absorbed away and cells were rinsed with ice PBS for 3 times. Then each well was added radioimmunoprecipitation assay (RIPA) to obtain total proteins. Then 12% separation gel and 5% spacer gel was used to perform SDS-PAGE gel electrophoresis. Sample was transferred to nitrocellulose filter membranes (Hybond, Escondido, CA, USA). Then membranes was placed in Tris-buffered saline Tween-20 (TBST) containing 5% skimmed milk powder and blocked for 1 h at room temperature. After 1 h, membranes were taken out and rinsed with TBST once. Antibodies were diluted with 5% Bovine Serum Albumin (BSA) and membranes were placed in antibody with appropriate concentration. Primary antibodies were determined by EZ-ECL chemiluminescence Detection kit for HRP (Biological Industries, Beit-Haemek, Israel).
Dual-luciferase reporter assay
Plasmids (0.8 μg) with 3’-UTR and mutant 3’-UTR DNA sequences were transfected into THP-1 cells, followed by transfection with miR-27a mimics (100 nM) before incubation for 24 h. Cells were lysed for the determination of fluorescence intensity using GloMax 20/20 luminometer (Promega, Madison, WI, USA). Using Renilla fluorescence as internal reference, dual-luciferase reporter assay was performed by Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s manual.
ELISA analysis
We measured the levels of IFN-γ, IL-6, IL-β and TNF-α using ELISA assay. To explore the influence of Mtb on the expression of cytokines, we determined the concentration of IFN-γ, IL-1β, IL-6 and TNF-α in macrophages after infection with Mtb. Moreover, we transfected the Mtb infected macrophages with miR-27a inhibitor or mimics.
Colony-forming unit (CFU) assay
To assay bacterial viability within human macrophages, the cells were infected with Mtb at an MOI of 10 for 24 h or 48 h at 37°C, and then washed with PBS three times to remove extracellular mycobacteria. The infected cells were lysed with sterile distilled water. Quantitative culturing was performed using 10-fold serial dilutions. Aliquots of each dilution were inoculated in triplicate on Middlebrook 7H10 agar plates containing 10% OADC and incubated at 37°C for three weeks. CFUs were calculated in triplicate using standard procedures. The survival rate was calculated as compared to the control.
Statistical analysis
Statistical evaluation for data analysis was determined by unpaired Student’s t test. Data were presented as the means ± SD and P values < 0.05 were considered significant.
Results
MiR-27a was down-regulated in macrophages infected with Mtb
MicroRNA chip technology was used to screen the differential expressed miRNAs in macrophages infected with Mtb. The heat map was shown in Figure 1A. As shown, 33 miRNAs were down-regulated and 29 miRNAs were up-regulated. Among the 29 down-regulated miRNAs, miR-27a was the most down-regulated. To investigate the expression pattern of miR-27a in response to Mtb-infection, human macrophage cell lines THP-1 cells were infected with Mtb at an MOI of 10 for different periods of time as indicated, or at the different MOI for 24 h, and the expression levels of miR-27a were detected by qRT-PCR analysis. The results were shown in Figure 1B and 1C. As shown, the expressions of miR-27a in Mtb-infected THP-1 were remarkably downregulated with the increase of time and dose. These findings indicated that Mtb infection robustly reduced the expression of miR-27a in human macrophages, implicating a potential correlation of miR-27a with mycobacterial infection.
Figure 1.
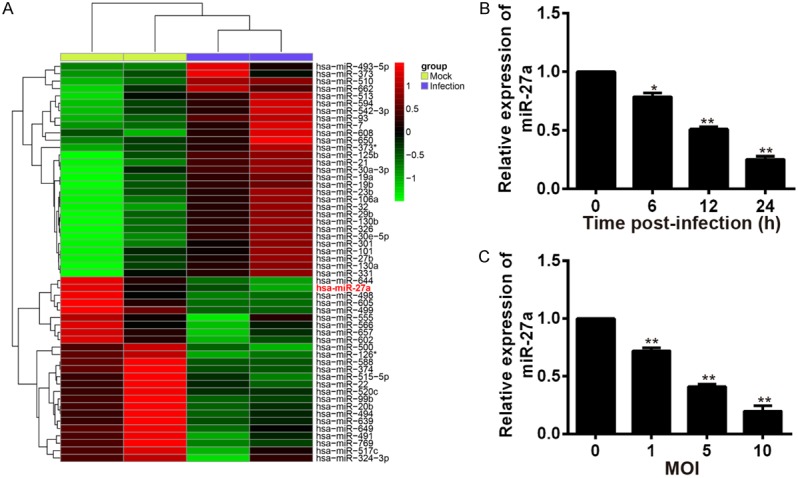
Screening of differential miRNAs and the expression of miR-27a was reduced in human macrophages infected with Mtb. A. Differential expressed miRNAs were screened by microRNA chip technology in macrophages infected with Mtb. B. The relative expression of miR-27a in human macrophages after infected with Mtb for 0, 6, 12 and 24 h, determined by qRT-PCR. *P < 0.05 and **P < 0.01, vs. the time of 0 h. C. The relative expression of miR-27a in human macrophages after infected with different concentrations of Mtb. *P < 0.05 and **P < 0.01, vs. the MOI of 0.
MiR-27a inhibits the release of inflammatory factors and promotes mycobacterial survival in Mtb-infected macrophages
IFN-γ, IL-β, IL-6, and TNF-α were the secretion of inflammatory cytokines. To explore the influence of miR-27a on immune response, we determined the concentrations of IFN-γ, IL-β, IL-6, and TNF-α in THP-1 cells after infection with Mtb and transfection with miR-27a mimics simultaneously. The results were shown in Figure 2A-D. As shown, the concentrations of IFN-γ, IL-β, IL-6, and TNF-α were increased in both H37Rv infected macrophages. However, further transfection with miR-27a mimics decreased the concentration of IFN-γ, IL-β, IL-6, and TNF-α. In order to gain insight into the potential role of miR-27a in cellular immune response during Mtb infection, we determined the effect of miR-27a on mycobacterial survival using CFU assay in THP-1 cells transfected with the control mimic and miR-27a mimic during infection of Mtb. CFU data showed that the abundance of miR-27a greatly facilitated intracellular growth of mycobacteria in Mtb-infected THP-1 at 24 h and 48 h (Figure 2E).
Figure 2.
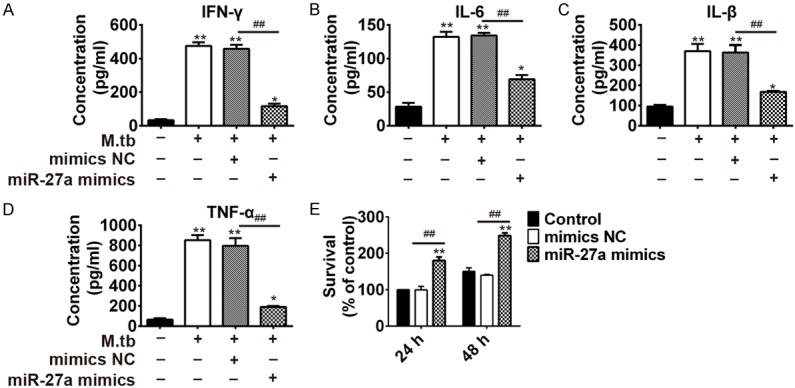
MiR-27a mimics inhibited the immune response activated by mycobacterium tuberculosis activated the in THP-1 cells. THP-1 cells were transfected with miR-27a mimic and control mimic for 24 h, followed by Mtb challenge. IFN-γ (A), IL-6 (B), IL-β (C) and TNF-α (D) in THP-1 cells was examined by ELISA at 48 h. (E) Mycobacterial viability was determined by CFU assay in THP-1 cells. *P < 0.05 and **P < 0.01, vs. the control. ##P < 0.01, vs. Mtb + mimics NC.
Moreover, we determined the concentrations of IFN-γ, IL-β, IL-6, and TNF-α in THP-1 cells after infection with Mtb and transfection with miR-27a inhibitor simultaneously. Results showed infection of Mtb to THP-1 cells increased the concentrations, and simultaneous transfection of miR-27a inhibitor further promoted the increase. (Figure 3A-D). We also studied the effect of miR-27a on mycobacterial survival using CFU assay in THP-1 cells transfected with the control inhibitor and miR-27a inhibitor during Mtb infection. As CFU data shown, transfection of miR-27a inhibitor to THP-1 cells decreased the survival percent of cells. Moreover, the percent decreased with the increase of time verified from 24 h to 48 h (Figure 3E). These results indicated that miR-27a promoted the survival rate of intracellular mycobacteria via inhibiting immune responses in Mtb-challenged macrophages.
Figure 3.
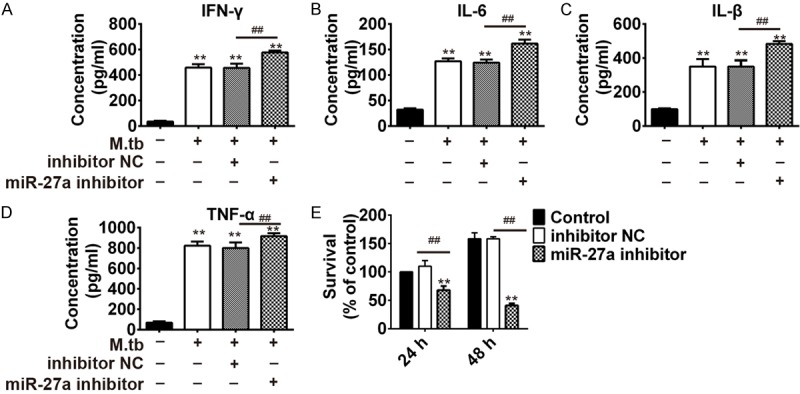
MiR-27a inhibitor promotes the immune response activated by mycobacterium tuberculosis infection. THP-1 cells were transfected with control inhibitor and miR-27a inhibitor for 24 h, followed by Mtb challenge. IFN-γ (A), IL-6 (B), IL-β (C) and TNF-α (D) in THP-1 cells was examined by ELISA at 48 h. (E) Mycobacterial viability was determined by CFU assay in THP-1 cells. *P < 0.05 and **P < 0.01, vs. the control. ##P < 0.01, vs. Mtb + inhibitor NC.
IRAK4 is a direct target of miR-27a in macrophages infected with Mtb
Nuclear factor kappa B (NF-κB) play an important role in regulating genes encoding cytokines, which influencing immune and inflammatory responses [13]. IRAK-4 protein plays an important role in Toll-like receptor-mediated immune responses, and is essentially required for signal transduction to NF-κB downstream of MyD88 [14]. To explore if IRAK4 is a direct target for miR-27a, we constructed the 3’-UTR reporter plasmids coupled with full length of IRAK4 3’-UTR with wild-type (wt) or mutant (mut) miR-27a binding sites (Figure 4A). Luciferase assay showed that miR-27a could repress the expression of reporter gene containing wt 3’-UTR but not that containing mut 3’-UTR (Figure 4B). To further verify the relationship between IRAK4 and miR-27a, we used qRT-PCR and western blotting to determine the mRNA level and protein expression of IRAK4 in macrophages after transfection with control mimic and miR-27a mimic or control inhibitor and miR-27a inhibitor. Results showed that level of IRAK4 was dramatically decreased after miR-27a mimic transfection, whereas increased after miR-27a inhibitor transfection in THP-1 cells (Figure 4C and 4D). In conclusion, there is a negative relationship between IRAK4 and miR-27a.
Figure 4.
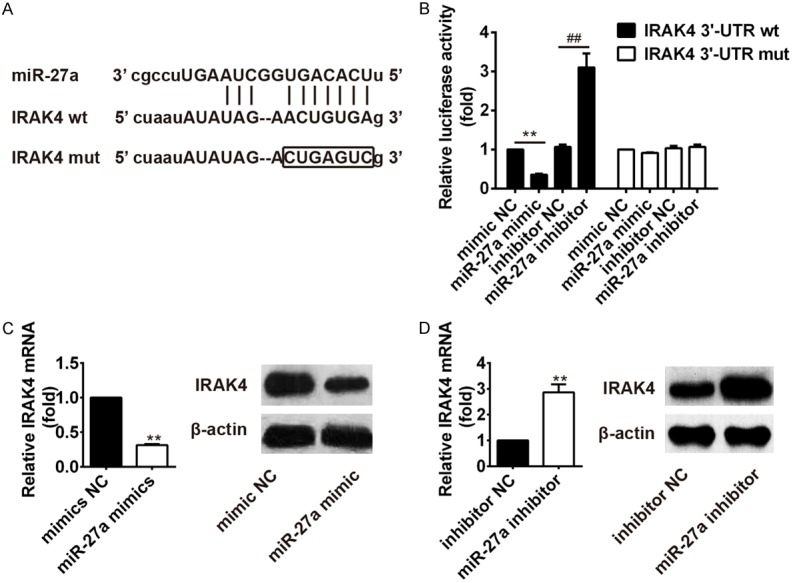
IRAK4 is the target gene of miR-27a. (A) Diagram of the miR-27a putative binding sites and corresponding mutant sites in the 3’-UTR of IRAK4. (B) Effects of miR-27a on the expression of IRAK4 3’-UTR-containing reporter genes. Relative mRNA level or protein expression of IRAK4 in macrophage infected with mycobacterium tuberculosis after transfection with miR-27a mimic (C) or inhibitor (D).
MiR-27a regulates inflammatory cytokine via targeting IRAK4
To explore whether miR-27a promote the activity of immune-related cytokines in Mtb infected macrophage by targeting IRAK4, we transfected THP-1 cells simultaneously with miR-27a mimics and pcDNA-IRAK4 after infection with Mtb. Then, we determined the relative luciferase activity and concentrations of IFN-γ, IL-β, IL-6, and TNF-α. Results showed the concentration of IFN-γ, IL-1β, IL-6, and TNF-α were significantly increased after macrophage infected with Mtb H37Rv, which indicated immune response was activated after infection of Mtb in macrophage. Further transfection with miR-27a mimics reduced the concentration of IFN-γ, IL-1β, IL-6, and TNF-α which were induced by Mtb. It indicated miR-27a mimics inhibited immune response. However, further transfection of pcDNA-IRAK4 abolished the inhibition of miR-27a mimics on immune response (Figure 5A-E). We also studied the effect of miR-27a on mycobacterial survival using CFU assay in THP-1 cells transfected with the miR-27a mimics or pcDNA-IRAK4 during Mtb infection (MOI of 10). Results (Figure 5F) showed the overexpression of miR-27a dramatically promoted intracellular growth of mycobacteria in Mtb-infected THP-1 at 24 h and 48 h. Further transfection of pcDNA-IRAK4 abolished the promotion of miR-27a mimics on growth of mycobacteria. In conclusion, miR-27a could inhibit the activity of immune-related cytokines and survival rate of mycobacterias by targeting IRAK4.
Figure 5.
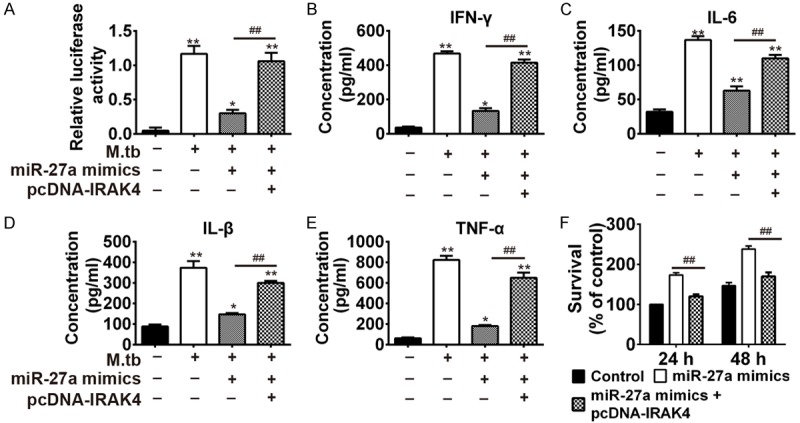
MiR-27a regulated inflammatory cytokines via targeting IRAK4. (A) Detection on the activity of NF-κB by luciferase report assay. The concentration of IFN-γ (B), IL-6 (C), IL-β (D) and TNF-α (E) in THP-1 cells after infection with Mtb, Mtb + 27a mimic or Mtb + 27a mimic + pcDNA-IRAK4. *P < 0.05 and **P < 0.01, vs. the control group; ##P < 0.01, vs. Mtb + 27a mimic. (F) Mycobacterial viability was determined by CFU assay in THP-1 cells. ##P < 0.01, vs. 27a mimic.
Discussion
Mtb is an intracellular mycobacterium and can infect macrophages. But the studies about the immune mechanism of Mtb infected macrophages is confined [15]. Some miRNAs is important in the immune response of macrophages [16,17]. We screened the differential expressed miRNAs in Mtb infected macrophages with miRNAs chip technology. MiR-27a was screened the most downregulated molecular. Moreover, the expressions of miR-27a in Mtb-infected THP-1 were remarkably downregulated in a time- and dose-dependent manner. MiR-27a can regulate immune responses via targeting important immune-related genes [18]. Ni et al. reported miR-27a is important in immune response pathway and the expression is reduced in Mtb infected macrophages [19]. Therefore, we supposed downregulated miR-27a in Mtb infected host cell may activate the immune response of macrophages to clear off Mtb.
Some studies reported cytokines were contained in immune responses of TB, such as IFN-γ, IL-4, TNF-α, IL-1 and IL-6 [20]. In this study, we showed that the concentration values of IFN-γ, IL-β, IL-6, and TNF-α were enhanced in macrophages after infection with Mtb. However, overexpression of miR-32-5p noticeably attenuated the secretion of these inflammatory factors in response to Mtb, thus ameliorating inflammatory responses in human macrophages. Moreover, the abundance of miR-27a greatly facilitated intracellular growth of mycobacteria in Mtb infected THP-1 at 24 h and 48 h. These findings suggest that miR-27a played a key role in regulating Mtb induced inflammatory response during the host innate defense against mycobacteria, and miR-27a overexpression enhanced Mtb survival.
NF-κB contains numerous of transcription factors and can regulate many biological responses. It can regulate immune responses and inlammation, and plays a key role in regulate the immune responses of epithelial cell after infection with enteroinvasive bacteria [12]. In Drosophila, NF-κB protein could regulate the JNK-mediated immune response via targeting TAK1 [21]. IRAK4 is a kinase and also important in immune responses [22]. In innate and acquired immunity, IRAK4 can activate NF-κB [23]. The small molecule inhibitors of IRAK4 kinase could extinguish NF-κB activation [24]. Our study revealed IRAK4 was the target gene of miR-27a. After transfection with miR-27a mimic, the IRAK4 in Mtb infected macrophages decreased c. The upregulation of IFN-γ, IL-β, IL-6, and TNF-α in Mtb infected macrophages was suppressed in the presence of a miR-27a mimic and enhanced when cells are transfected with pcDNA-IRAK4, suggesting that overexpression of miR-27a could inhibit the activity of NF-κB, while transfection of IRAK4 abolished the suppression of miR-27a mimics. IRAK4 is critical in conducting signal to the downstream of NF-κB. NIK and IKK-α were found play key roles in activating NF-κB via inflammatory cytokines. In this study, overexpression of IRAK4 activated the expression of inflammatory cytokines IFN-γ, IL-β, IL-6 and TNF-α, which then activated IKK. The activated IKK phosphorylates IκB proteins, which leads to the degradation of IκB. It releases p50/p65 heterodimer, which enter into the nucleus and act as a transcription factor. Moreover, IKK phosphorylates the Ser536 of p65, so it increases the transactivation activity of NF-κB [25].
In conclusion, this study indicates the level of miR-27a is reduced in macrophages because of Mtb infection. The miR-27a mimics attenuate IFN-γ, IL-β, IL-6 and TNF-α secretion in macrophages by targeting IRAK4. Our study indicated miR-27a is important in generating inflammatory factors, which help us to find new method to treat TB.
Acknowledgements
This study was financially supported by the Science and Technology Project Foundation of Lanzhou (Grant No.: 2014-1-57).
Disclosure of conflict of interest
None.
References
- 1.Rajaram MV, Ni B, Dodd CE, Schlesinger LS. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol. 2014;26:471–485. doi: 10.1016/j.smim.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 3.Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, Speelman P, Van Deventer SJ, Van der Poll T. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis. 2000;182:902–908. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 4.Song Q, Li H, Shao H, Li C, Lu X. MicroRNA-365 in macrophages regulates Mycobacterium tuberculosis-induced active pulmonary tuberculosis via interleukin-6. Int J Clin Exp Med. 2015;8:15458. [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Li JT, Pan X, Wei L, Wu JY. Candidate Mycobacterium tuberculosis genes targeted by human microRNAs. Protein Cell. 2010;1:419–421. doi: 10.1007/s13238-010-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisognin A, Sales G, Coppe A, Bortoluzzi S, Romualdi C. MAGIA2: from miRNA and genes expression data integrative analysis to microRNA-transcription factor mixed regulatory circuits (2012 update) Nucleic Acids Res. 2012;40:W13–21. doi: 10.1093/nar/gks460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Guo L, Tang J, Zhang A, Liu X, Xu G. miR-144 regulates BCG-and rapamycin-induced autophagy by targeting Atg4a in RAW264. 7 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31:163–167. [PubMed] [Google Scholar]
- 8.Xie N, Cui H, Banerjee S, Tan Z, Salomao R, Fu M, Abraham E, Thannickal VJ, Liu G. miR-27a regulates inflammatory response of macrophages by targeting IL-10. J Immunol. 2014;193:327–334. doi: 10.4049/jimmunol.1400203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CT, Oyang YJ, Huang HC, Juan HF. MicroRNA-mediated networks underlie immune response regulation in papillary thyroid carcinoma. Sci Rep. 2014;4:6495. doi: 10.1038/srep06495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, Jiang L. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol. 2014;290:190–195. doi: 10.1016/j.cellimm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 12.Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–1466. [PubMed] [Google Scholar]
- 13.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortega J, Uzal FA, Walker R, Kinde H, Diab SS, Shahriar F, Pamma R, Eigenheer A, Read DH. Zygomycotic lymphadenitis in slaughtered feedlot cattle. Vet Pathol. 2010;47:108–115. doi: 10.1177/0300985809352975. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Wang J, Zhang X. The involvement of MiR-1-clathrin pathway in the regulation of phagocytosis. PLoS One. 2014;9:e98747. doi: 10.1371/journal.pone.0098747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. PLoS One. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furci L, Schena E, Miotto P, Cirillo DM. Alteration of human macrophages microRNA expression profile upon infection with Mycobacterium tuberculosis. Int J Mycobacteriol. 2013;2:128–134. doi: 10.1016/j.ijmyco.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Ni B, Rajaram MV, Lafuse WP, Landes MB, Schlesinger LS. Mycobacterium tuberculosis decreases human macrophage IFN-γ responsiveness through miR-132 and miR-26a. J Immunol. 2014;193:4537–4547. doi: 10.4049/jimmunol.1400124. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Park JM, Brady H, Ruocco MG, Sun H, Williams D, Lee SJ, Kato T, Richards N, Chan K, Mercurio F. Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004;18:584–594. doi: 10.1101/gad.1168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lye E, Dhanji S, Calzascia T, Elford AR, Ohashi PS. IRAK-4 kinase activity is required for IRAK-4-dependent innate and adaptive immune responses. Eur J Immunol. 2008;38:870–876. doi: 10.1002/eji.200737429. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki N, Saito T. IRAK-4-a shared NF-κB activator in innate and acquired immunity. Trends Immunol. 2006;27:566–572. doi: 10.1016/j.it.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies. Immunol Rev. 2012;246:359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]


