Abstract
Primary aldosteronism (PA) is the most common form of endocrine hypertension. This study was to investigate the gene expression profile in PA adrenal glands and normal controls using RNA-Sequencing. By performing transcriptome analyses for 3 PA adrenal glands and 3 controls on Illumina platform, we identified 1,093 transcripts as significantly differently expressed genes (DEGs), which provided clues for further study of these transcript changes during PA pathogenesis. Further, Gene Set Enrichment Analysis (GSEA) identified 35 significant Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathways, including ‘ribosome’, ‘oxidative phosphorylation’, ‘histidine metabolism’, ‘xenobiotics metabolism by Cytochrome P450’, ‘drug metabolism by Cytochrome P450’, ‘tyrosine metabolism’ and ‘glutathione metabolism’. In summary, we identified novel genes that are associated with PA phenotype, as well as differently regulated biological pathways relating to protein synthesis, energy acquisition and metabolism. Our study provides new candidates for further elucidation of the molecular mechanisms underlying PA pathogenesis.
Keywords: Primary aldosteronism, RNA sequencing, gene set enrichment analysis
Introduction
Primary aldosteronism (PA), which was first described by Conn in 1955 [1], is the most common form of endocrine hypertension with a prevalence of 5% to 10% of all hypertensive patients [2]. Idiopathic hyperaldosteronism (IHA) and aldosterone-producing adenoma (APA) are two major forms of PA [3]. PA is characterized by excessive and autonomous aldosterone secretion, causing increased renal sodium retention and potassium excretion, hypervolemia, suppressed plasma renin activity (PRA) and hypertension [1,4]. PA has long been considered relatively benign associated with a low incidence of cardiovascular events [5]. However, more recent studies suggest that prolonged exposure to elevated aldosterone might cause cardiovascular complications, renal damage and metabolic sequelae [6-8].
Gene expression profile analysis techniques offer powerful tools to identify biomarkers and understand the pathophysiology of various diseases at the molecular level [9-13]. Using microarray, several genes associated with APA phenotype have been identified [14-18], such as CYP11B2 (Cytochrome P450, family 11, subfamily B, polypeptide 2), PCP4 (Purkinje cell protein 4), PRRX1 (paired related homeobox 1), AKR1C3 (17β-hydroxysteroid dehydrogenase type 5), CYP17 (17α-hydroxylase/17, 20 lyase) and CYB5 (cytochrome b5). RNA-sequencing (RNA-seq) technology offers substantially enhanced sensitivity to analyze gene expression and RNA-seq has not been employed for PA samples. The aim of the present study was to investigate the general pattern of the gene expression profile in PA adrenal glands and normal controls using RNA-Seq. We also conducted enrichment analysis to identify biological pathways that are preferentially associated with PA. Our study demonstrated differential expressed genes (DEGs) was involved in key metabolic pathways regulating histidine metabolism, Cytochrome P450-mediated xenobiotics and drug metabolism, tyrosine metabolism and glutathione metabolism.
Materials and methods
Study participants
Thirty patients with diagnosed PA (ages: 38-63 years) were enrolled into the study following written informed consent. The study received ethical approval from the ethics committee of Ruijin Hospital. The initial diagnosis of PA was based on the presence of hypertension, hypokalemia (< 3.5 mmol/l), suppressed plasma renin activity (PRA, < 0.2 ng/L/s) and high concentration of plasma aldosterone (> 400 pmol/l) followed by positron emission tomography and CT scanning (PET/CT). Clinical characteristics of the patients were summarized in Table 1. The patients were diagnosed with aldosterone-producing adenoma (APA) and underwent unilateral adrenalectomy based on adrenal venous sampling results. Adrenal tumor tissue and adjacent non-affected control tissues were surgically removed from these patients, snap-frozen using liquid nitrogen and stored at -70°C until use.
Table 1.
Clinical and biochemical parameters of patients
| All patients (n=30) | Patients for RNA-sequencing (n=3) | |
|---|---|---|
| Age (Years) | 52 ± 9 | 52 ± 4 |
| Gender, M/F | 16/14 | 2/1 |
| Systolic BP (mm Hg) | 191.3 ± 17.1 | 198.0 ± 7.5 |
| Diastolic BP (mm Hg) | 114.6 ± 10.2 | 113.7 ± 9.5 |
| K+ (mmol/L) | 2.7 ± 0.5 | 3.0 ± 0.6 |
| Supine PRA (ng • L-1 • s-1) | 0.094 ± 0.040 | 0.065 ± 0.031 |
| Upright PRA (ng • L-1 • s-1) | 0.149 ± 0.065 | 0.099 ± 0.044 |
| Supine aldosterone (pmol/L) | 556.7 ± 119.0 | 655.5 ± 160.4 |
| Upright aldosterone (pmol/L) | 1101.8 ± 269.5 | 922.0 ± 88.8 |
RNA extraction
RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) per the manufacturer’s instructions, and treated with RNase-free DNase (Sigma, St. Louis, MO, USA) to ensure degradation of DNA. RNA quality was assessed using the ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The integrity of the RNA samples was assessed by 2% agarose gel electrophoresis.
cDNA library preparation and sequencing
The cDNA libraries for 3 paired samples were constructed using Illumina’s TruSeq Sample Preparation Kit (San Diego, CA, USA) according to the manufacturer’s guide. In brief, poly (A) mRNA was isolated from total RNA and fragmented into small fragments. The RNA fragments were reverse-transcribed into first strand cDNA with random hexamer-primers, and then second-strand cDNA synthesis was performed with DNA polymerase I. The cDNAs were ligated to Illumina sequencing adapters, purified by agrose gel, and then amplified by PCR. The cDNA library was sequenced on an Illumina Genome Analyzer II with the standard protocol and 36-bp RNA-Seq reads were obtained as previously described [19].
Gene set enrichment analysis (GSEA)
We identified differentially expressed genes (DEGs) between PA and control samples based on the following criteria: P < 0.05 and fold change > 1.5. Bioinformatics pathway analysis of DEGs was conducted with the Gene Set Enrichment Analysis (GSEA) software package. GSEA has been widely used to identify enriched gene-sets (pathways) in transcriptomics by calculating a weighted Kolmogorov-Smirnov test, adjusted for gene-set size (known as the Normalized Enrichment Score, NES) for each gene-set [20].
Real-time PCR
Total RNA extracted from 30 PA and 30 control subjects was reverse transcribed with RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Rockford, IL, USA). Real-time PCR was carried out using SYBR-green PCR Master Mix (Thermo Scientific) on an ABI 7300 instrument (Applied Biosystems, Foster City, CA, USA). The real-time PCR thermo cycling was 95°C for 10 min, 40 cycles at 95°C for 15 s, 60°C for 45 s and 72°C for 10 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was served as reference gene and statistical significance was evaluated using the Student’s t-test. All real-time PCR primers were listed in as follows:
Cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1), 5’-GCAGTGTCTCGGGACTTCG-3’ and 5’-GGCAAAGCGGAACAGGTCA-3’; Aldehyde dehydrogenase 3 family member B1 (ALDH3B1), 5’-GGGCTGTGGTTATGCGATAGG-3’ and 5’-GCTTTGGCTGAGTGGATGG-3’; Aldehyde dehydrogenase 2 family (ALDH2), 5’-GATCCTCGGCTACATCAACAC-3’ and 5’-TCATGCCATCCTGCACATC-3’; CYP11B2, 5’-TTCAACCGCCCTCAACACTAC-3’ and 5’-GGAAACGCTGTCGTGTCCA-3’; Glutathione S-transferase omega 2 (GSTO2), 5’-AGACCAGCCAATGTCAAC-3’ and 5’-GCCAGAGGAGGTAATCAATC-3’; Glutathione peroxidase 3 (GPX3), 5’-TGGTCATTCTGGGCTTTC 3’ and 5’-GGAGGACAGGAGTTCTTTAG-3’; Isocitrate dehydrogenase (NADP(+)) 2 (IDH2), 5’-GGCAGTGGTGTCAAGGAGTG-3’ and 5’-CGCCCATCGTAGGCTTTCAG -3’; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5’-CACCCACTCCTCCACCTTTG-3’ and 5’-CCACCACCCTGTTGCTGTAG-3’.
Western blot analysis
To extract protein, the tissue sample was lysed in RIPA lysis buffer (Beyotime, Shanghai, China) at 4°C. The protein concentration was quantified by using the Bradford method, and 50 μg protein of each sample was loaded on a SDS-PAGE gel for electrophoresis and then transferred to nitrocellulose membrane (Millipore, Bredford, MA, USA). The membranes were incubated with 5% shimmed milk at room temperature for 1 h to block non-specific background binding, followed by incubating overnight at 4°C with primary antibody, respectively: anti-CYP11A1 (no. ab75497; Abcam, Cambridge, MA, USA), anti-ALDH3B1 (no. ab84961; Abcam), anti-ALDH2 (no. ab108306; Abcam), anti-GSTO2 (no. ab191156; Abcam), anti-GPX3 (no. ab27325; Abcam), anti-IDH2 (no. ab55271, Abcam) and anti-GAPDH (no. 5174; Cell Signaling Technology Inc., Danvers, MA, USA). The membranes were then incubated with goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (Beyotime) at room temperature for 1 h. Specific protein bands were detected with ECL detection kits (Millipore). The intensity of the bands was quantified by ImageJ software (Bethesda, MD, USA) and normalized to densitometric values of GAPDH in each sample. Statistical significance was evaluated using the Student’s t-test.
Results
Analysis of gene expression by RNA-Seq
We sequenced cDNA of 3 PA adrenal glands and 3 normal control. We identified 1,093 out of 56,643 transcripts displayed distinct expression different patterns between normal and PA subjects. 724 transcripts were up-regulated (Table S1), whereas 369 transcripts were shown to be down-regulated in PA samples (Table S2). The heat map generated indicated the differential expression profile of 50 genes between normal and PA subjects (Figure 1). The following transcripts showed the highest increase (Table 2): ATAD3C (ATPase Family, AAA Domain Containing 3C), ZC2HC1B (Zinc Finger C2HC-Type Containing 1B), PROM1 (Prominin 1) and LINC00664 (Long Intergenic Non-Protein Coding RNA 664), whereas these transcripts were decreased the most (Table 3): HAS2 (Hyaluronan Synthase 2), HOXD10 (Homeobox D10) and DDX50P1 (DEAD-Box Helicase 50 Pseudogene 1). ATAD3C encodes a mitochondrial membrane bound ATPase whose role is vital for embryonic development and tumor progression [21,22]. PROM1 expression is linked to a resistant phenotype of lung cancer [23]. HOXD10 may suppress tumor invasive growth [24]. However, the roles of these transcripts in the PA progression have not been characterized. Our comprehensive analysis of different transcripts in PA adrenal glands provided clues for further study of these transcript changes during PA development.
Figure 1.
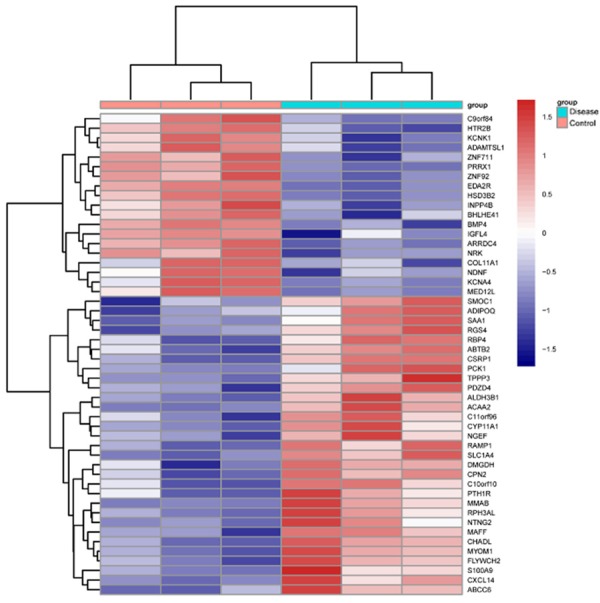
Hierarchical clustering of 50 transcripts and samples. The relative expression levels of each transcript (rows) in each sample (column) were shown.
Table 2.
Top 50 up-regulated genes in primary aldosteronism
| Rank | Gene name | Fold change | P value |
|---|---|---|---|
| 1 | ATAD3C | 46.6845 | 0.0171 |
| 2 | ZC2HC1B | 41.9095 | 0.0446 |
| 3 | PROM1 | 41.4890 | 0.0036 |
| 4 | LINC00664 | 41.3117 | 0.0044 |
| 5 | KCNB1 | 35.4931 | 0.0004 |
| 6 | IGLON5 | 34.0998 | 0.0087 |
| 7 | RP11-380L11.4 | 32.9287 | 0.0015 |
| 8 | TM4SF4 | 32.9287 | 0.0015 |
| 9 | SCRT1 | 32.1499 | 0.0029 |
| 10 | CDH1 | 32.0383 | 0.0370 |
| 11 | RP1-69D17.3 | 30.2225 | 0.0306 |
| 12 | HOXC9 | 27.7151 | 0.0027 |
| 13 | TCL1A | 27.3733 | 0.0092 |
| 14 | SV2C | 26.7295 | 0.0114 |
| 15 | RP11-348H3.2 | 25.5858 | 0.0344 |
| 16 | MB | 25.1747 | 0.0042 |
| 17 | RP5-1021I20.2 | 24.0022 | 0.0066 |
| 18 | CD79A | 22.9409 | 0.0179 |
| 19 | ACSM2A | 21.9568 | 0.0483 |
| 20 | RP11-540A21.2 | 21.9077 | 0.0427 |
| 21 | SLC25A41 | 21.7466 | 0.0159 |
| 22 | LINC00842 | 19.4911 | 0.0292 |
| 23 | C1orf186 | 19.3075 | 0.0412 |
| 24 | CTD-2576D5.4 | 19.3075 | 0.0412 |
| 25 | NEURL1 | 18.6808 | 0.0001 |
| 26 | MIRLET7DHG | 18.2149 | 0.0338 |
| 27 | PRAME | 18.1746 | 0.0073 |
| 28 | KIAA1644 | 17.8406 | 0.0003 |
| 29 | CMTM5 | 17.5691 | 0.0447 |
| 30 | PLEKHB1 | 17.0321 | 0.0008 |
| 31 | RAMP1 | 16.8890 | 0.0000 |
| 32 | AC004069.2 | 16.3100 | 0.0418 |
| 33 | CRABP1 | 15.9249 | 0.0457 |
| 34 | MYBPC1 | 15.7775 | 0.0439 |
| 35 | MYOM1 | 15.3843 | 0.0000 |
| 36 | ADIPOQ | 14.5844 | 0.0000 |
| 37 | PRDM8 | 14.1741 | 0.0071 |
| 38 | TCF15 | 14.1673 | 0.0052 |
| 39 | CXCR2 | 14.1657 | 0.0382 |
| 40 | RP11-797A18.4 | 13.9485 | 0.0493 |
| 41 | PABPN1L | 13.7754 | 0.0014 |
| 42 | LRRC4B | 13.4370 | 0.0030 |
| 43 | SCGB2A1 | 12.6185 | 0.0129 |
| 44 | CIDEA | 12.6129 | 0.0118 |
| 45 | C19orf35 | 12.3534 | 0.0462 |
| 46 | C14orf180 | 12.3178 | 0.0010 |
| 47 | MIR3648 | 12.0648 | 0.0398 |
| 48 | LHB | 11.7551 | 0.0363 |
| 49 | GPR97 | 11.7532 | 0.0493 |
| 50 | FOLR1 | 11.7308 | 0.0003 |
Table 3.
Top 50 down-regulated genes in primary aldosteronism
| Rank | Gene name | Fold change | P value |
|---|---|---|---|
| 1 | HAS2 | 0.0507 | 0.0014 |
| 2 | HOXD10 | 0.0560 | 0.0449 |
| 3 | DDX50P1 | 0.0573 | 0.0332 |
| 4 | GALNT5 | 0.0587 | 0.0063 |
| 5 | PTPRT | 0.0627 | 0.0044 |
| 6 | IGFL4 | 0.0707 | 0.0000 |
| 7 | IGFN1 | 0.0731 | 0.0010 |
| 8 | RIMKLBP2 | 0.0923 | 0.0024 |
| 9 | RP11-219B17.1 | 0.0925 | 0.0087 |
| 10 | HEXA-AS1 | 0.0932 | 0.0396 |
| 11 | DHRS9 | 0.0942 | 0.0374 |
| 12 | PTPN20CP | 0.1011 | 0.0457 |
| 13 | AC018766.6 | 0.1025 | 0.0445 |
| 14 | COL11A1 | 0.1067 | 0.0001 |
| 15 | RP5-1184F4.5 | 0.1084 | 0.0158 |
| 16 | CLRN1-AS1 | 0.1088 | 0.0278 |
| 17 | QRFPR | 0.1138 | 0.0026 |
| 18 | RP11-701H24.3 | 0.1195 | 0.0253 |
| 19 | RP11-164N3.3 | 0.1210 | 0.0293 |
| 20 | ANGPT2 | 0.1225 | 0.0007 |
| 21 | SP5 | 0.1235 | 0.0059 |
| 22 | HS6ST3 | 0.1258 | 0.0130 |
| 23 | CLIC6 | 0.1306 | 0.0230 |
| 24 | RP11-171A24.3 | 0.1366 | 0.0280 |
| 25 | CABP7 | 0.1374 | 0.0039 |
| 26 | SEMA3D | 0.1397 | 0.0005 |
| 27 | PRSS35 | 0.1405 | 0.0009 |
| 28 | RP11-673E1.1 | 0.1410 | 0.0483 |
| 29 | HOXA11 | 0.1420 | 0.0135 |
| 30 | LMAN1L | 0.1445 | 0.0483 |
| 31 | RP11-134K13.2 | 0.1475 | 0.0121 |
| 32 | AP000688.29 | 0.1557 | 0.0007 |
| 33 | SPACA6P-AS | 0.1559 | 0.0328 |
| 34 | NDNF | 0.1562 | 0.0003 |
| 35 | RP11-276H19.1 | 0.1625 | 0.0128 |
| 36 | CDKL5 | 0.1636 | 0.0357 |
| 37 | ENPP6 | 0.1769 | 0.0253 |
| 38 | HIST2H2BA | 0.1847 | 0.0106 |
| 39 | PCDHB12 | 0.1908 | 0.0157 |
| 40 | MAL2 | 0.1925 | 0.0058 |
| 41 | RP1-78O14.1 | 0.1933 | 0.0163 |
| 42 | SLC9A2 | 0.1933 | 0.0020 |
| 43 | RP11-384P7.7 | 0.1998 | 0.0327 |
| 44 | RP3-468K18.6 | 0.2013 | 0.0351 |
| 45 | AP001468.1 | 0.2048 | 0.0449 |
| 46 | RP11-61A14.2 | 0.2052 | 0.0279 |
| 47 | KCNA4 | 0.2056 | 0.0001 |
| 48 | RELN | 0.2066 | 0.0165 |
| 49 | ATP2B2 | 0.2134 | 0.0028 |
| 50 | DDIT4L | 0.2269 | 0.0059 |
Multiple pathways were altered in PA samples
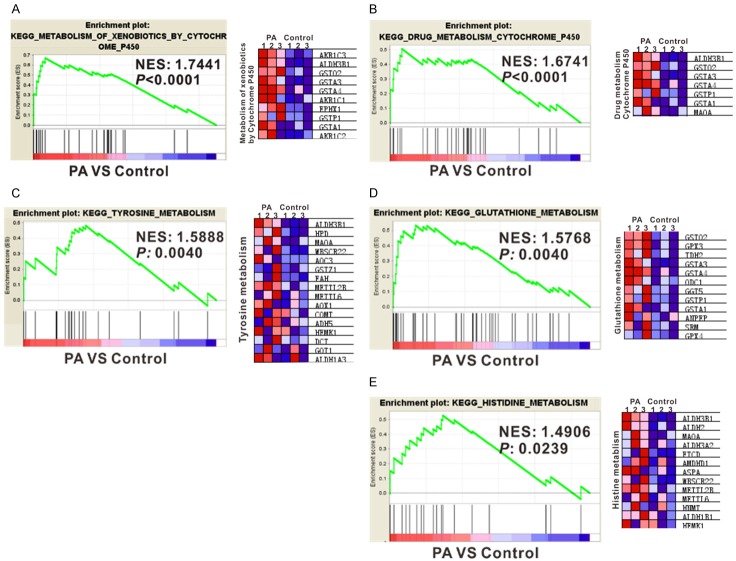
GSEA was then performed to investigate functional associations of gene expression changes in the tissue samples (PA and normal control). We generated a gene list with greatest changes using RNA-seq data, and the enrichment of Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathways was evaluated by GSEA. GSEA analysis indicated that 38 pathways were significantly altered in PA tissues, with P value less than 0.05. 35 KEGG pathways were enriched among genes differentially expressed in PA samples versus normal controls (Table 4), including ‘ribosome’, ‘oxidative phosphorylation’, ‘histidine metabolism’, ‘xenobiotics metabolism by Cytochrome P450’, ‘drug metabolism by Cytochrome P450’, ‘tyrosine metabolism’ and ‘glutathione metabolism’ (Figure 2). Control group had 3 signaling pathways enriched, including the ‘basal cell carcinoma’, ‘cell cycle’ and ‘Wnt’ signaling pathway (Table 5).
Table 4.
Enriched regulated (KEGG) biological pathways in primary aldosteronism
| Pathway | Size | Nes | Nom p-val | FDR q-val |
|---|---|---|---|---|
| Ribosome | 88 | 1.9619 | 0.0000 | 0.0000 |
| Oxidative_phosphorylation | 117 | 1.8973 | 0.0000 | 0.0000 |
| Proteasome | 46 | 1.8277 | 0.0000 | 0.0027 |
| Metabolism_of_xenobiotics_by_cytochrome_p450 | 70 | 1.7441 | 0.0000 | 0.0198 |
| Cardiac_muscle_contraction | 73 | 1.6957 | 0.0021 | 0.0363 |
| Parkinsons_disease | 114 | 1.6879 | 0.0000 | 0.0335 |
| Drug_metabolism_cytochrome_p450 | 72 | 1.6741 | 0.0000 | 0.0364 |
| Olfactory_transduction | 386 | 1.6561 | 0.0000 | 0.0418 |
| Tyrosine_metabolism | 42 | 1.5888 | 0.0040 | 0.0808 |
| Glutathione_metabolism | 50 | 1.5768 | 0.0040 | 0.0843 |
| Neuroactive_ligand_receptor_interaction | 271 | 1.5416 | 0.0000 | 0.1109 |
| Type_i_diabetes_mellitus | 43 | 1.5294 | 0.0103 | 0.1167 |
| Long_term_depression | 70 | 1.4925 | 0.0039 | 0.1583 |
| Histidine_metabolism | 28 | 1.4906 | 0.0239 | 0.1497 |
| Prion_diseases | 35 | 1.4563 | 0.0279 | 0.1924 |
| Huntingtons_disease | 173 | 1.4525 | 0.0000 | 0.1869 |
| Glycine_serine_and_threonine_metabolism | 31 | 1.4483 | 0.0367 | 0.1825 |
| Pathogenic_escherichia_coli_infection | 56 | 1.4373 | 0.0111 | 0.1923 |
| Autoimmune_thyroid_disease | 52 | 1.4060 | 0.0268 | 0.2384 |
| Graft_versus_host_disease | 40 | 1.4035 | 0.0397 | 0.2316 |
| Dilated_cardiomyopathy | 90 | 1.3973 | 0.0141 | 0.2321 |
| Retinol_metabolism | 64 | 1.3915 | 0.0273 | 0.2299 |
| Complement_and_coagulation_cascades | 69 | 1.3895 | 0.0204 | 0.2139 |
| Bladder_cancer | 42 | 1.3883 | 0.0412 | 0.2074 |
| Hematopoietic_cell_lineage | 87 | 1.3826 | 0.0108 | 0.2090 |
| Drug_metabolism_other_enzymes | 51 | 1.3672 | 0.0448 | 0.2192 |
| Arginine_and_proline_metabolism | 53 | 1.3617 | 0.0453 | 0.2205 |
| Alzheimers_disease | 157 | 1.3439 | 0.0147 | 0.2340 |
| Hypertrophic_cardiomyopathy_hcm | 83 | 1.3361 | 0.0270 | 0.2262 |
| Glycolysis_gluconeogenesis | 61 | 1.3302 | 0.0467 | 0.2305 |
| Mapk_signaling_pathway | 265 | 1.2885 | 0.0000 | 0.2754 |
| Chemokine_signaling_pathway | 189 | 1.2875 | 0.0169 | 0.2703 |
| Cell_adhesion_molecules_cams | 133 | 1.2819 | 0.0343 | 0.2687 |
| Calcium_signaling_pathway | 176 | 1.2666 | 0.0183 | 0.2741 |
| Cytokine_cytokine_receptor_interaction | 261 | 1.2600 | 0.0108 | 0.2634 |
Figure 2.
Gene set enrichment analysis (GSEA) of signaling pathways strongly associated with PA (A-E). Normalized Enrichment score (NES) and P value are shown as indicated (left panels). The heatmaps of gene expression in KEGG signaling pathways were shown in the right panels. Gene expression was normalized for each row. Higher expression was represented in red and lower expression in blue.
Table 5.
Enriched regulated (KEGG) biological pathways in control
| Pathway | Size | Nes | Nom p | Fdr q-val |
|---|---|---|---|---|
| Basal_cell_carcinoma | 39 | -1.8667 | 0.0039 | 0.0485 |
| Cell_cycle | 101 | -1.5038 | 0.0118 | 0.6349 |
| Wnt_signaling_pathway | 111 | -1.4218 | 0.0203 | 0.7496 |
Confirmation of expression measurements with real-time PCR and Western blotting
To confirm the results of RNA-seq, real-time PCR was performed to detect the mRNA expression of 6 up-regulated genes. As shown in Figure 3, CYP11A1, ALDH3B1, ALDH2, GSTO2, GPX3 and IDH2 were up-regulated in all 30 PA samples (Group A) and the 3 PA samples used for RNA-seq (Group B), although the difference of IDH2 mRNA expression between PA and Control in Group B was not significant. The mRNA levels of CYP11B2, which is a well-known up-regulated gene [25,26] and was not identified by RNA-seq, were also tested. CYP11B2 mRNA expression was significantly increased in PA samples of Group A as compared to Control samples. In Group B, CYP11B2 mRNA expression had an up-regulated trend in PA samples, although the change was not statistically significant.
Figure 3.
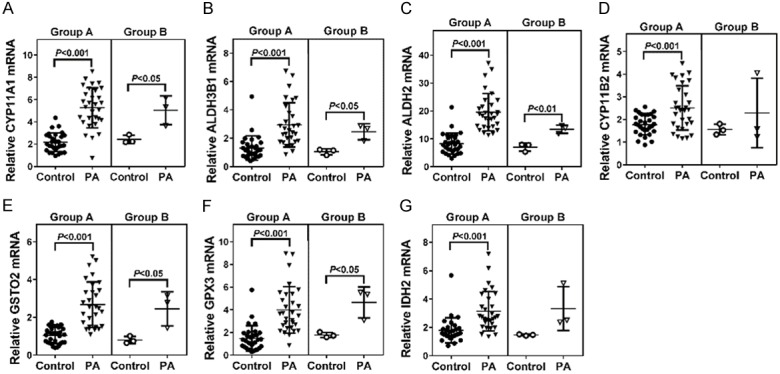
The mRNA levels of CYP11A1 (A), ALDH3B1 (B), ALDH2 (C), CYP11B2 (D), GSTO2 (E), GPX3 (F) and IDH2 (G) were detected by real-time PCR. GAPDH was served as an internal control. Statistical significance was evaluated using the Student’s t-test. Group A: 30 pairs of Adrenal tumor tissue and adjacent normal adrenal samples. Group B: 3 pairs of Adrenal tumor tissue and adjacent normal adrenal samples which were also used for RNA-seq.
Western blot analysis was also performed on 4 pairs of samples, which were not included for RNA-seq. Similar results were obtained at translational levels of CYP11A1, ALDH3B1, ALDH2, GSTO2, GPX3 and IDH2 (Figure 4). These findings were consistent with our RNA-seq results.
Figure 4.
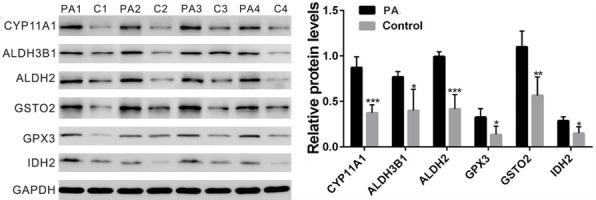
The protein levels of CYP11A1, ALDH3B1, ALDH2, GSTO2, GPX3 and IDH2 in PA (PA1, PA2, PA3 and PA4) and control samples (C1, C2, C3 and C4) were detected by Western blot. GAPDH was served as a loading control. Statistical significance was evaluated using the Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Several studies have described expression profiling analysis of aldosterone-producing adenomas compared to adjacent normal adrenal gland by using microarray [14-18]. To our knowledge, this is the first investigation using RNA-seq methodology to conduct gene expression profiling of human adrenal glands from patients with PA. Using RNA-seq analysis, 1,093 transcripts were significantly changed in PA affected samples. Some genes, such as CYP11A1 (Cytochrome P450 Family 11 Subfamily A Member 1) [27], VSNL1 (Visinin Like 1) [22,28], KCNJ5 (Potassium Voltage-Gated Channel Subfamily J Member 5) [14,29], ATP2B3 (ATPase Plasma Membrane Ca2+ Transporting 3) [30] AKR1C3 (Aldo-Keto Reductase Family 1 Member C3) [15], RGS4 (Regulator Of G-Protein Signaling 4) [17] have been identified by previous transcriptome studies and linked to PA pathogenesis, and numerous genes, such as ATAD3C, PROM1 and HOXD10, have not previously been studied in PA. Our data suggest that the next generation RNA-sequencing is powerful and sensitive to detect changes in gene expression. Real-time PCR and Western blot assays confirmed the RNA-seq results for the genes of interest. The application of GSEA provided additional information of the biological processes that are differentially regulated in PA and control samples. Here, we also identified 38 signaling pathways altered in PA samples, some of which (e.g., ‘ribosome’, ‘oxidative phosphorylation’, ‘xenobiotics metabolism by Cytochrome P450’, ‘drug metabolism by Cytochrome P450’ and ‘Wnt’ pathways) were consistent with previous studies. Aldosterone is a well-known hormone to stimulate energy acquisition [31]. The observed up-regulation of the ‘KEGG ribosome’ pathway in PA subjects is consistent with a response to increased metabolism by excessive aldosterone secretion in PA patients [32]. A previous study showed that a single intraperitoneal injection of aldosterone produced a rapid oxidative phosphorylation in mouse liver mitochondria [33]. Cytochrome P450 family proteins can regulate aldosterone biosynthesis and are involved in the pathogenesis of PA [27,34]. Oxidative stress was increased in PA patients [35]. It is not surprising that the ‘KEGG oxidative phosphorylation’, ‘KEGG xenobiotics metabolism by Cytochrome P450’ and ‘KEGG drug metabolism by Cytochrome P450’ and ‘glutathione metabolism’ pathways were up-regulated in PA subjects. Finally, the down-regulated of ‘KEGG Wnt’ pathway is consistent with a previous study that the transcriptome profiles of Wnt pathway genes in APA adrenals were distinct from control adrenals [36]. More interestingly, several KEGG metabolism pathways, including histidine metabolism and tyrosine metabolism pathways were enriched in PA samples, which were discovered for the first time.
In summary, gene expression profiling in adrenal glands demonstrated significant differences in transcript levels between PA and control. GSEA further identified differences in biological pathways relating to protein synthesis, energy acquisition and metabolisms between the two groups. Our current study greatly extents the range of potential genes involved in PA pathogenesis, which have not been previously considered.
Acknowledgements
This work was supported by Research Grant of Huangpu Health and Family Planning Commission of Shanghai (Grant No.HKW201605).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17. [PubMed] [Google Scholar]
- 2.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 3.Mulatero P, Dluhy RG, Giacchetti G, Boscaro M, Veglio F, Stewart PM. Diagnosis of primary aldosteronism: from screening to subtype differentiation. Trends Endocrinol Metab. 2005;16:114–119. doi: 10.1016/j.tem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Rossi GP. Diagnosis and treatment of primary aldosteronism. Endocrinol Metab Clin North Am. 2011;40:313–332. vii–viii. doi: 10.1016/j.ecl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg. 1964;107:159–172. doi: 10.1016/0002-9610(64)90252-1. [DOI] [PubMed] [Google Scholar]
- 6.Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19:88–90. doi: 10.1016/j.tem.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 8.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes CJ, Im H, Cao A, Hennigs JK, Wang L, Sa S, Chen PI, Nickel NP, Miyagawa K, Hopper RK. RNA sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:356–366. doi: 10.1164/rccm.201408-1528OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickrell JK, Marioni JC, Pai AA, Degner JF, Engelhardt BE, Nkadori E, Veyrieras JB, Stephens M, Gilad Y, Pritchard JK. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 2010;464:768–772. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Satoh F, Morimoto R, Nakamura Y, Sasano H, Auchus RJ, Edwards MA, Rainey WE. Gene expression profiles in aldosteroneproducing adenomas and adjacent adrenal glands. Eur J Endocrinol. 2011;164:613–619. doi: 10.1530/EJE-10-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- 16.Lenzini L, Seccia TM, Aldighieri E, Belloni AS, Bernante P, Giuliani L, Nussdorfer GG, Pessina AC, Rossi GP. Heterogeneity of aldosteroneproducing adenomas revealed by a whole transcriptome analysis. Hypertension. 2007;50:1106–1113. doi: 10.1161/HYPERTENSIONAHA.107.100438. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, Yoshimoto T, Nakabayashi K, Tsuchiya K, Minami I, Bouchi R, Izumiyama H, Fujii Y, Abe K, Tayama C. Integration of transcriptome and methylome analysis of aldosterone-producing adenomas. Eur J Endocrinol. 2015;173:185–195. doi: 10.1530/EJE-15-0148. [DOI] [PubMed] [Google Scholar]
- 18.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosaand zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez D, François P, Farinelli L, Østerås M, Schrenzel J. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 2008;18:802–809. doi: 10.1101/gr.072033.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Rousseau D. ATAD3, a vital membrane bound mitochondrial ATPase involved in tumor progression. J Bioenerg Biomembr. 2012;44:189–197. doi: 10.1007/s10863-012-9424-5. [DOI] [PubMed] [Google Scholar]
- 22.Goller T, Seibold UK, Kremmer E, Voos W, Kolanus W. Atad3 function is essential for early post-implantation development in the mouse. PLoS One. 2013;8:e54799. doi: 10.1371/journal.pone.0054799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Chen S, Xue M, Zhong J, Wang X, Gan L, Lam EK, Liu X, Zhang J, Zhou T. Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med. 2012;18:389. doi: 10.2119/molmed.2011.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallo F, Pezzi V, Barzon L, Mulatero P, Veglio F, Sonino N, Mathis J. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 2002;147:795–802. doi: 10.1530/eje.0.1470795. [DOI] [PubMed] [Google Scholar]
- 26.Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98:1567–1574. doi: 10.1210/jc.2012-3726. [DOI] [PubMed] [Google Scholar]
- 27.Zennaro MC, Jeunemaitre X, Boulkroun S. Integrating genetics and genomics in primary aldosteronism. Hypertension. 2012;60:580–588. doi: 10.1161/HYPERTENSIONAHA.111.188250. [DOI] [PubMed] [Google Scholar]
- 28.Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59:833–839. doi: 10.1161/HYPERTENSIONAHA.111.188532. [DOI] [PubMed] [Google Scholar]
- 29.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. 444e1–2. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 31.Santana P, Akana S, Hanson E, Strack AM, Sebastian R, Dallman M. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology. 1995;136:2214–2222. doi: 10.1210/endo.136.5.7720670. [DOI] [PubMed] [Google Scholar]
- 32.Devenport L, Manes G, Thomas T, Mena S, Kem D, Knehans A. Aldosterone and the mobilization of energy. Appetite. 1987;8:81–90. doi: 10.1016/s0195-6663(87)80001-6. [DOI] [PubMed] [Google Scholar]
- 33.Bedrak E, Samoiloff V. Aldosterone and oxidative phosphorylation in liver mitochondria. J Endocrinol. 1966;36:63–71. doi: 10.1677/joe.0.0360063. [DOI] [PubMed] [Google Scholar]
- 34.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Molecular and Cellular Endocrinology. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, Solis M, García L, Lavandero S, Jalil J. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28:2120–2126. doi: 10.1097/HJH.0b013e32833d0177. [DOI] [PubMed] [Google Scholar]
- 36.Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, Lefebvre H, Louiset E, Jeunemaitre X, Meatchi T, Benecke A, Lalli E, Zennaro MC. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.