Abstract
Ovarian cancer is a gynaecological cancer with a high mortality rate. In recent years, circulating tumour cells (CTCs) have attracted attention from scientists because of their significant association with metastasis. However, due to the low CTC enrichment rate of the conventional CellSearch system and limited clinical sample sizes, only a small number of studies have focused on CTCs and epithelial ovarian cancer (EOC). Here, we apply a microfluidic system with immunomagnetic beads preconjugated with an anti-EpCAM antibody to enrich CTCs from whole blood and then analyse the enriched cells by immunofluorescence staining and automatic fluorescence microscope scanning. The average recovery rate of SK-OV-3 EOC cells was 70.2%±13.3%. When using blood samples from EOC patients and healthy volunteers, CTC counts of more than 8 cells were detected in 20 of 23 EOC patients (87.0%) but in none of the 16 healthy volunteers (0%). Total CTC counts were found to be significantly (P<0.05) elevated in the EOC group (median =55.0 [29.5, 123.0] CTCs/7.5 mL) compared with the healthy control group (median =0.5 [0,3.5] CTCs/7.5 mL). In conclusion, this is the first study to use the IsoFlux system on ovarian cancer samples. This system can efficiently capture EOC CTCs from a majority of patients and may provide a potential tool for further biological studies and for the development of in vitro EOC diagnostic products.
Keywords: Epithelial ovarian cancer, circulating tumour cells, immunomagnetic beads, microfluidic, anti-EpCAM
Introduction
Ovarian cancer is one of the most common malignant tumours with a high mortality in females [1,2], posing a serious threat to women’s health and even life expectancy [3]. More than 90% of ovarian cancer cases are epithelial ovarian cancer (EOC) [4]. Although diverse methodologies, such as image-based assessment, serum tumour marker measurements, cytological diagnosis and histopathologic biopsy, have been used to detect ovarian cancer [5,6], a considerable proportion of patients (almost 70%) are diagnosed at an advanced stage (phase III or IV) [7] with a high metastasis rate and poor prognosis, and the five-year survival rate for these patients is less than 30% [8-12]. This phenomenon indicates that current approaches are insufficient to meet the needs of early diagnosis of ovarian cancer; more effective and sensitive methods therefore should be developed to overcome this challenge.
Previous studies have identified circulating tumour cells (CTCs) in patient peripheral blood [13], and these cells can be transported to diverse tissues and organs by the circulation system and subsequently develop into novel solid tumours [14,15]. CTCs are thus closely associated not only with clinical stages, early recurrence and metastasis of tumours but also with progress-free survival time, overall survival time, and even drug efficacy [15-17]. CTC detection is a novel technique that involves the isolation and analysis (e.g., cell counting and gene sequencing) of CTCs from the peripheral blood. This technique has demonstrated prognostic value in numerous solid tumours. Compared with the previously mentioned traditional methods, the detection of ovarian cancer CTCs has the significant advantages of being sensitive, effective, non-invasive and convenient. However, CTCs are so rare that they account for just 1/106 ~1/107 of the white blood cells (WBCs) in the peripheral blood, which makes detecting these cells difficult.
The IsoFlux System (Fluxion Biosciences) combines immunomagnetic beads with microfluidic technology to isolate CTCs from whole blood with high efficiency. CTCs are coupled to immunomagnetic particles and then passed through a flow channel traversing a magnetic field, resulting in a magnetic flow cytometry setup. Immunofluorescence staining is performed on recovered samples to identify tumour cells. The IsoFlux System was designed to enhance CTC capture and reduce WBC background, and it has been shown to increase CTC detection sensitivity in prostate cancer, colorectal cancer [18] and bladder cancer [19].
CTC detection in EOC has thus far attracted little attention, and publications have almost exclusively used the CellSearch system. In a representative study conducted prior to the start of therapy [20], >2 CTCs were detected in only 14% of patients tested, but some correlation to clinical outcomes was observed. Another study [21] reported the overall recovery of a small number of CTCs (0-8 cells) and no correlation to outcomes.
In this study, a microfluidic separation system (IsoFlux) was used to detect CTCs in advanced EOC for the first time. We performed a spike-in experiment with SK-OV-3 (an EOC cell line) to assess the recovery rate of the IsoFlux System. Then, clinical validation was performed using blood samples from healthy volunteers and patients with ovarian cancer to evaluate the sensitivity and specificity of CTC detection. This study explored the potential role of the IsoFlux System in the diagnosis of EOC, which offers a non-invasive and convenient method of identifying individuals with known or suspected EOC.
Materials and methods
Participants
The present study was conducted at Sun Yat-sen Memorial Hospital of Sun Yat-sen University from October 2015 to August 2016. A total of 39 participants were enrolled in the study, with an average age of 57.9. There were 23 patients with known or suspected EOC and 16 healthy volunteers. This study was approved by the local research ethics committee, and informed consent was obtained from each participant. The baseline characteristics of the ovarian cancer patients are summarized in Table 1.
Table 1.
Patient characteristics at the time of primary diagnosis of ovarian cancer
| Total no. of patients | 23 | 100% |
| Age | Average, 57.9 years | SD, 11.7 years |
| FIGO stage | ||
| FIGO I-II | 6 | 26.1% |
| FIGO III-IV | 16 | 69.6% |
| Unknown | 1 | 4.3% |
| Ovarian infiltration | ||
| Yes | 16 | 69.6% |
| No | 5 | 21.7% |
| Unknown | 2 | 8.7% |
| Cancer type | ||
| Epithelial | 23 | 100% |
| Any other type | 0 | 0 |
| CA125 | ||
| Up | 20 | 87.0% |
| Normal | 3 | 13.0% |
| Unknown | 0 | 0 |
| HE4 | ||
| Up | 16 | 69.6% |
| Normal | 0 | 0 |
| Unknown | 7 | 30.4% |
Clinical blood samples used to isolate and enumerate CTCs were collected in 10 mL BD Vacutainer® ethylenediaminetetraacetic acid (EDTA) tubes and shipped overnight at room temperature for processing within 36 hours of the initial blood draw. The actual blood volume was 6 to 10 mL for IsoFlux enumeration tests.
Cell culture
The SK-OV-3 cell line was obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in McCoy’s medium (Gibco, Thermo Fisher Scientific, USA) containing 10% foetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT) and 1% penicillin-streptomycin at 37°C in a 5% CO2 humidified atmosphere.
Cell line sample preparation
A spike-in test was performed to assess the performance of the IsoFlux system with regard to CTC isolation and enrichment. In brief, peripheral blood from healthy donors was collected into EDTA Vacutainer tubes (Kangjian, China) and subsequently spiked with SK-OV-3 cells. Cultured SK-OV-3 cells were collected and suspended in a stock solution and counted using a haemocytometer. A series of 7.5 mL blood samples were then subsequently spiked with 20, 40, 60, 80, 100, 150 or 300 cells, while the clinical samples from the ovarian cancer patients remained free of SK-OV-3 cells [18].
Isolation of CTCs
The peripheral blood mononuclear cell fraction of the blood samples was obtained using LeucoSep tubes (Greiner Bio-One, Monroe, NC) with 15 mL of Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA). These cells were subsequently resuspended in 1 mL of binding buffer (CTC Enrichment Kit; Fluxion Biosciences, Inc.). After adding immunomagnetic beads preconjugated with an anti-EpCAM antibody (CTC Enrichment Kit; Fluxion Biosciences, Inc.), the samples were shaken gently on a rotator and incubated for 1.2 hours at 4°C.
Next, the samples were loaded into the inlet well of the microfluidic cartridge’s inlet well. As a head pressure of 2 psi was applied, the samples thus passed through the channel at a flow rate of 20 μL per minute, and the isolation process took less than 45 minutes. Finally, the isolated target cells of each sample were collected in 1.5 mL Eppendorf tubes for further study [18].
Enumeration analysis
For target cell samples, isolated target cells were recovered from the IsoFlux disk through pipetting and dispensed onto a standard slide for imaging. Immunofluorescence staining was performed using anti-cytokeratin (CK) and anti-CD45 antibodies and Hoechst 33342 (for nuclear staining) (CTC Enumeration Kit; Fluxion Biosciences, Inc.). Recovered CTCs were fixed with binding buffer (BB) containing 1.8% formaldehyde, washed, and blocked with 10% normal donkey serum in BB. The cells were stained with a rabbit polyclonal anti-human CD45 antibody followed by a Cy3-conjugated donkey anti-rabbit antibody. After permeabilization with 0.1% Triton X-100, the cells were then stained with a fluorescein isothiocyanate-conjugated anti-CK antibody. For CK staining, we used the antibody clone CK3-6H5, a pancytokeratin-specific antibody that is likely to recognize all simple epithelium CKs and that has been shown to bind CKs [22,23]. Stained CTCs were mounted in SlowFade Gold mounting medium with Hoechst 33342 (Life Technologies, Inc., Foster City, CA) to SensoPlate glass-bottom multiwall plates (Greiner Bio-One) for imaging.
Images were captured using an inverted fluorescence microscope (Nikon ECLIPSE Ti-E, Nikon, Japan), and the cells were measured by the software (Ti-E scanning, Nikon, Japan) based on fluorescence [24]. Cells were scored as CTCs if they were CK+, CD45-, nucleated, and morphologically intact. The total cell count was determined by an automated counting of all nuclei and reported as a measure of sample purity.
Statistical analysis
The data were analysed using SPSS version 20.0 (IBM, Armonk, NY, USA) and expressed as the median and mean ± standard deviation. Random analysis of variance was also performed; the result was considered statistically significant when P<0.05, and expression differences were considered statistically significant when P<0.01. Graphs were plotted by GraphPad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Analysis of CTC recovery
As CTCs exist at a very low concentration in the peripheral blood of cancer patients, a high recovery efficiency is critical for diagnostic testing. Here, cell-spiking experiments were conducted to determine the recovery rate of the IsoFlux System using cultured cells that allow for control of the starting tumour cell number and EpCAM expression level. We observed an EpCAM expression of nearly 100% in the cell line. CTCs were defined as Hoechst+, CK+, CD45-, and morphologically intact after immunofluorescence staining.
SK-OV-3 cells were spiked into 7.5 mL of healthy donors’ blood as described above. The measured recovery rate ranged from 61.2 (±17.0)% to 77.7 (±11.1)% (Figure 1). The average recovery percentage across all spike-in levels was 70.2%, with an overall SD of 13.3%. Linear fitting to the results yielded an R2 value of 0.97, with a slope of 0.77.
Figure 1.
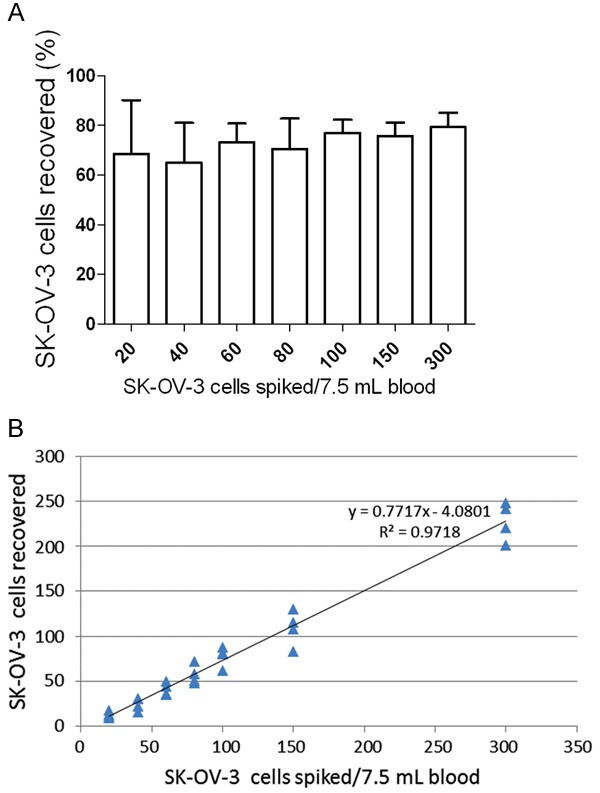
The capture efficiency and linearity of the current methodology. A: Model CTC samples were prepared by spiking different amounts of SK-OV-3 cells into 7.5 mL of healthy donors’ blood; the samples were then processed by the IsoFlux System. The average recovery percentage across all spike-in levels was 70.2 (±13.3)%. Standard errors of 4 parallel samples are shown for each spiked concentration. B: The R2 value was 0.97 with a slope of 0.77 (77% of cells captured).
Recovery of CTCs in clinical ovarian cancer samples
Blood samples were collected from 39 participants, including 23 (59.0%) patients diagnosed with EOC, with a mean age of 57.9±11.7, and 16 (41.0%) healthy volunteers. Of the EOC patients, 26.1% had Stage I-II disease, 69.6% had Stage III-IV disease, and 4.3% had an unknown disease stage. Patients with ovarian infiltration, high CA125 and high HE4 accounted for 69.6%, 87.0% and 69.6% of the EOC patients, respectively.
The clinical specimens were processed using the IsoFlux platform as described previously. Representative images of target CTCs and background WBCs after immunofluorescence staining are shown in Figure 2, in which nuclei are stained blue by Hoechst 33342, CK-positive cells are stained green by fluorescein isothiocyanate, and CD45-positive cells are stained red by Cy3. The CTCs were defined as Hoechst+, CK+, CD45-, nucleated and morphologically intact, while WBCs were identified as being Hoechst+, CK-, and CD45+. According to the enumeration analysis, the CTC recovery rate of each specimen was assessed, and the results are shown in Table 2 and Figure 3.
Figure 2.
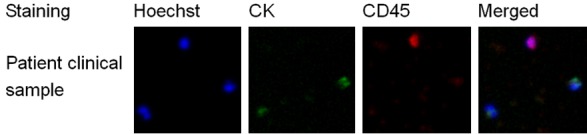
Enumeration analysis of CTCs from an ovarian cancer patient. After immunofluorescence staining, Hoechst+, CK+, CD45-, and morphologically intact cells were defined as CTCs, while Hoechst+, CK-, and CD45+ cells were defined as WBCs.
Table 2.
CTC count in 7.5 mL of blood from EOC patients and healthy volunteers
| Patient Identifier | CTC Count | Total Cell Count | Proportion | Healthy Identifier | CTC Count | Total Cell Count | Proportion |
|---|---|---|---|---|---|---|---|
| P1 | 31 | 2136 | 1.45% | H1 | 0 | 1876 | 0.00% |
| P2 | 43 | 1213 | 3.54% | H2 | 0 | 2020 | 0.00% |
| P3 | 228 | 4061 | 5.61% | H3 | 3 | 1795 | 0.17% |
| P4 | 1 | 10983 | 0.01% | H4 | 7 | 4037 | 0.17% |
| P5 | 48 | 9874 | 0.49% | H5 | 3 | 2756 | 0.11% |
| P6 | 75 | 2305 | 3.25% | H6 | 0 | 4349 | 0.00% |
| P7 | 63 | 1735 | 3.63% | H7 | 0 | 12358 | 0.00% |
| P8 | 158 | 10194 | 1.55% | H8 | 0 | 21558 | 0.00% |
| P9 | 49 | 3895 | 1.26% | H9 | 0 | 21969 | 0.00% |
| P10 | 626 | 43671 | 1.43% | H10 | 0 | 3231 | 0.00% |
| P11 | 1208 | 16383 | 7.37% | H11 | 0 | 2332 | 0.00% |
| P12 | 10 | 13174 | 0.08% | H12 | 8 | 5462 | 0.15% |
| P13 | 254 | 9555 | 2.66% | H13 | 1 | 3319 | 0.03% |
| P14 | 85 | 6341 | 1.34% | H14 | 8 | 4157 | 0.19% |
| P15 | 55 | 4083 | 1.35% | H15 | 1 | 2255 | 0.04% |
| P16 | 28 | 4169 | 0.67% | H16 | 5 | 2651 | 0.19% |
| P17 | 88 | 6402 | 1.37% | ||||
| P18 | 26 | 2760 | 0.94% | ||||
| P19 | 283 | 4080 | 6.94% | ||||
| P20 | 55 | 2518 | 2.18% | ||||
| P21 | 48 | 15242 | 0.31% | ||||
| P22 | 2 | 1654 | 0.12% | ||||
| P23 | 0 | 1851 | 0.00% | ||||
| Median | 55.0 | 4083.0 | 1.37% | 0.5 | 3275.0 | 0.02% | |
| Quartile | 29.5, 123.0 | 2411, 10034 | 0, 3.5 | 2313, 4627 | |||
| Mean | 150.6 | 7751.3 | 2.70% | 2.3 | 6007.8 | 0.07% | |
| SD | 269.1 | 9072.7 | 0.00% | 3.1 | 6645.1 | 0.08% | |
| Independent | |||||||
| Sample t test | P<0.05 (CTC Count) | ||||||
Figure 3.
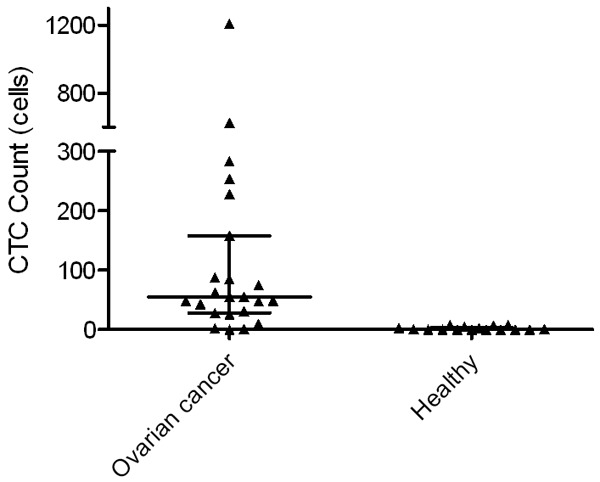
Distribution of CTC counts. The median number of CTCs in EOC patients was 55.0, while the median number in healthy volunteers was 0.5.
CTCs were isolated from all patients except P23. The median number of CTCs was 55 (29.5, 123.0) CTCs/7.5 mL sample; however, fewer CTCs were detected in the healthy group, which exhibited a median of 0.5 (0, 3.5) CTCs/7.5 mL sample. The average number of CTCs was 150.6 (±269.1) CTCs/7.5 mL in the EOC group and 2.1 (±3.1) CTCs/7.5 mL sample in the healthy group. In addition, the CTCs comprised 2.7% and 0.07% of the total cells in the EOC group and the healthy group, respectively. The difference in the number of CTCs was statistically significant between the 2 groups (P<0.05). Furthermore, 20 of the 23 (87.0%) EOC patients had a relatively high number of CTCs (more than 10 in 7.5 mL of blood), while none of the healthy donors had a CTC count above 8.
Discussion
Different methodologies have been used to isolate and collect CTCs according to their physical and biological properties. For example, Cell Search, developed by Johnson & Johnson (JNJ) and approved by the FDA in 2004 as a representative technique of the first-generation testing platform for CTC detection, has been primarily used in the prognostic evaluation of diverse cancers such as breast cancer [25], colorectal cancer [26,27] and prostate cancer [28]. However, due to its low sensitivity and inflexible system, CellSearch can be used almost exclusively for advanced-stage cancer detection, as it does not efficiently detect cancer at the early and middle stages of progression. Furthermore, low yield and purity are also drawbacks of the system, posing challenges for downstream analyses, especially molecular detection. For instance, CellSearch has previously been used to detect CTCs in serum from women with ovarian cancer. Although CTCs could be detected in 64 of the 78 (82%) patients, only a few cells (0-8) were isolated by the CellSearch technique, which uses a cut-off of 2 cells [21]. Another study reported an even lower detection rate, with 2 or more CTCs (range 2-566) detected in only 31 of 216 (14.4%) ovarian cancer patients prior to the start of therapy [20].
The IsoFlux platform, as a representative of the application of microfluidics technology to CTC capture, has been previously used to detect prostate cancer, colorectal cancer [18] and bladder cancer [19]. Nevertheless, the utility of IsoFlux analysis of CTCs in the assessment of ovarian cancer has not yet been described. In this pilot study, we performed cell spike-in experiments to determine the recovery rate of the platform. As shown in Figure 1, when we spiked different numbers of SK-OV-3 cells into 7.5 mL of blood from healthy donors, the recovery rate of the device ranged from 61.2 (±17.0)% to 77.7 (±11.1)% with an average of 70.2% (the overall SD was 13.3%). We noticed that with increasing amounts of spike-in cells, the recovery rate exhibited an overall increasing trend. When the spike-in cell number was equal to or greater than 100, the recovery rate was relatively stable and was maintained at above 72%. The R2 value of our data was 0.97, indicating a sufficient goodness of fit. We found that when fewer cells (e.g., 20 and 40 cells) were spiked into the healthy blood, the recovery rate was lower, and greater variation was observed between 4 parallel experiments. This was likely because the estimated number of spiked-in SK-OV-3 cells was not completely accurate, which may have had a greater impact on the recovery rate when the number of spiked-in cells was small.
Twenty-three clinical blood samples and 16 healthy blood samples were included in this pilot study. The IsoFlux System recovered >8 CTCs from 7.5 mL of blood in 87.0% (20/23) of the matched clinical samples tested, compared to 0% (0/16) of the healthy donor samples. The data indicate that due to its effective design, the IsoFlux microfluidic system provides significant improvements in sensitivity and specificity for the detection of EOC.
The CTC fraction isolated by the IsoFlux platform had less background interference from WBCs, regardless of the cell line or clinical samples used. This feature of the IsoFlux System provided ideal conditions for immunofluorescence staining of CTCs and was non-invasive and convenient for downstream analysis (Figure 2).
This is the first study to use a commercial microfluidic cell separation system (IsoFlux) on ovarian cancer samples. Analytical testing indicated that the IsoFlux microfluidic device could be used to isolate EOC cells at a high recovery rate. A small-sample-size clinical feasibility study was performed to test the sensitivity and specificity of this device in the detection of individuals with known or suspected EOC, and the results showed superior sensitivity/specificity compared with previous studies performed using other strategies. Further studies should include large-sample-size clinical trials correlating CTC results with clinical outcomes to identify the utility of the IsoFlux System in the early diagnosis and therapeutic strategy determination of EOC.
Acknowledgements
This study was funded by the Peacock Project of Shenzhen Science and Technology Innovation Committee (KQCX2015033016451172), a project of Shenzhen Strategic Emerging Industries and Future Industry Development Special Funds (number 887, 2016, Shenzhen Science and Technology Innovation Committee).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai A, Xu J, Aysola K, Qin Y, Okoli C, Hariprasad R, Chinemerem U, Gates C, Reddy A, Danner O, Franklin G, Ngozi A, Cantuaria G, Singh K, Grizzle W, Landen C, Partridge EE, Rice VM, Reddy ES, Rao VN. Epithelial ovarian cancer: an overview. World J Transl Med. 2014;3:1–8. doi: 10.5528/wjtm.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 6.Miller RW, Ueland FR. Risk of malignancy in sonographically confirmed ovarian tumors. Clin Obstet Gynecol. 2012;55:52–64. doi: 10.1097/GRF.0b013e31824970cf. [DOI] [PubMed] [Google Scholar]
- 7.Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102:222–229. doi: 10.1093/jnci/djp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piver MS, Malfetano J, Baker TR, Hempling RE. Five-year survival for stage IC or stage I grade 3 epithelial ovarian cancer treated with cisplatin-based chemotherapy. Gynecol Oncol. 1992;46:357–360. doi: 10.1016/0090-8258(92)90232-8. [DOI] [PubMed] [Google Scholar]
- 9.Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–438. [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 11.Krzystyniak J, Ceppi L, Dizon DS, Birrer MJ. Epithelial ovarian cancer: the molecular genetics of epithelial ovarian cancer. Ann Oncol. 2016;27(Suppl 1):i4–i10. doi: 10.1093/annonc/mdw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80:609–616. [PubMed] [Google Scholar]
- 13.Lu DY, Chen XL, Ding J. Individualized cancer chemotherapy integrating drug sensitivity tests, pathological profile analysis and computational coordination - an effective strategy to improve clinical treatment. Med Hypotheses. 2006;66:45–51. doi: 10.1016/j.mehy.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat Rev Clin Oncol. 2014;11:401–412. doi: 10.1038/nrclinonc.2014.82. [DOI] [PubMed] [Google Scholar]
- 15.Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer--clinical applications. Nat Rev Clin Oncol. 2010;7:693–701. doi: 10.1038/nrclinonc.2010.171. [DOI] [PubMed] [Google Scholar]
- 16.Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabieres C, Janni W, Messina C, Paoletti C, Muller V, Hayes DF, Piccart M, Pierga JY. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32:179–188. doi: 10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 18.Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, Ionescu-Zanetti C. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol. 2013;6:528–538. doi: 10.1593/tlo.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alva A, Friedlander T, Clark M, Huebner T, Daignault S, Hussain M, Lee C, Hafez K, Hollenbeck B, Weizer A, Premasekharan G, Tran T, Fu C, Ionescu-Zanetti C, Schwartz M, Fan A, Paris P. Circulating tumor cells as potential biomarkers in bladder cancer. J Urol. 2015;194:790–798. doi: 10.1016/j.juro.2015.02.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P, Monk BJ. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent ad-vanced ovarian cancer. Gynecol Oncol. 2011;122:567–572. doi: 10.1016/j.ygyno.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol. 2013;131:352–356. doi: 10.1016/j.ygyno.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Arrington AK, Heinrich EL, Lee W, Duldulao M, Patel S, Sanchez J, Garcia-Aguilar J, Kim J. Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int J Mol Sci. 2012;13:12153–12168. doi: 10.3390/ijms131012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valkenburg KC, Amend SR, Verdone JE, van der Toom EE, Hernandez JR, Gorin MA, Pienta KJ. A simple selection-free method for detecting disseminated tumor cells (DTCs) in murine bone marrow. Oncotarget. 2016;7:69794–69803. doi: 10.18632/oncotarget.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SH, Becker TM, Chua W, Caixeiro NJ, Ng WL, Kienzle N, Tognela A, Lumba S, Rasko JE, de Souza P, Spring KJ. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett. 2014;346:24–33. doi: 10.1016/j.canlet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, Xu H, Wang Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer. 2015;15:202. doi: 10.1186/s12885-015-1218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldkorn A, Ely B, Quinn DI, Tangen CM, Fink LM, Xu T, Twardowski P, Van Veldhuizen PJ, Agarwal N, Carducci MA, Monk JP 3rd, Datar RH, Garzotto M, Mack PC, Lara P Jr, Higano CS, Hussain M, Thompson IM Jr, Cote RJ, Vogelzang NJ. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]


