Abstract
This study was to investigate the effects of microRNA-21 (miR-21) on ox-LDL-induced HUVECs apoptosis. MTT assay was performed to evaluate the proliferation of HUVECs. Quantitative RT-PCR was conducted to quantify the expression of miR-21. Western blotting was used to determine protein expression. Annexin V/propidium iodide double staining was adopted to detect cell apoptosis. We found that ox-LD significantly induced HUVECs apoptosis and reduced miR-21 expression. MiR-21 mimic attenuated the apoptosis of HUVECs under ox-LDL treatment compared with the NC groups while miR-21 inhibitors promoted that of HUVECs. MiR-21 directly targeted PDCD4 in HUVECs. Moreover, miR-21 significantly regulated the downstream apoptotic proteins like bax, bad, bcl-2 and caspase3, meanwhile it enhanced the phosphorylation of ERK.
Keywords: microRNA-21, HUVECs, ox-LDL, apoptosis, PDCD4
Introduction
Endothelial cells line the inner layer of vascular walls and have a key role in the maintenance of vascular homeostasis. Endothelial dysfunction is significantly associated with atherosclerosis and cardiovascular disease, for which oxidized low-density lipoprotein (Ox-LDL) has been identified as a risk factor in the pathogenesis of atherosclerosis. Ox-LDL causes vascular endothelial apoptosis by triggering lipid accumulation, local inflammation, and oxidative events in the pathogenesis of atherosclerosis and atherosclerotic plaque rupture [1]. Meanwhile, the increased superoxide in vessels during oxidative stress facilitates the oxidation of LDL and generates higher amount of ox-LDL, which cause further detrimental effects on endothelial cells.
MicroRNAs (miR) are a group of small endogenous non-coding RNAs interfering with transcriptional and posttranscriptional processes. These RNAs negatively regulate gene expression typically by binding to the 3’UTR of their target mRNAs causing translational inhibition or degradation of the mRNA. Increasing evidence indicates the importance of miRNAs in the regulation of atherosclerotic developmental and pathological processes. For example, miR-181b was found to be over-expressed in symptomatic human atherosclerotic plaques and abdominal aortic aneurysms, and correlated with decreased expression of predicted miR-181b targets TIMP-3 and elastin [2]. Moreover, miR-495 significantly promoted HUVECs proliferation by altering cell cycle distribution, and it also inhibited HUVECs apoptosis by affecting the expression of cleaved caspase3 [3]. More recently, Yang XF et al. reported that miR-155 deficiency lead to decreased atherosclerosis in a novel mouse model of obesity paradox [4]. These results suggest that miRNAs have a fundamental role in the development of atherosclerosis. Recently, miR profiling studies revealed that a miRNA model associating miR-20a, miR-21, and miR-155, accurately discriminated early-stage NSCLC patients from controls with a higher accuracy in advanced stage and squamous carcinoma subgroups [5]. What’s more, miR-21 was over-expressed in primary intrahepatic cholangiocarcinoma patients and considered as potential diagnostic markers in these patients [6]. Nevertheless, the signature of miR-21 expression and possible roles of miRNAs in atherosclerosis are not well elucidated. The potential role of miR-21 in ox-LDL-injured HUVECs has not been reported in recent studies.
Therefore, we performed an in vitro experiment to detect the expression pattern of miR-21 in ox-LDL-injured HUVECs. Surprisingly, in ox-LDL-injured HUVECs, miR-21 levels were found to be significantly decreased. Thus, it would be very interesting to elucidate the possible roles of miR-21 in ox-LDL-injured HUVECs. Overexpression of miR-21 resulted in decreased apoptosis of ox-LDL-injured HUVECs, whereas inhibition of miR-21 using antisense methodology increased HUVECs injury induced by ox-LDL. Using luciferase/GFP reporter assay, we identified PDCD4 as a real target for miR-21. It could target PDCD4 and modulate the phosphorylation of p65 and ERK to suppress the apoptosis of ox-LDL-injured HUVECs. Taken together, our findings implicate miR-21 as a potential therapeutic target for atherosclerosis.
Materials and methods
Cell culture
HUVECs (EA. Hy926 cells) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (Hyclone, UT, USA) supplemented with 10% fetal bovine serum (Gibco, CA, USA) and 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere containing 5% CO2 at 37°C. Cells were passaged at 80% confluence. HUVECs in ox-LDL treatment group were treated with 10 μg/mL ox-LDL.
HUVECs were seeded on a 24-well plate at a density of 5×104 cells per well for 24 hours. Then plasmid at a concentration of 50 ng/μL and the miR-21 mimic of 50 nM along with negative control was transfected into cells using Lipofectamine 2000 (Invitrogen, USA) following the instructions. Culture was removed 4 hours after transfection, and fresh culture medium was added.
RNA isolation and quantitative real-time PCR
Total RNA from HUVECs was extracted using Trizol (Invitrogen, CA, USA) and subsequent ethanol purification for analysis of relative mRNA levels. After DNase I (Takara, Otsu, Japan) treatment, RNA was reverse-transcribed with reverse transcriptase kit. The resulting cDNAs were subjected to qRT-PCR using SYBR Premix Ex Taq (Takara, China) to measure miR-21 and PDCD4 mRNA according to the manufacturer’s instructions. The relative expression levels of miR-21 were normalized to those of U6 small nuclear RNA (U6-snRNA). GAPDH mRNA was calculated with the 2-ΔΔCt method.
Cell apoptosis assay
HUVECs apoptosis were detected using a FITC-Annexin V/PI staining kit (Sungene, Tianjin, China) by flow cytometry. The cultured HUVECs were digested with trypsin and washed, then suspended in Annexin V binding buffer. Cells were stained with FITC-Annexin V and PI according to the manufacturer’s instructions, and then analyzed on the BD FACS Verse flow cytometer (Becton Dickinson Co., NJ, USA).
Western blot
Cells were lysed for 30 min on ice in a cell lysis buffer (Beyotime, Jiangsu, China) and protein concentration was determined using the BCA assay (Beyotime). Equal amounts of protein extract were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membrane. The membrane was then blocked with 5% nonfat milk in TBST buffer (Tris-buffered saline with 0.2% Tween-20) for 1 hour. Blots were probed using the primary antibodies including anti-p-ERK (Abcam, Cambridge, UK), anti-ERK (Abcam, Cambridge, UK), anti-p-p65 (Abcam, Cambridge, UK), anti-p65 (Abcam, Cambridge, UK) and anti-β-actin (Santa Cruz Biotechnology). After being washed 3 times, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. The membranes were rinsed, and the signals were detected using ECL detection system (Tannon, Shanghai, China).
Luciferase activity assay
The wild-type and mutant-type 3’UTR sequence of human PDCD4 containing miR-21 binding sites was amplified by PCR and cloned into the pmiRGLO vector (Promega, USA). HUVECs were transfected with 100 ng of the wild-type pmiRGLO-PDCD4-3’-UTR, or mutant-type pmiRGLO-PDCD4-3’-UTR, along with 100 nM of miR-21 mimics or mimics control (Genepharma, Shanghai). 50 ng of pRL-TK vector was transfected as an internal control. After 48 hours transfection, luciferase activities were determined with a Dual Luciferase Assay kit (Promega, USA) according to manufacturer’s instructions.
Statistics
All statistical analyses were performed using the SPSS 13.0. The results are represented as means ± SD of at least three independent experiments. Differences between two experimental groups were analyzed by T-test. Differences among more than two experimental groups were assessed by one-way ANOVA analysis of variance. P<0.05 was considered statistically significant.
Results
MiR-21 was down-regulated in ox-LDL-injured HUVECs
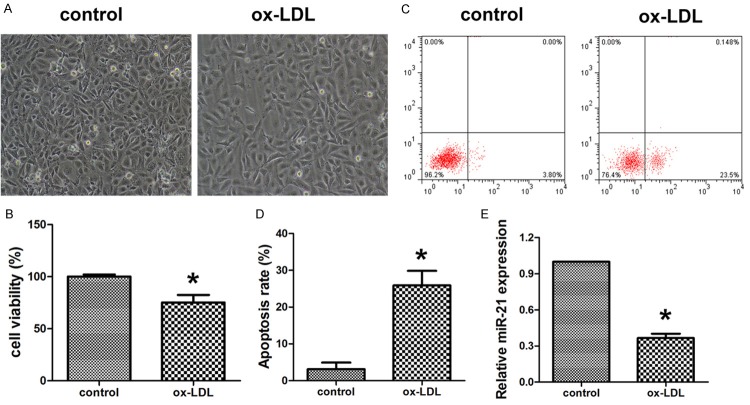
After being exposed to ox-LDL for 36 hours, HUVECs viability was significantly decreased (Figure 1A and 1B). Flow cytometry analysis showed the apoptosis rates of ox-LDL-injured HUVECs were significantly increased to 23.6% (Figure 1C and 1D). However, the relative expression of miR-21 was obviously decreased (Figure 1E). These results suggested that miR-21 could be down-regulated in HUVECs injured by ox-LDL.
Figure 1.
Ox-LDL induced HUVECs apoptosis and inhibited miR-21 expression. A. The cellular morphology after treatment with ox-LDL and control. B. MTT was used to evaluate the cell viability of HUVECs after treatment with ox-LDL and control. C, D. Flow cytometry was used to detect the apoptosis of HUVECs after treatment with ox-LDL and control. E. Quantitative realtime PCR was applied to evaluate the expression of miR-21. Data are expressed as mean ± SD; n = 8, *P<0.05.
Overexpression of miR-21 in HUVECs increased cell viability and inhibited apoptosis
To investigate the potential role of miR-21 in the cell viability and apoptosis of HUVECs, cells were transfected with miR-21 mimic and inhibitor and then exposed to ox-LDL. Flow cytometry analysis showed the gain of miR-21 could partially inhibit HUVECs apoptosis whereas the loss of miR-21 could increase the apoptosis rates of HUVECs (Figure 2A and 2B). In contrast, MTT assay exhibited the gain of miR-21 significantly increased cell viability whereas the loss of miR-21 suppressed cell viability (Figure 2C).
Figure 2.
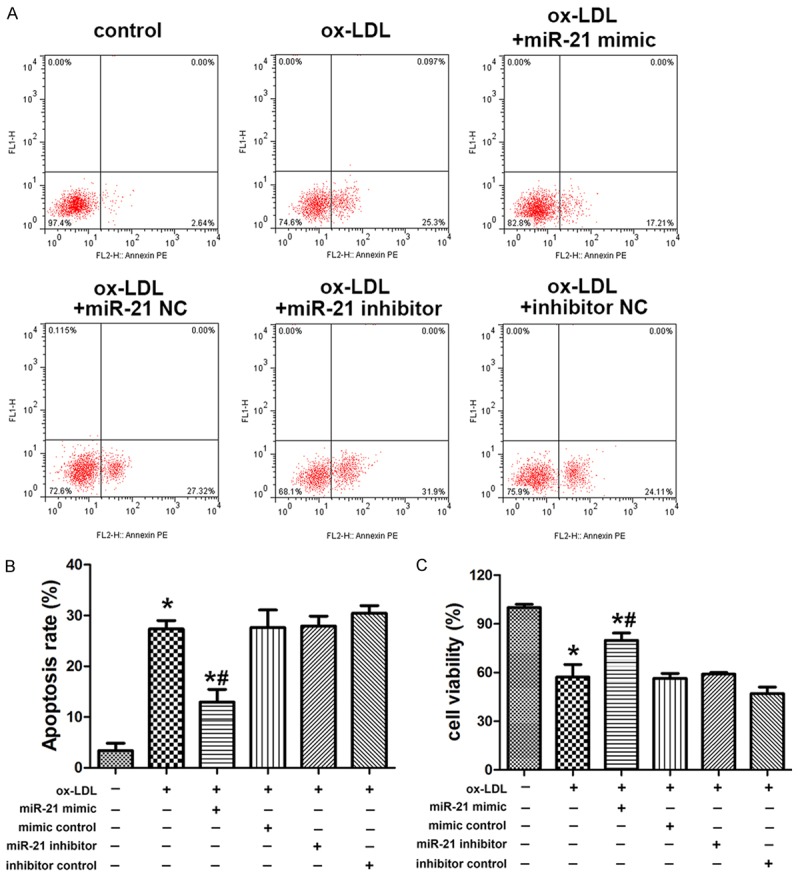
Aberrant miR-21 expression in HUVECs altered cell viability and apoptosis. A, B. Flow cytometry was used to detect the apoptosis of HUVECs transfected with miIR-21 mimics, miR-21 inhibitor and negative controls under treatment with ox-LDL and control. C. MTT was used to evaluate the cell viability of HUVECs transfected with miR-21 mimics, miR-21 inhibitor and negative controls under treatment with ox-LDL and control. Data are expressed as mean ± SD; n = 8, *P<0.05.
PDCD4 was identified as potential target gene of miR-21
To explore the mechanism by which miR-21 suppress the apoptosis of HUVECs induced by ox-LDL, potential mRNA targets of miR-21 were searched using the online bioinformatics TargetScan algorithm software. PDCD4 was predicted to be a target of miR-21. To determine whether PDCD4 was negatively regulated by miR-21, the 3’-UTR of PDCD4 containing wildtype (WT) or mutant miR-21 target sequences was cloned into the psiCHECK-2 vector (Figure 3A). After transfection with miR-21 mimics, the luciferase activity of the WT 3’-UTR reporter gene significantly decreased, whereas the luciferase activity of the mutant reporter gene was not affected (Figure 3B). To investigate the potential role of miR-21 in PDCD4 expression in HUVECs, cells were transfected with miR-21 mimic and inhibitor. Flow cytometry analysis showed miR-21 mimic group could suppress the expression of PDCD4 whereas miR-21 inhibitor group could increase the expression of PDCD4 in HUVECs (Figure 3C and 3D). These data suggested that PDCD4 was the potential target gene of miR-21 and could possibly affect HUVECs survival.
Figure 3.
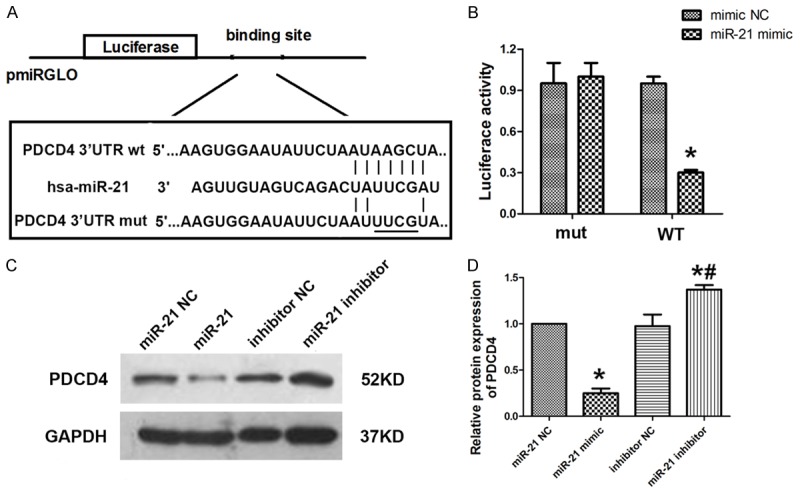
MiR-21 directly targeted PDCD4. A. Sequence alignment between miR-21 and the 3’UTR of PDCD4. B. Luciferase reporter activities of pmiRGLO vectors carrying luciferase gene and a fragment of HFG 3’UTR containing the binding sites of miR-21. C, D. Western blot was carried out to analysis the level of PDCD4 after miR-21 mimic or inhibitor transfected into HUVECs. Data are expressed as mean ± SD; n = 8, *P<0.05.
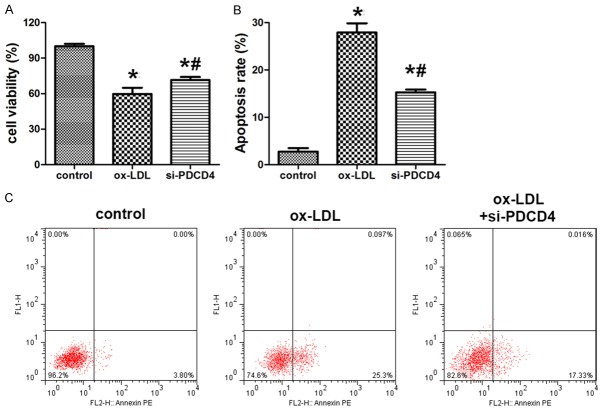
Si-PDCD4 increased HUVECs survival
To elucidate the function of PDCD4 in HUVECs apoptosis induced by ox-LDL, knock down of PDCD4 was introduced in the experiment. MTT assay showed that the cell viability in ox-LDL group and si-PDCD4 group were significantly decreased. However, when compared with ox-LDL group, si-PDCD4 group showed increased cell viability (Figure 4A). Flow cytometry analysis showed the apoptosis rate of HUVECs was decreased after the knock down of PDCD4 (Figure 4B and 4C).
Figure 4.
Si-PDCD4 altered HUVECs survival and apoptosis induced by ox-LDL. A. MTT was used to evaluate the cell viability of HUVECs with knock down of PDCD4 after ox-LDL treatment. B, C. Flow cytometry was used to evaluate the cell apoptosis of HUVECs with knock down of PDCD4 after ox-LDL treatment. Data are expressed as mean ± SD; n = 8, *P<0.05.
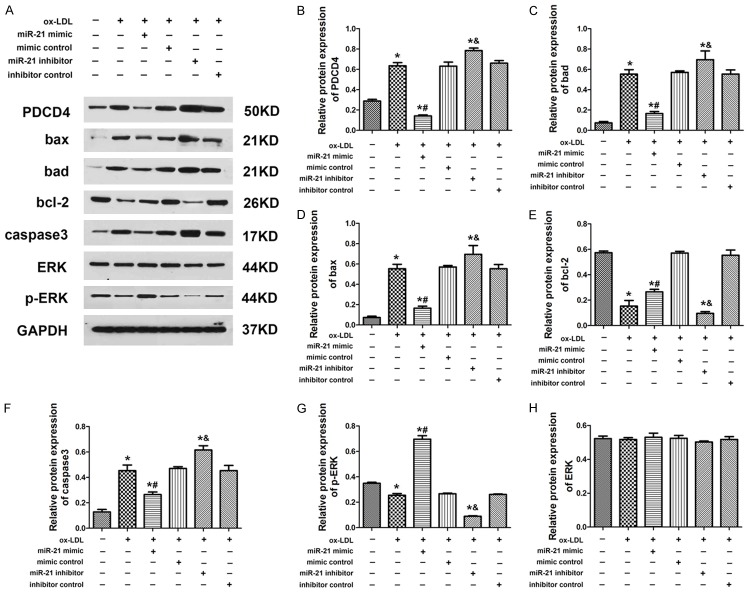
Overexpression of miR-21 inhibited PDCD4 expression but increased bcl-2 and p-ERK expression in HUVECs
Western blot experiment was performed to further examine the expression of relevant proteins in HUVECs. Results showed that the expression of PDCD4, Bad, Bax and caspase3 was increased in ox-LDL group compared with control, but the expression of these proteins in miR-21 mimic group was significantly suppressed. However, the miR-21 inhibitor exhibited the opposite effect to miR-21 mimic group (Figure 5A-D and 5F). Interestingly, the expression of bcl-2 and p-ERK was down-regulated in HUVECs after exposure to ox-LDL. The miR-21 mimic could increase the expression of bcl-2 and p-ERK to attenuate the ox-LDL injury. In contrast, the miR-21 inhibitor suppressed the expression of bcl-2 and p-ERK in HUVECs and the expression of ERK was not significantly changed (Figure 5A, 5E, 5G and 5H). These data suggested miR-21 could suppress ox-LDL-induced HUVECs apoptosis by targeting PDCD4. What’s more, miR-21 could protect HUVECs from ox-LDL injury by increasing the expression of bcl-2 and the phosphorylation of ERK proteins.
Figure 5.
Aberrant expression of miR-21 changed expression level or phosphorylation of proteins under ox-LDL treatment. A-H. Overexpression or knock down of miR-21 altered the expression level of PDCD4, bax, bad, bcl-2, caspase3 and the phosphorylation of ERK. Data are expressed as mean ± SD; n = 8, *P<0.05.
Discussion
Atherosclerosis contributes to the events cardiovascular diseases such as myocardial infarction and stroke which is the leading cause of death and disability worldwide [7]. Apoptosis of endothelial cells involves in the development of atherosclerosis tightly during which it initiates the atherosclerotic lesions by inducing neointima formation, inflammatory cell infiltration, lipid transport, and plaque rupture [8].
Accordingly, endothelial cells apoptosis could be induced by ox-LDL [9]. Increasing evidences have indicated that miRNAs regulate various physiopathologic processes such as by targeting the expression of mRNA targets. Considering this, gene therapies based on altering the miRNAs expression have been developed rapidly. As in atherosclerosis, a number of miRNAs have been identified as important regulators such as miR-433, miR-106b-5p, miR-125a, miR-200c and so on [10-13]. However, the potential role of miR-21 and its target PDCD4 have been limited.
In the current study, we first evaluated the expression of miR-21 in HUVECs under ox-LDL treatment. We found that ox-LDL notably induced the HUVECs apoptosis meanwhile inhibited its viability. The expression of miR-21 was significantly reduced through ox-LDL treatment. To discover whether miR-21 involves in the ox-LDL-induced-apoptosis in HUVECs, we altered the miR-21 expression by transfection of HUVECs with miR-21 mimic or miR-21 inhibitor as well as their negative controls. As expected, miR-21 mimic inhibited apoptosis and promoted cell viability in HUVECs under ox-LDL treatment, on the other hand, miR-21 inhibitor promoted apoptosis and attenuated cell viability in HUVECs.
MiR-21 has been found expressed in heart, spleen, colon and small intestine [14]. Aberrant expression of miR-21 was indicated to contribute to atherosclerosis, cardiac hypertrophy, heart failure and myocardial infarction [15-17]. PDCD4 inhibits translation initiation via binding to the translation initiation factor eIF4A or translation elongation by directly or indirectly binding to the coding region of specific RNAs [18]. PDCD4 is a critical mediator of apoptosis and lost expression of PDCD4 protein has been identified in many different human cancers. PDCD4-deficient cells were significantly less sensitive to apoptosis [19]. A wide range of studies demonstrated that PDCD4 plays a critical role in cardiovascular diseases.
Lin et al. demonstrated that PDCD4 is an important target gene of miR-21 associated with anti-apoptotic effects on cardiac cells under H2O2 treatment. Moreover, PDCD4 gene had a positive regulatory effect in atherosclerosis by increasing the expression of IL-10 [20]. MiR-155 regulated inflammation response by the SOCS1-STAT3-PDCD4 axis in atherogenesis. PDCD4 deficiency enhances macrophage lipoautophagy and attenuates from cell formation and atherosclerosis in mice [21].
In the present study, we first predicted and verified that miR-21 directly targeted PDCD4 in HUVECs. Knock down of PDCD4 attenuated the apoptosis of HUVECs induced by ox-LDL which further confirmed our finding.
MiR-21 has been shown to augment ERK-MAP kinase activity through inhibition of sprout homologue 1 and to promote ERK-MAP kinase-mediated cell survival in cardiac fibroblasts [22]. MiR-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cell [23]. Thus, we next investigated the ERK expression and the phosphorylation of ERK. The results revealed that miR-21 inhibited apoptotic proteins expression such as Bax, bad and caspase3 meanwhile promoted expression of anti-apoptotic protein Bcl-2. Furthermore, miR-21 did not alter the expression of ERK, however, it enhanced the phosphorylation of ERK.
In conclusion, our work indicated that ox-LDL induced apoptosis through reducing miR-21 expression and then regulating the PDCD4 expression which is the target of miR-21 in HUVECs. MiR-21 changed the phosphorylation of ERK and apoptosis related protein expression. However, whether the phosphorylation of ERK alteration was regulated by PDCD4 or directly by miR-21 was unclear. It is the further investigation we will carry out.
Acknowledgements
The research was supported by National Natural Science Foundation of China (81570304).
Disclosure of conflict of interest
None.
References
- 1.Muller C, Salvayre R, Negre-Salvayre A, Vindis C. Oxidized LDLs trigger endoplasmic reticulum stress and autophagy: prevention by HDLs. Autophagy. 2011;7:541–543. doi: 10.4161/auto.7.5.15003. [DOI] [PubMed] [Google Scholar]
- 2.Di Gregoli K, Mohamad AN, Bianco R, White SJ, Newby AC, George SJ, Johnson JL. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ Res. 2017;120:49–65. doi: 10.1161/CIRCRESAHA.116.309321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D, Zhang XL, Yan CH, Li Y, Tian XX, Zhu N, Rong JJ, Peng CF, Han YL. MicroRNA-495 regulates the proliferation and apoptosis of human umbilical vein endothelial cells by targeting chemokine CCL2. Thromb Res. 2015;135:146–154. doi: 10.1016/j.thromres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Virtue A, Johnson C, Lopez-Pastrana J, Shao Y, Fu H, Li X, Li YF, Yin Y, Mai J, Rizzo V, Tordoff M, Bagi Z, Shan H, Jiang X, Wang H, Yang XF. MicroRNA-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: a novel mouse model of obesity paradox. J Biol Chem. 2017;292:1267–1287. doi: 10.1074/jbc.M116.739839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng Q, Fan T, Zhang B, Wang W, Xu Y, Hu H. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir Res. 2014;15:149. doi: 10.1186/s12931-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa-Gallego C, Maddalo D, Doussot A, Kemeny N, Kingham TP, Allen PJ, D’Angelica MI, DeMatteo RP, Betel D, Klimstra D, Jarnagin WR, Ventura A. Circulating plasma levels of MicroRNA-21 and MicroRNA-221 are potential diagnostic markers for primary intrahepatic cholangiocarcinoma. PLoS One. 2016;11:e163699. doi: 10.1371/journal.pone.0163699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 9.Schmiedel JM, Klemm SL, Zheng Y, Sahay A, Bluthgen N, Marks DS, van Oudenaarden A. Gene expression. MicroRNA control of protein expression noise. Science. 2015;348:128–132. doi: 10.1126/science.aaa1738. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang Y, Zhang X, Zhang Y, Jiang Y, Xiao X, Tan J, Yuan W, Liu Y. MicroRNA-433 inhibits the proliferation and migration of HUVECs and neurons by targeting hypoxia-inducible factor 1 alpha. J Mol Neurosci. 2017;61:135–143. doi: 10.1007/s12031-016-0853-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Li SF, Chen H, Song JX. MiR-106b-5p inhibits tumor necrosis factor-alpha-induced apoptosis by targeting phosphatase and tensin homolog deleted on chromosome 10 in vascular endothelial cells. Chin Med J (Engl) 2016;129:1406–1412. doi: 10.4103/0366-6999.183414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensson D, Gidlof O, Turczynska KM, Erlinge D, Albinsson S, Nilsson BO. Inhibition of microRNA-125a promotes human endothelial cell proliferation and viability through an antiapoptotic mechanism. J Vasc Res. 2014;51:239–245. doi: 10.1159/000365551. [DOI] [PubMed] [Google Scholar]
- 13.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissuespecific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 17.Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. Febs Lett. 2008;582:4137–4142. doi: 10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Jiang Y, Song X, Guo C, Zhu F, Wang X, Wang Q, Shi Y, Wang J, Gao F, Zhao W, Chen YH, Zhang L. Pdcd4 deficiency enhances macrophage lipoautophagy and attenuates foam cell formation and atherosclerosis in mice. Cell Death Dis. 2016;7:e2055. doi: 10.1038/cddis.2015.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F, Shi W, Chen YH. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A. 2011;108:12030–12035. doi: 10.1073/pnas.1101450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE-/- mice. J Am Heart Assoc. 2012;1:e3376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye J, Guo R, Shi Y, Qi F, Guo C, Yang L. miR-155 regulated inflammation response by the SOCS1-STAT3-PDCD4 axis in atherogenesis. Mediators Inflamm. 2016;2016:8060182. doi: 10.1155/2016/8060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Luo F, Ling M, Lu L, Shi L, Lu X, Xu H, Chen C, Yang Q, Xue J, Li J, Zhang A, Liu Q. MicroRNA-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cells. Toxicol Lett. 2016;252:1–10. doi: 10.1016/j.toxlet.2016.04.015. [DOI] [PubMed] [Google Scholar]