Abstract
This study was undertaken to investigate the distribution of mast cells (MCs) and the expression of transforming growth factor-β (TGF-β) on tryptase positive MCs in different types of human periapical diseases. Periapical tissues of 78 participates were used in this study, including healthy control (n=28), periapical cyst (n=25), and periapical granuloma (n=25). The tissue samples were fixed in 10% formalin for at least 48 h, followed by staining with hematoxylin and eosin (HE) for histopathological examination. Then, they were stained with toluidine blue for MCs and MCs degranulation examination, or stained with double immunofluorescence for identification of tryptase-TGF-β double positive MCs. The results showed that the density of tryptase-TGF-β double positive MCs in periapical lesions was significantly higher than that of healthy control (P<0.01). The number of TGF-β positive MCs in the periapical cyst was potently higher than that in the periapical granuloma (P<0.01). In addition, compared with toluidine blue staining, the number of MCs with double immunofluorescence staining was significantly increased (P<0.01). The TGF-β positive MCs may play an important role in the pathogenesis of human chronic periapical diseases, particularly in the formation of fibrous tissues in periapical cyst. Double immunofluorescence staining is more sensitive than the traditional toluidine blue staining for identifying MCs.
Keywords: Chronic periapical diseases, mast cells, tryptase, TGF-β
Introduction
Periapical disease is initiated primarily by the bacteria or their products in the infected root canals. The immune system of organisms could not completely eradicate the infections, thus resulting in periapical bone destruction and reabsorption [1]. Transforming growth factor-β (TGF-β) is a biofunctional growth regulator, which plays a significant role in the inflammation regulation, embryonic development, tissue repair, immune system, cell growth, and differentiation. TGF-β is also closely associated with tumor prognosis, autoimmune conditions, and fibrosis diseases. In view of the effects mast cells (MCs) playing in the allergic inflammatory diseases and the inhibiting role of TGF-β in immune cells, TGF-β probably inhibites the activity of MCs through autocrine and paracrine [2]. Our previous study revealed that the collection, degranulation, and the stem cell factor (SCF) of MCs may contribute to the progression of periodontal disease, arguing that MCs and their associated cytokines play an important role in the pathogenesis and progression of periapical diseases [3]. However, the expression of TGF-β in MCs involved in periapical diseases has not been reported.
In this study, we identified the distribution of MCs in tissues with different human chronic periapical diseases by the toluidine blue and double immunofluorescence staining. Moreover, we analyzed the expression of TGF-β in MCs to investigate effects of Tryptase-TGF-β double positive MCs on the immune pathogenesis of chronic periapical disease. We intended to provide novel targets for the treatment of periapical disease.
Materials and methods
Study subject
A total of 50 subjects who were diagnosed with periapical cyst and periapical granuloma in the Liwan Stomatological Hospital of Jinan University from June 2013 to July 2014 were enrolled in this study. All subjects received the radicular curettage and all diagnosis had been confirmed by pathologic examination. Among these subjects, 25 cases (20-60 years old, with a mean age of 43.2 ± 11.9) were diagnosed with periapical granuloma, and 25 cases (24-64 years old, with a mean age of 45.9 ± 11.8) were diagnosed with periapical cysts. Twenty-eight cases (18-23 years old, with a mean age of 20.5 ± 4.3) with healthy periapical tissues were enrolled into the healthy control group. The healthy samples were derived from periodontal membrane of premolars extracted for orthodontics from June to September 2013, and premolars had healthy periapical tissues and fully developed tooth root. The subjects were systemic-disease free and no antibiotic therapy for the past three months. All subjects signed informed consents for this study.
Group and specimen
All subjects were divided into three groups. The healthy control group (n=28): specimens were derived from the healthy periodontal membrane of premolars extracted for orthodontics. The periapical granuloma group (n=25): X ray examination revealed that round or ovoid shaped low-density radiolucent shadows were less than 1 cm in size and there was well-defined boundary with the teeth root apex, meanwhile histological examinations showed no explicit inflammatory cell infiltration and no squamous epithelial lining. The periapical cyst group (n=25): X ray examination revealed that the teeth root apex was with white-plagued lines and round or ovoid shaped low-density radiolucent shadows were less than 1 cm in size. Besides, histological examination showed cysts fully or partially covered with stratified squamous epithelium in periapical tissues. There was obvious inflammatory cell infiltration in epithelial layers, and there was no explicit infiltration of inflammatory cells in the fibrous tissue layer dominated by collagen fibers.
Tissue specimen collection and treatment
The specimens of periapical granulomas and periapical cysts were derived from the tissues obtained during the periapical curettage. The specimens of healthy control group were derived from the root of healthy premolar teeth which was extracted for orthodontics. The collected periapical tissues were fixed in 10% buffered formalin for more than 48 h. Then after dehydration, cleaning, wax immersion and embedding, the tissues were made into 5 μm thick serial slices.
Hematoxylin-eosin (HE) staining
Histological changes of each specimen were observed by 2 independent pathologists in blind method. For HE staining, the above mentioned slices were stained with hematoxylin and eosin (HE). Then histological changes of three groups were observed under a light microscopy (Olympus, Tokyo, Japan).
Toluidine blue staining
After a series of routine processing including dewaxing and alcohol rehydration, the tissue biopsies were stained with toluidine blue in room temperature overnight, and then enveloped with neutral gum. The morphology and degranulation of MCs were observed and counted by 2 independent pathologists with an optical microscope. The MCs counting and tissue area in every specimen were conducted in the high power field (×400). Then the density (cells/mm2) of MCs and the degranulated MCs were calculated in each group. Where: MCs density (cells/mm2) = total number of MCs in the slice/the slice area (mm2); degranulated MCs density (cells/mm2) per slice = total number of degranulated MCs in each slice/the slice area (mm2).
Double immunofluorescence staining for identification of tryptase-TGF-β double positive MCs
The primary antibodies used in double immunofluorescence staining were tryptase (Abcam, UK; 1:200) and TGF-β (Santa Cruz Biotechnology, USA; 1:100). The second antibodies were goat anti-mouse IgG (H+L) Alex Flour 555® (Cell Signaling Technology, USA; 1:200).
Tissue biopsies underwent a series of processes including routine dewaxing, alcohol rehydration, microwave repair by citrate buffer, normal sheep serum blocking, incubation of antibodies, and fluorescence staining in dark condition. After anti-fluorescence quenching liquid sealing, tissue slices were immediately observed under a fluorescence microscope. The 2 dependent pathologists identified the expression of TGF-β on MCs in immunofluorescence microscope, then counted the tryptase-TGF-β positive MCs and calculated the cell density (cells/mm2). The tryptase-TGF-β double positive MCs counting and area recording of every tissue biopsy were conducted under the high power field (×400). Where: tryptase-TGF-β positive MCs density (cells/mm2) = total number of tryptase-TGF-β positive MCs in the slice/the slice area (mm2).
Statistical analysis
Data were represented with mean ± standard error (mean ± SEM) and analyzed with SPSS 13.0. Non-parametric test (Kruskal-Wallis H test) was applied to compare the density of MCs with toluidine blue staining between groups; if there was no significant difference between the three independent groups, Nemenyi test was adopted in the multiple comparisons. Non-parametric test (Kruskal-Wallis H test) was applied to compare the density of tryptase-TGF-β positive MCs between groups; if there was no significant difference between the 3 independent groups, Nemenyi test was adopted in the multiple comparisons. Non-parameters Wilcoxon matched pairs signed rank test was used to compare the density of MCs with toluidine blue staining or double immunofluorescence. P<0.05 was considered to be significantly different.
Results
Histological observation
In order to find out the difference of three groups, histological observation was performed using a light microscope. Noobvious infiltration of inflammation cells was detected in healthy control group (Figure 1A). There was significant inflammatory cell infiltration in the periapical granuloma group (Figure 1B). Most inflammatory cells were comprised of monocytes, lymphocytes, and plasmocytes, which were characterized with focal infiltration. Other cells including macrophage-like cells and neutrophils were mainly characterized with scattering infiltration. Microscopically, the periapical cyst was covered with the epithelial layer of intercellular edema (Figure 1C). Mass chronic inflammatory cell infiltration could be found below the epithelial layer. Moreover, there was no obvious infiltration of inflammatory cells in the fibrous tissue layer which was dominated by collagen fibers (Figure 1D).
Figure 1.
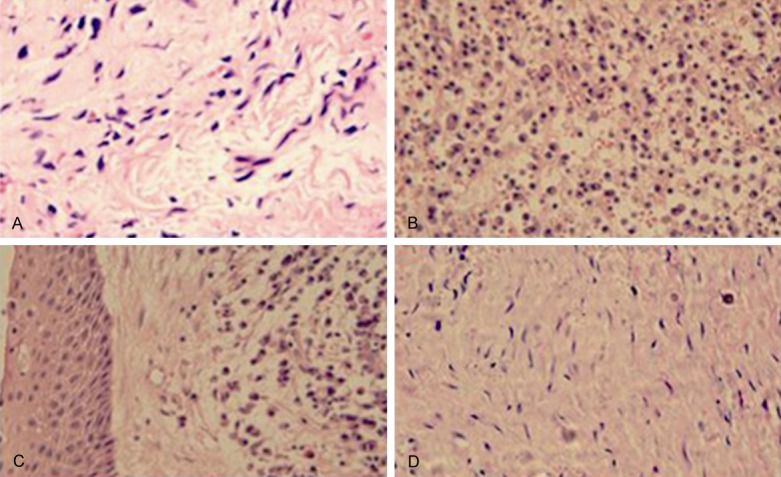
The histological changes of the specimens. A: Healthy control; B: Periapical granuloma; C: The epithelial layer of Periapical cyst; D: The fibrous tissue area of Periapical cyst. Hematoxylin and eosin staining, original magnification ×400.
Toluidine blue staining
To investigate the distribution of mast cells (MCs) in each group, the slices were going through a series of routine processing including dewaxed, alcohol rehydrated, stained with toluidine blue, and enveloped with neutral gum. Some MCs were visible in the healthy periodontal tissue of Control group with limited number (Figure 2A, 2B). The MCs density in the periapical cyst was significantly higher than that of healthy control (Figure 2C, 2D). MCs were round or oval in shape with even edges, smooth surface and uniform cytoplasm granules, some of which were showed mild or moderate degranulation. In Figure 2E, 2F, large numbers of MCs were visible below the cystic epithelium layers and in the connective tissues. MCs were manifested to be dark staining, various shapes and overt degranulation. Some of these cells broke down and released the metachromatic granules around cells.
Figure 2.
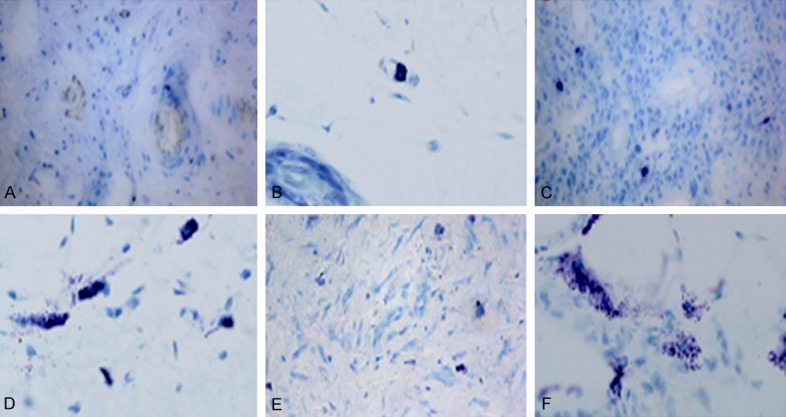
Representative photomicrographs of the mast cells infiltration with toluidine blue staining. (A, B) Healthy control; (C, D) Periapical granuloma; (E, F) Periapical cyst. Toluidine blue staining, original magnification (A, C, E) ×400, (B, D, F) ×1000.
The comparison of the MCs density or degranulation was shown in Figure 3. The density of MCs in two Periapical lesion groups was potently higher than that in healthy control group (P<0.01), and the density of MCs in the periapical cyst was significantly higher than that in the periapical granuloma group (P<0.05). The density of degranulated MCs in two Periapical lesion groups was significantly higher than that in healthy control group (P<0.01). The density of degranulation MCs in the periapical cyst group was significantly higher than that in the periapical granuloma group (P<0.05). These results indicated that MCs were involved in the pathologic process of Periapical diseases.
Figure 3.
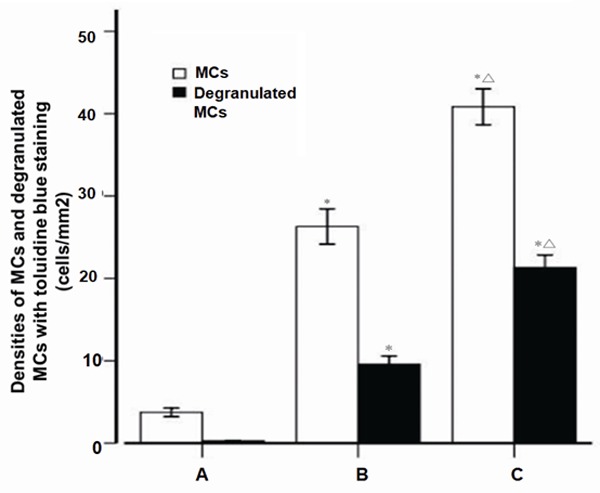
The densities of mast cells and degranulated mast cells with toluidine blue staining. A: Healthy control (n=28); B: Periapical granuloma (n=25); C: Periapical cyst (n=25); MC, mast cell; *P<0.01 vs healthy control; ΔP<0.05 vs periapical granuloma.
Double immunofluorescence staining of tryptase-TGF-β double positive MCs
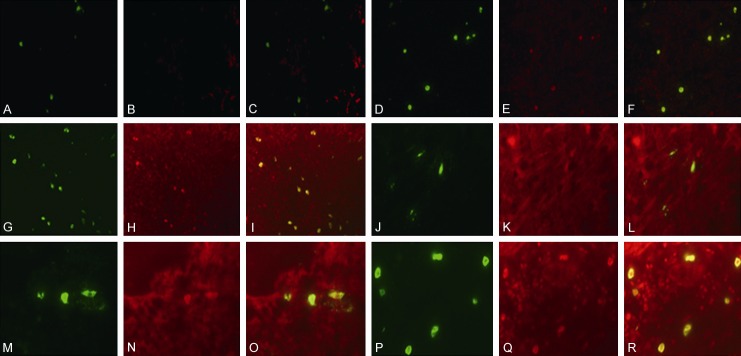
In order to observe the expression of TGF-β on tryptase positive MCs in all three groups, tissue biopsies were underwent fluorescence staining in dark condition. After anti-fluorescence quenching liquid sealing, tissue slices were immediately observed under a fluorescence microscope. Expression of TGF-β on MCs was visible in all three groups. There were only a few double-positive cells distributed in the periapical tissues of the healthy control group (Figure 4A-C, 4J-L). The number of TGF-β positive MCs in the periapical cyst was significantly higher than that in the other two groups (Figure 4D-F, 4M-O; P<0.01). Abundance of tryptase-TGF-β positive MCs were visible below the cystic epithelium and inside the connective tissues and epithelium in periapical cyst group (Figure 4G-I, 4P-R).
Figure 4.
Representative photomicrographs of mast cells infiltration with tryptase-TGF-β immunofluorescence double staining. A few tryptase-positive MCs (A, J) and tryptase-TGF-β positive MCs (C, L) in healthy controls with integrated membrane and unapparent degranulation, more tryptase-positive MCs (D, M) and tryptase-TGF-β positive MCs (F, O) in the periapical granuloma with a portion of membranolysis and degranulation, and even more tryptase-positive MCs (G, P) and tryptase-TGF-β positive MCs (I, P) with strong degranulation in the periapical cyst. Original magnification, (A-I) ×400, (J-R) ×1000.
The results of tryptase-TGF-β double positive cells in each group were shown in Figure 5, suggesting that there was a statistical significant difference in the density of tryptase-TGF-β positive MCs among three groups (X2=67.61, P<0.001). The density of typtase-TGF-β MCs was the highest in periapical cyst group among the three groups (average rank of 65.68). Nemenyi test results showed that there was significant difference in the density of tryptase-TGF-β positive MCs between the healthy control group and the granuloma group (X2=18.50, P=0.002), also between the healthy control group and the cyst group (X2=67.37, P<0.001). Besides, the number of tryptase-TGF-β positive MCs in the periapical cyst was significantly higher than that in the periapical granuloma (X2=14.45, P=0.001). These results demonstrated that TGF-β positive MCs might play an important role in the pathogenesis of human chronic periapical diseases, particularly in the formation of fibrous tissues in periapical cyst.
Figure 5.
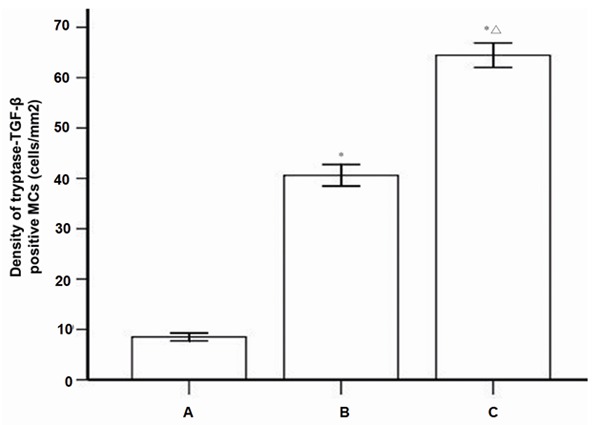
The density of tryptase-TGF-β positive mast cells with immunofluorescence double staining. A: Healthy control (n=28), B: Periapical granuloma (n=25); C: Periapical cyst (n=25); MC, mast cell; *P<0.01 vs healthy control; ΔP<0.05 vs periapical granuloma.
Density comparison between MCs with toluidine blue and double immunofluorescence staining
To choose an optimal staining method of MCs, the density of MCs between toluidine blue and double immunofluorescence staining was compared, as shown in Figure 6. Compared with toluidine blue staining, the number of MCs with double immunofluorescence staining was significantly increased (P<0.01). The result suggested that the tryptase immunofluorescence staining was more sensitive than the traditional toluidine blue staining for identifying MCs.
Figure 6.
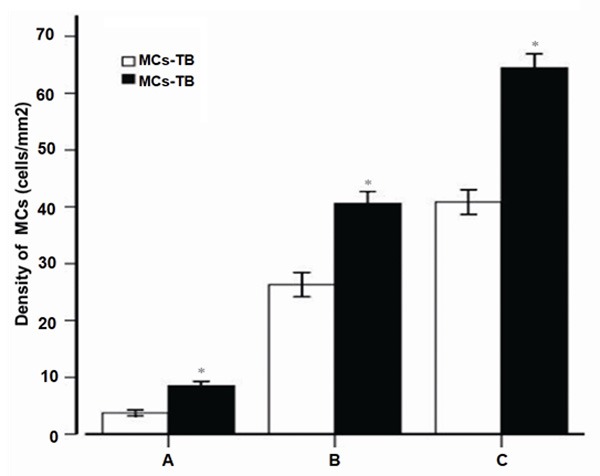
The comparison of mast cell densities determined by toluidine blue and double immunofluorescence staining. A: Healthy control (n=28); B: Periapical granuloma (n=25); C: Periapical cyst (n=25); MC, mast cell; TB, toluidine blue; IF, immunofluorescence; *P<0.01 vs toluidine blue staining.
Discussions
The root-canal-infected microorganisms were the main contributing factor for periapical diseases. Bacteria and their toxic metabolites spread into the periapical tissues through the root canal system and then activated the immune system, which led to the infiltration of various inflammatory cells and osteoclasts. These cells then produce various creatine kinases (CKs) which accelerate periapical tissue damages and bone reabsorption [4]. Lipopolysaccharide (LPS) in the cell wall of root-canal-infected bacteria could stimulate neutrophils, macrophages, and MCs to release a variety of pro-inflammatory cytokines, including interleukin-1 (IL-1), tumor necrosis factor (TNF)-α, TGF-β, prostaglandin E2 (PGE2) and so on. Meanwhile, LPS could activate osteoclasts, resulting in periapical and Alveolar bone reabsorption [5].
Conventional view is that MCs are mainly associated in type I hypersensitivity. After binding with the IgE on the MCs surface, antigenic ma-terial caused changes in MCs membrane structure, which leads to cell degranulation and bioactive peptide release, including histamine and leukotrienes. These bioactive peptides meditate the anaphylaxis, which are aggravating clinical symptoms including eriapical tissue edema, painful feeling, tooth stretchy feeling, and occlusive tooth pain [6]. The identification of MCs among the inflammatory cells in chronic periapical granuloma and cyst suggests that MCs are associated with the pathogenic mechanism of chronic periapical diseases [7]. However, some scholars did not agree with the role that the MCs-mediated anaphylaxis played in periapical diseases [8,9]. Therefore, there is no consensus view about the role of MCs in the periapical diseases.
Emerging evidence had shown that MCs and macrophages are involved in the pathogenesis of apical cyst [10]. Márton et al. identified a large number of MCs in the fibrous capsule of periapical lesions, but none in the periapical granuloma [8]. De Noronha et al. identified rise in the MC number and a significant degranulation in the deep tissue of radicular cysts of inflammatory lesions, suggesting that MCs were related with the inflammation of odontogenic lesions and cystic diseases [11]. Drazi et al. observed that the presence of MCs was greater in cysts than in granulomas, and MCs mainly existed in the inflamed periapical lesions and the fibroblast area [12]. Ledesma-Montes et al. found that MCs number was higher in proliferating epithelium of granulomas and that degranulation was frequently found in these zones, suggesting that MCs were closely related with the initiation, development, and persistence of the periapical inflammatory process [13]. In this study, we tested the parodontium of 25 periapical granuloma cases, 25 periapical cyst cases and 28 healthy cases by the staining. The results showed that the density of degranulation of MCs in two periapical lesion groups was significantly higher than that in healthy control group, and the density of degranulation of MCs in the periapical cyst group was significantly higher than that in the periapical granuloma group. Our results were consistent with most of previous study results, suggesting that MCs were involved in the pathologic process of periapical diseases.
TGF-β superfamily, composed of polypeptide growth factors which share similar structures, is a protein family regulating cell proliferation and differentiation. Recently, TGF-β was considered to be associated with the pathogenesis of the autoimmune diseases, fibrotic lesions and tumor [2]. TGF-β has a double regulatory role in the growth and differentiation of various cells. Inhibiting effects or activating effects of TGF-β are depended on cell types, differentiation states, and experiment conditions. Given the role of MCs played in the allergic inflammatory diseases and the role of TGF-β played in inhibiting immune cells, TGF-β probably inhibited the activity of MCs through autocrine and paracrine ways [2]. A previous study suggested that TGF-β might have opposing functions on MC-mediator release, depending on the type of MC and its state of maturation and activation [14]. Another previous study supported the theory that TGF-β1 produced by MCs or other cells is involved in the inflammatory cascade providing a delayed and reversible inhibition of IgE-mediated MCs inflammatory function [15]. TGF-β exerts a significant effect on chronic kidney disease (CKD) by mediating the renal fibrosis [16]. TGF-β1 could regulate the inflammation of the kidneys, and also promote the development of renal fibrosis, which is a potential drug target for reducing and preventing the deterioration of CKD [17].
The pathogenic mechanism of periapical diseases is, as yet, unclear and the expression of TGF-β on MCs involved in periapical diseases has not been reported. This study investigated the expression of TGF-β on MCs with double immunofluorescence staining. Our results showed that TGF-β positive MCs might play an important role in the pathogenesis of human chronic periapical diseases, particularly in the formation of fibrous tissues in periapical cyst. Therefore, we inferred that TGF-β was one of the main anti-inflammatory cytokines and that the biological significance of TGF-β expression on MCs was in inflammation stablization and repair of damaged tissues. In periapical diseases, TGF-β could counter pro-inflammatory cytokines such as IL-1 and TNF-α, as a macrophage inactivator. The study had theoretical significances and clinic guidance values in illuminating the pathogenesis of periapical diseases, particularly the periapical cyst. Controlling the TGF-β-positive MCs or regulating the MCs function could become the promising treatment target for preventing the development of periapical diseases. Thus, this study could provide new therapeutic targets and more efficient therapeutic directions for periapical disease treatment.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (No. 2013B021800043; No. 2014A020212212).
Disclosure of conflict of interest
None.
References
- 1.Marton IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000;15:139–50. doi: 10.1034/j.1399-302x.2000.150301.x. [DOI] [PubMed] [Google Scholar]
- 2.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 3.Huang S, Lu F, Chen Y, Huang B, Liu M. Mast cell degranulation in human periodontitis. J Periodontol. 2013;84:248–55. doi: 10.1902/jop.2012.120066. [DOI] [PubMed] [Google Scholar]
- 4.Nair PN. Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontol 2000. 1997;13:121–48. doi: 10.1111/j.1600-0757.1997.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson M, Reddi K, Henderson B. Cytokineinducing components of periodontopathogenic bacteria. J Periodontal Res. 1996;31:393–407. doi: 10.1111/j.1600-0765.1996.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca-Silva T, Santos CC, Alves LR, Dias LC, Brito M Jr, De Paula AM, Guimarães AL. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int Endod J. 2012;45:859–64. doi: 10.1111/j.1365-2591.2012.02043.x. [DOI] [PubMed] [Google Scholar]
- 7.Kabashima H, Nagata K, Maeda K, Iijima T. Involvement of substance P, mast cells, TNFalpha and ICAM-1 in the infiltration of inflammatory cells in human periapical granulomas. J Oral Pathol Med. 2002;31:175–80. doi: 10.1034/j.1600-0714.2002.310309.x. [DOI] [PubMed] [Google Scholar]
- 8.Marton I, Nemes Z, Harmati S. Quantitative significance of IgE-producing plasma cells and tissue distribution of mast cells in apical periodontitis. Oral Microbiol Immunol. 1990;5:46–8. doi: 10.1111/j.1399-302x.1990.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 9.Colic M, Gazivoda D, Vucević D, Vasilijić S, Rudolf R, Lukić A. Proinflammatory and immunoregulatory mechanisms in periapical lesions. Mol Immunol. 2009;47:101–13. doi: 10.1016/j.molimm.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Bracks IV, Armada L, Gonçalves LS, Pires FR. Distribution of mast cells and macrophages and expression of interleukin-6 in periapical cysts. J Endod. 2014;40:63–8. doi: 10.1016/j.joen.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 11.de Noronha Santos Netto J, Pires FR, da Fonseca EC, Silva LE, de Queiroz Chaves Lourenço S. Evaluation of mast cells in periapical cysts, dentigerous cysts, and keratocystic odontogenic tumors. J Oral Pathol Med. 2012;41:630–6. doi: 10.1111/j.1600-0714.2012.01126.x. [DOI] [PubMed] [Google Scholar]
- 12.Drazic R, Sopta J, Minic AJ. Mast cells in periapical lesions: potential role in their pathogenesis. J Oral Pathol Med. 2010;39:257–62. doi: 10.1111/j.1600-0714.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Ledesma-Montes C, Garcés-Ortíz M, Rosales-García G, Hernández-Guerrero JC. Importance of mast cells in human periapical inflammatory lesions. J Endod. 2004;30:855–9. doi: 10.1097/01.don.0000134207.67360.fc. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-beta1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–72. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, Kepley CL, Ryan JJ. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–93. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Sanchez O, Lopez-Hernandez FJ, Lopez-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77:950–5. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]