Abstract
Background: Long non-coding RNAs (lncRNAs) are noncoding RNAs that regulate cellular processes during the progression of tumors. Among various lncRNAs, lncRNA HOXA-AS2 (HOXA-AS2) has been reported to be involved in many critical processes of human malignancies. This study aimed to evaluate the significance and prognostic value of HOXA-AS2 in human non-small cell lung cancer (NSCLC). Methods: A total of 103 NSCLC tissues samples and matched adjacent non-tumor tissues specimens were obtained from NSCLC patients and the quantitative real-time PCR (qRT-PCR) were performed to investigate expression levels of HOXA-AS2. The correlation between HOXA-AS2 expression and survival outcomes of NSCLC patients was performed by Kaplan-Meier analysis, univariate and multivariate analysis. Results: HOXA-AS2 expression was significantly increased in NSCLC tissues compared with that in matched non-tumor adjacent tissues (P<0.05). In addition, high expression of HOXA-AS2 was demonstrated to be associated with larger tumors size, advanced TNM stages and distant metastasis of NSCLC patients. Survival analysis revealed that patients with high expression of HOXA-AS2 showed a significantly lower survival rate for OS, DFS and RFS, respectively (all, log rank test, P<0.05) and HOXA-AS2 could be an independent prognostic indicator for NSCLC patients. Conclusion: The results suggest that HOXA-AS2 has the clinical significance in the progression of NSCLC and could be a potential prognostic biomarker for NSCLC patients.
Keywords: HOXA-AS2, NSCLC, prognosis, biomarker, qRT-PCR
Introduction
As a malignant cancer, non-small cell lung cancer (NSCLC) accounts for 85% of primary lung cancer which is the leading cause of cancer-related deaths worldwide [1,2]. Actually, lung carcinogenesis is a complicated biological process due to mutual dysregulation of different tumor-related genes [3]. Although advances in combination treatment strategies involving surgery, radiotherapy and chemotherapy for NSCLC, the five-year overall survival rate remains at a low level [4]. Therefore, it is still urgent to explore precise and special markers for improving the survival of NSCLC patients.
LncRNAs are a class of transcripts which are larger than 200 nucleotides in size and lack the potential of encoding proteins [5]. Growing evidence has shown that aberrant lncRNA play an important role in various pathological processes, such as cell proliferation, differentiation, metabolism and apoptosis, indicating their function as suppressor genes or oncogenes [6]. Currently, increasing evidence has provided that lncRNAs are involved in different human malignancies including lung cancer [7]. For example Yang et al showed that lncRNA PVT1 was upregulated in NSCLC tissues and promoted NSCLC cells tumorigenesis [8]. Han et al showed low expression of lncRNA GAS6-AS1 could predict a poor prognosis in patients with NSCLC [9]. Lin et al showed that increased expression of the lncRNA ANRIL promoted lung cancer cell metastasis and correlated with poor prognosis [10]. However, the expression level of HOXA-AS2 and weather HOXA-AS2 has the clinical significance in NSCLC has not been reported.
Therefore, the aim of the study was to measure the expression level of HOXA-AS2 in NSCLC tissues and investigate the correlation of HOXA-AS2 level with clinicopathological features as well as the prognostic value of HOXA-AS2 in NSCLC patients.
Materials and methods
Patients and samples
A total of 103 NSCLC tissue samples and matched non-tumor adjacent tissues specimens were obtained from patients who had pathologically confirmed as NSCLC in Huaihe Hospital of Henan University. None of the patients underwent adjuvant treatments including radiotherapy, chemotherapy or immunotherapy before surgical resection. All patients underwent complete tumor resection with systematic lymph node dissection. TNM classification of the International Union Against Cancer was used for determination of disease stages. None of these patients had received preoperative and postoperative adjuvant therapy before tumor relapse. Written and informed consent was obtained from all patients and the investigation was approved by the ethical committee of our hospital.
RNA extraction and real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cell lines and tissues with TRIzol reagent (Invitrogen) according to the instructions and cDNA was gained with the PrimeScript RT reagent Kit (TaKaRa). qRT-PCR was performed using a StepOne Plus system following the standard protocol. Expression of GAPDH was detected to normalize the transcription levels. Changes of gene expression levels were calculated by 2-ΔΔCt method.
Statistical analysis
The relationship between HOXA-AS2 expression and clinical-pathological characteristics was analyzed with the Chi-square test. Survival curves were obtained using the Kaplan-Meier method. Multivariate analyses were performed using the Cox regression model to identify independent prognostic factors. All statistical analyses were carried out using SPSS software (version 13.0) for Windows. The statistical differences at P<0.05 were considered to be statistically significant.
Results
LncRNA HOXA-AS2 was up-regulated in NSCLC
In the present study, we explored the expression of HOXA-AS2 in 103 samples of NSCLC tissues and matched adjacent non-tumor specimens by qRT-PCR. As shown in Figure 1A, HOXA-AS2 expression was significantly higher in human NSCLC tissues than that in adjacent non-tumor tissues (P<0.05).
Figure 1.
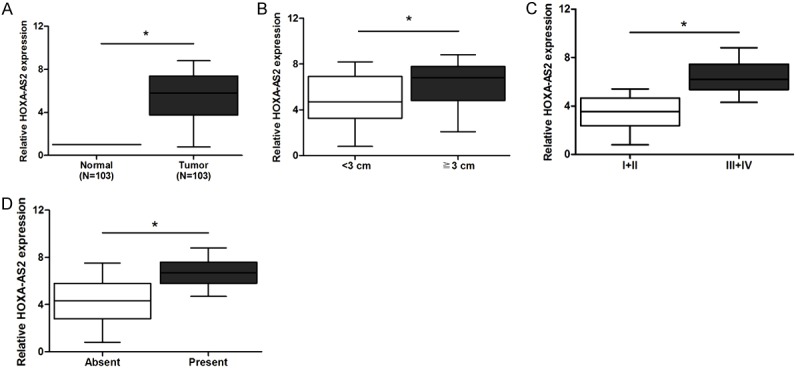
The expression of HOXA-AS2 in NSCLC tissues. (A) Relative expression of HOXA-AS2 in NSCLC tissues was significantly decreased compared with that in adjacent non-tumor tissues. (B-D) HOXA-AS2 expression was significantly higher in patients with large tumor size (B), advanced TNM stage (C) and distant metastasis (D).
Association of HOXA-AS2 expression with clinical characteristics
To analyze whether HOXA-AS2 was associated with the development and progression of NSCLC, we divided patients into two groups based on the median value of HOXA-AS2 expression levels: a high HOXA-AS2 expression group (n = 52) and a low HOXA-AS2 expression group (n = 51), and then investigated its relationship with the clinical characteristics. As shown in Table 1, HOXA-AS2 expression was remarkably related with tumor size, TNM stages, and distant metastasis (P<0.05). Concretely, high level of HOXA-AS2 expression was significantly correlated with larger tumor size (≥3 cm), advanced TNM stages (III, IV), and present distant metastasis (P<0.05; Figure 1B-D). All these results demonstrated that HOXA-AS2 was related to NSCLC progression and it might act as an oncogene. However, no significant relationship had been found between HOXA-AS2 expression and other clinical characteristics (P>0.05; Table 1).
Table 1.
Relationship between HOXA-AS2 expression and clinical factors of NSCLC patients
| Variables | N | HOXA-AS2 expression | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Age | 0.750 | |||
| ≥65 | 61 | 30 | 31 | |
| <65 | 42 | 22 | 20 | |
| Gender | 0.771 | |||
| Male | 54 | 28 | 26 | |
| Female | 49 | 24 | 25 | |
| Smoking | 0.733 | |||
| Yes | 67 | 33 | 34 | |
| No | 36 | 19 | 17 | |
| Histology | 0.651 | |||
| Squamous carcinoma | 47 | 22 | 25 | |
| Adenocarcinoma | 41 | 23 | 18 | |
| Other type | 15 | 7 | 8 | |
| Tumor size | 0.001 | |||
| ≥3 cm | 57 | 37 | 20 | |
| <3 cm | 46 | 15 | 31 | |
| TNM | 0.000 | |||
| I, II | 63 | 22 | 41 | |
| III, IV | 40 | 30 | 10 | |
| Differentiation | 0.140 | |||
| Well/moderately | 58 | 33 | 25 | |
| Poorly | 45 | 19 | 26 | |
| Lymph node metastasis | 0.094 | |||
| Absent | 50 | 21 | 29 | |
| Present | 53 | 31 | 22 | |
| Distant metastasis | 0.001 | |||
| Absent | 67 | 26 | 41 | |
| Present | 36 | 26 | 10 | |
The correlation between HOXA-AS2 level and survival of NSCLC patients
To further explore the correlation between HOXA-AS2 expression and clinical outcomes, we determined the prognostic value of HOXA-AS2 expression on overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS) in NSCLC patients. As determined by Kaplan-Meier method with log rank test, the patients with high expression of HOXA-AS2 presented a poor OS (P<0.05; Figure 2A), poor DFS (P<0.05; Figure 2B) and poor RFS (P<0.05; Figure 2C) respectively than those with low HOXA-AS2 expression, indicating overexpression of HOXA-AS2 predicted a poor prognosis in patients with NSCLC.
Figure 2.
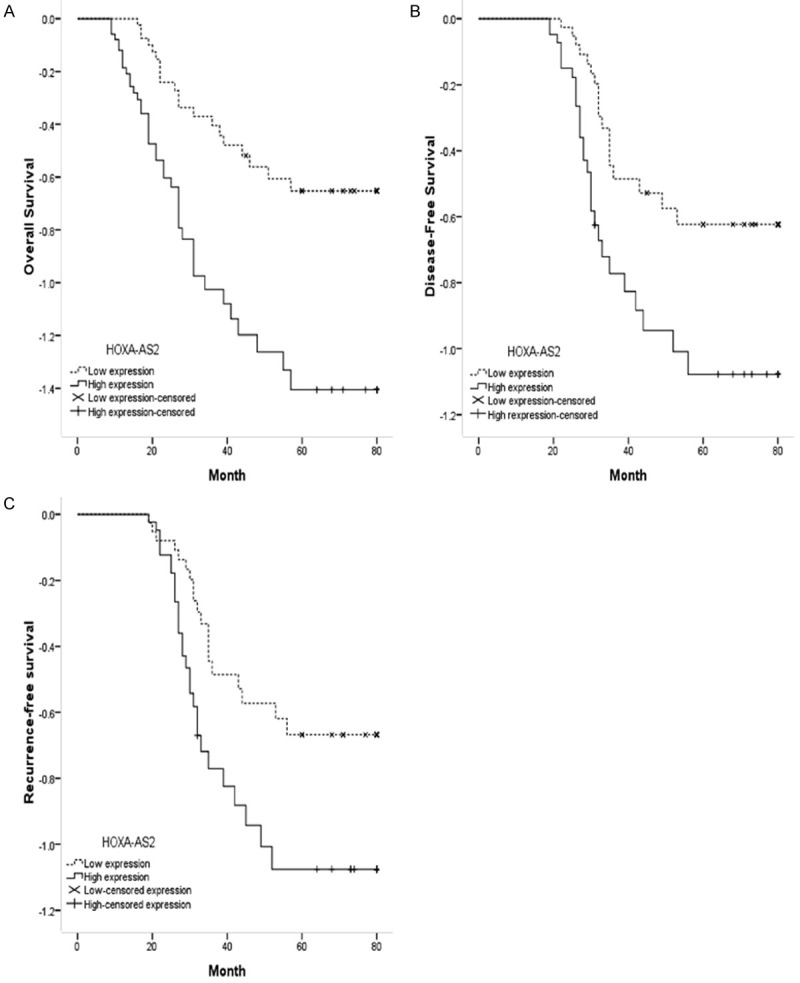
Survival analysis. NSCLC patients with high HOXA-AS2 expression had poor overall survival (OS, A) disease-free survival (DFS, B) and recurrence-free survival (RFS, C) than patients with low HOXA-AS2 expression.
Moreover, as respect to the influence of HOXA-AS2 levels and clinicopathological characteristics on patient survival, we performed univariate Cox regression analysis. As shown in Tables 2, 3 and 4, the tumor size, TNM stage, distant metastasis and HOXA-AS2 expression were all associated with the OS, DFS and RFS, respectively. Further multivariate analysis indicated that, for NSCLS patients, upregulated HOXA-AS2 level was an independent factor for OS (P<0.05; Table 2), DFS (P<0.05; Table 3) and RFS (P<0.05; Table 4). Furthermore, tumor size, TNM stage and distant metastasis were independent factors associated with OS, RFS and DFS in NSCLC patients (Tables 2, 3 and 4).
Table 2.
Univariate and multivariate survival analysis of OS with NSCLC
| Clinical factors | P value | Univariate HR (95% CI) | P value | Multivariate HR (95% CI) |
|---|---|---|---|---|
| Age | 0.918 | 1.211 (0.491-2.098) | ||
| Gender | 0.571 | 1.391 (0.625-2.816) | ||
| Histology | 0.739 | 1.092 (0.812-1.937) | ||
| Tumor size | 0.012 | 1.715 (0.913-3.083) | 0.002 | 2.971 (1.531-6.365) |
| TNM | 0.008 | 2.173 (1.386-5.437) | 0.001 | 2.793 (1.485-5.991) |
| Lymph node metastasis | 0.069 | 2.061 (0.774-5.038) | ||
| Differentiation | 0.083 | 1.917 (0.506-5.876) | ||
| Distant metastasis | 0.015 | 2.914 (0.831-4.173) | 0.004 | 3.761 (1.172-4.996) |
| Smoking | 0.087 | 1.783 (0.920-3.457) | ||
| HOXA-AS2 | 0.001 | 4.708 (2.913-14.451) | 0.001 | 6.711 (3.526-17.019) |
Table 3.
Univariate and multivariate survival analysis of DFS with NSCLC
| Clinical factors | P value | Univariate HR (95% CI) | P value | Multivariate HR (95% CI) |
|---|---|---|---|---|
| Age | 0.785 | 1.121 (0.612-2.013) | ||
| Gender | 0.352 | 1.162 (0.712-2.315) | ||
| Histology | 0.596 | 1.273 (0.752-1.903) | ||
| Tumor size | 0.018 | 1.632 (0.789-3.025) | 0.006 | 2.194 (1.432-5.830) |
| TNM | 0.025 | 3.212 (1.463-5.426) | 0.011 | 3.712. (1.744-6.217) |
| Lymph node metastasis | <0.001 | 2.417 (1.123-4.291) | ||
| Differentiation | 0.068 | 1.564 (0.963-4.215) | ||
| Distant metastasis | 0.002 | 2.256 (1.732-4.653) | <0.001 | 3.118 (1.899-5.713) |
| Smoking | 0.052 | 1.235 (0.856-3.647) | ||
| HOXA-AS2 | <0.001 | 5.132 (2.536-15.168) | <0.001 | 7.167 (2.926-19.027) |
Table 4.
Univariate and multivariate survival analysis of RFS with NSCLC
| Clinical factors | P value | Univariate HR (95% CI) | P value | Multivariate HR (95% CI) |
|---|---|---|---|---|
| Age | 0.856 | 1.321 (0.845-2.414) | ||
| Gender | 0.096 | 1.032 (0.456-1.963) | ||
| Histology | 0.492 | 1.731 (0.663-2.306) | ||
| Tumor size | 0.002 | 1.526 (0.812-2.856) | 0.000 | 2.719 (1.437-5.892) |
| TNM | 0.011 | 2.856 (1.326-4.632) | 0.003 | 3.065 (1.347-6.250) |
| Lymph node metastasis | 0.006 | 2.621 (1.325-4.521) | ||
| Differentiation | 0.089 | 1.972 (0.651-4.727) | ||
| Distant metastasis | 0.001 | 3.072 (1.075-6.619) | <0.001 | 3.331 (1.621-9.4638) |
| Smoking | 0.126 | 1.426 (0.889-3.212) | ||
| HOXA-AS2 | <0.001 | 5.893 (2.618-19.773) | <0.001 | 6.737 (2.926-20.527) |
Discussion
As the most common malignant disease in the world, lung cancer is the leading cause of mortality in China [11]. According to the past researches, several prognostic factors and biomarkers for NSCLC have been identified [12-14], which have improved the outcome of the NSCLC patients. However, the 5-year survival rate for the NSCLC is still unsatisfactory. Therefore, it is still urgent to identify novel and reliable prognostic markers to improve the prognosis of NSCLC patients.
Long noncoding RNAs (lncRNAs), an important subtype of noncoding RNAs, is longer than 200 nucleotides (nt) and has no protein coding potential [5]. With the help of improvements in modern biotechnology such as high-throughput sequencing and microarray analysis, growing evidences indicated that lncRNAs participated in a surprisingly diverse collection of biological progresses [15]. Moreover, many reports suggested that dysregulated expressions of lncRNAs occur in various cancers. For example, Zhang et al showed that upregulation of lncRNA MALAT1 correlated with tumor progression and poor prognosis in clear cell renal cell carcinoma [16]. Li et al found that lncRNA HOTTIP was up-regulated and associated with poor prognosis in patients with osteosarcoma [17]. Li et al showed that lncRNA CASC2 suppressed the proliferation of gastric cancer cells by regulating the MAPK signaling pathway [18].
HOXA cluster antisense RNA 2 (HOXA-AS2), a lincRNA located between and antisense to the human HOXA3 and HOXA4 genes [19]. Recently, evidence has shown that HOXA-AS2 was up-regulated in various types of cancer tissues, and closely associated with the outcome of prognosis for several tumors. For example. Xie et al showed that lncRNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression [20]. Zhang et al showed that upregulation of lncRNA HOXA-AS2 promoted proliferation and induced epithelial-mesenchymal transition in gallbladder carcinoma [21]. Fang et al showed that lncRNA HOXA-AS2 promoted proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge [22]. However, the clinical significance of HOXA-AS2 with lung cancer is poor characterized.
In this study, we examined the expression of HOXA-AS2 and its clinical value in predicting survival outcome in patients with NSCLC. Our data showed that the HOXA-AS2 expression was significantly increased in NSCLC tissues compared to the adjacent non-tumor tissues. In addition, our data showed that HOXA-AS2 expression levels were closely correlated with tumor size, TNM stage and distant metastasis. In contrast, there was no correlation between HOXA-AS2 expression and other clinical features. All these findings indicated that HOXA-AS2 plays an important role in the progression of NSCLC. Moreover, survival analysis revealed that patients with high HOXA-AS2 expression have worse survival outcome. This result is consistent with a report examining the prognostic role of HOXA-AS2 [22]. According to multivariate analyses, HOXA-AS2 was an independent prognostic factor for OS, DFS and RFS.
In conclusion, our study was the first time to explore the relation between HOXA-AS2 expression and prognosis of NSCLC patients. We first confirmed the increased expression of HOXA-AS2 in NSCLC tissues using qRT-PCR. Besides, HOXA-AS2 expression may be a novel and promising prognostic biomarker for NSCLC patients.
Acknowledgements
We thank the members at Department of Oncology and Radiotherapy, Huaihe Hospital of Henan University for their help.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW Jr, Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW Jr, Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-smallcell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 4.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 6.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Lin J, Liu T, Chen T, Pan S, Huang W, Li S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer. 2014;85:110–115. doi: 10.1016/j.lungcan.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in nonsmall cell lung cancer. Int J Clin Exp Pathol. 2014;7:6929–6935. [PMC free article] [PubMed] [Google Scholar]
- 9.Han L, Kong R, Yin DD, Zhang EB, Xu TP, De W, Shu YQ. Low expression of long noncoding RNA GAS6-AS1 predicts a poor prognosis in patients with NSCLC. Med Oncol. 2013;30:694. doi: 10.1007/s12032-013-0694-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non-coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. doi: 10.1186/s13000-015-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Mimi CY. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 12.Phillips M, Altorki N, Austin JH, Cameron RB, Cataneo RN, Greenberg J, Kloss R, Maxfield RA, Munawar MI, Pass HI. Prediction of lung cancer using volatile biomarkers in breath1. Cancer Biomark. 2007;3:95–109. doi: 10.3233/cbm-2007-3204. [DOI] [PubMed] [Google Scholar]
- 13.Villalobos P, Wistuba II. Lung cancer biomarkers. Hematol Oncol Clin North Am. 2017;31:13–29. doi: 10.1016/j.hoc.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 15.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36:2947–2955. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Cao L, Hang D, Wang F, Wang Q. Long non-coding RNA HOTTIP is up-regulated and associated with poor prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2015;8:11414. [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Xue WJ, Feng Y, Mao QS. Long noncoding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8:3522–3529. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Zhang X, Frazão JB, CondinoNeto A, Newburger PE. HOX- antisense lincRNA HOXAAS2 is an apoptosis repressor in all Trans retinoic- acid treated NB4 promyelocytic leukemia cells. J Cell Biochem. 2013;114:2375–2383. doi: 10.1002/jcb.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie M, Sun M, Zhu YN, Xia R, Liu YW, Ding J, Ma HW, He XZ, Zhang ZH, Liu ZJ, Liu XH, De W. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6:33587–33601. doi: 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Cao P, Zhu X, Pan M, Zhong K, He R, Li Y, Jiao X, Gao Y. Upregulation of long noncoding RNA HOXA-AS2 promotes proliferation and induces epithelial-mesenchymal transition in gallbladder carcinoma. Oncotarget. 2017;8:33137–33143. doi: 10.18632/oncotarget.16561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Y, Wang J, Wu F, Song Y, Zhao S, Zhang Q. Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget. 2017;8:46090–46103. doi: 10.18632/oncotarget.17552. [DOI] [PMC free article] [PubMed] [Google Scholar]


