Abstract
Objective: To investigate the expression of long-chain non-coding RNAs DAPK1 and miR-182 in pancreatic cancer tissues and the role of DAPK1 and miR-182 in pancreatic cancer cell invasion and migration and its mechanism. Methods: The expression of DAPK1 and miR-182 in different pancreatic cancer and adjacent tissues and different pancreatic cancer cells were detected by qPCR. Transwell invasion assay was used to detect the invasion ability of pancreatic cancer cells after DAPK1 expression. The changes of the migration ability of pancreatic cancer cells after DAPK1 expression were detected by scratch test. Double luciferase reporter gene was used to detect the interaction between DAPK1 and miR-182. Transwell invasion assay showed that miR-182 overexpression of DAPK1 could restore the invasive ability of pancreatic cancer cells. Western blot was used to detect the expression of ROCK-1/RhoA pathway protein after overexpression of miR-182 in DAPK1 cells. Phalloidin was used to label the cytoskeleton. The effect of miR-182 overexpression of DAPK1 on tumor size and volume of pancreatic cancer was detected by subcutaneous tumor formation in nude mice. Results: The expression of DAPK1 was significantly decreased in pancreatic cancer tissues compared with adjacent tissues, and the expression of DAPK1 decreased gradually and the expression of miR-182 was opposite with the progression of tumor. DAPK1 was associated with pathological stage of pancreatic cancer and lymph node metastasis, while miR-182 was positively correlated. The expression level of DAPK1 in pancreatic cancer cell HS766T was the lowest. Overexpression of DAPK1 could inhibit the invasion and migration of pancreatic cancer cells. DAPK1 could bind specifically to 3’UTR of miR-182. Overexpression of miR-182 could restore the invasion and migration of pancreatic cancer cells after overexpression of DAPK1. The expression of ROCK-1/RhoA pathway protein was down-regulated by miR-182 after expression of DAPK1, and the expression of ROCK-1/RhoA pathway protein was restored. The expression of F-actin in LV5-DAPK1 group was significantly decreased, the formation of cell membrane wrinkles was significantly reduced, and the formation of pseudopodia was significantly reduced compared with LV5-DAPK1 + miR-182-mimic group. The tumor volume and weight of tumor-bearing mice in LV5-DAPK1 + miR-182-mimic group were significantly increased compared with LV5-DAPK1 group. Conclusion: DAPK1 plays an important role in the development and progression of pancreatic cancer. DAPK1 can regulate the invasion and migration of pancreatic cancer cells through the regulation of miR-82 through ROCK-1/RhoA signaling pathway.
Keywords: ROCK-1/RhoA, pancreatic cancer, miR-182, invasion, migration
Introduction
Pancreatic cancer is one of the most malignant tumors of the digestive tract, accounting for the 4th cause of death of malignant tumors accounted for sixth place in China. The 5 year survival rate of patients with pancreatic cancer was less than 5%, and most of the patients died within 1 year after diagnosis [1,2]. Surgical resection and chemotherapy have been used to treat pancreatic cancer for many years, but the overall prognosis has not improved significantly. 1 of the important causes of poor prognosis in pancreatic cancer is the high degree of invasion and metastasis of pancreatic cancer cells [3]. At present, the molecular mechanism of invasion and metastasis of pancreatic cancer has not been fully elucidated. It is very important to explore the molecular markers that regulate the invasion and metastasis of pancreatic cancer in order to reveal the malignant biological behavior of pancreatic cancer, to develop specific molecular targeted drugs and to improve the prognosis of pancreatic cancer.
Long non-coding RNA (lncRNA) is a class of RNA with a length of more than 200 nt, which itself does not encode the protein, but regulates the expression of genes in a variety of ways [4,5]. LncRNA not only in epigenetic modification, transcription and post transcriptional regulation, and play an important role in maintaining normal tissue and cell differentiation and development process, but also can regulate apoptosis of tumor cell proliferation, differentiation, invasion and migration. It is expected to be a potential diagnostic marker and therapeutic target [6]. Tahira et al. [7] found that pancreatic cancer tissue screening lncRNA in three lncRNAs and pancreatic cancer metastasis are closely related through the cDNA transcription group chip technology. They were transcribed in MAP3K14, PPP3CB, DAPK1 loci, and RT-PCR was used to confirm that these three lncRNAs were encoding protein genes. However, there are few researches on the expression of DAPK1 in pancreatic cancer and the invasion and migration of pancreatic cancer cells.
MicroRNA (miRNA) is a class of non-coding single-stranded small RNAs with a length of about 21 to 23 nucleotides. MiRNAs are highly conserved among species and participate in various biological processes such as cell proliferation, differentiation, apoptosis, metabolism etc. [8]. More and more data show that miRNA abnormal expression is closely related to the occurrence and development of various tumors. MiR-182 belongs to the miR-182 family. The miR-183 family was first reported to be involved in the development and function of nerve cilia organ organism, which is important for the development of animal sensory organs [9,10]. Lei et al. [11] have shown that low expression of miR-182 in breast cancer is associated with high metastatic and poor prognosis.
In this investigation, we investigated the expression of DAPK1 and miR-182 in pancreatic cancer and adjacent tissues by RT-PCR, and further investigated the role and mechanism of DAPK1 and miR-182 in the process of invasion and migration of pancreatic cancer cells.
Materials and methods
Samples collection
60 cases of pancreatic cancer in our hospital with complete clinical data of surgical resection specimens were collected in October 2015-September 2016, which were adjacent to the tissue as a control and fixed by RNA fixation liquid into the liquid nitrogen. All patients had no chemotherapy or radiotherapy before surgery; pathological sections were confirmed by two pathologists. 60 patients with postoperative pathology were confirmed as ductal adenocarcinoma. Patients with pancreatic cancer were divided into I (IA + IB), IIA, IIB and III according to the TNM staging guidelines for 2010 UICC.
Cell lines
Human pancreatic cancer cells PANC-1 and HS766T were purchased from the Chinese Academy of Sciences in Shanghai. Fetal bovine serum and RPMI 1640 medium were purchased from Gibco (Gibco, USA). Transwell chamber was purchased from Corning Company (Coning Costar Co., Cambridge, MA, USA). Matrigel was purchased from BD Corporation (BD Biosciences, USA). Liposectamine 2000 and miR-182-mimic were purchased from (Gnenpharma Co., Shanghai, China), Trizol was purchased from Invitrogen (Invitrogen, USA), reverse transcription kit was purchased from Fermentas (Fermentas, Lithuania). PCR kit was purchased from Sigma (Kapa Biosystems Inc., Boston, US). DAPK1 and miR-182 primers were designed by Takara, Japan. The luciferase activity assay kit was purchased from Promega Biotech Co., Beijing, China. The luciferase reporter vector was synthesized by Promega Corporation (Promega Biotech Co., Beijing, China). LV5-DAPK1 was synthesized by Shanghai Genji Gene (Gnenpharma Co., Shanghai, China).
Cell culture
All cell monolayers were grown in culture medium containing 10% fetal bovine serum, penicillin G concentration 100 U/L, streptomycin concentration 100 µg/L RPMI-1640, incubated in 37°C, 5% CO2 constant temperature closed incubator. The medium was changed the next day and the logarithmic growth phase cells were tested.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from pancreatic cancer tissues and pancreatic cancer cells, and the concentration and purity were measured using an ultraviolet spectrophotometer. Take the total RNA 500 ng reverse transcription, configure the reverse transcription reaction solution, the 37°C 15 min reverse transcription: 85°C, 5 s to reverse the reverse transcriptase, room temperature after cooling -20°C preservation of the reverse transcription products. DAPK1 upstream primer: 5’-ACAGGCACGGCAATACTCCCCT-3’, downstream primer: 5’-ACGTGCTCGTGCTGTTCCGA-3’. MiR-182 upstream primer: 5’-TGCGGTTTGGCAATGGTAGAAC-3’, downstream primer: 5’-CCAGTGCAGGGTCCGAGGT-3’. The reaction system was prepared according to the operating instructions of GoTaq @ qPCR Master Mix. The volume of each reaction system was 20 μL, and three wells were set. Each group of samples had to carry out the target RNA and the internal reference gene GAPDH Fluorescence quantitative PCR amplification. According to ΔΔCT method, the reaction conditions were as follows: 37°C 15 min, 98°C 5 min. PCR was performed according to the PCR kit instructions to obtain the mRNA expression level with RQ = 2 - ΔΔCT.
Cell transfection
Pancreatic cancer cells with about 80% fusion were cultured and 0.25% trypsin was digested into single cell suspension one day before transfection. The cells were inoculated into 24-well plates at 10 * 104 and 20 * 104/well, respectively. The cells were transfected when the degree of fusion was about 50%. Lipofectamine 2000 kit instructions were used for transfection. The transfection complex was discarded and the complete medium was supplemented with 10% fetal bovine serum after 4-6 h. The relevant qPCR and WB were detected after 24-48 h.
Western blot analysis
The cells were lysed with cell lysis buffer and centrifuged at 12000 r/min for 15 min. The supernatant was collected and assayed for protein concentration (BCA method). 20 µg protein was 10% SDS-PAGE gel after separation, wet transferred to PVDF membrane. The target strip area was cut according to the marker, 5% skimmed milk powder closed 2 h, primary antibody incubated overnight, membrane was washed three times then goat anti-mouse IgG was horseradish peroxidase labeled, incubated for 1 h. The ECL luminescent solution was added and exposed to color after the membrane was washed three times, and the experiment was repeated three times.
Transwell invasion assays
The preformed 8 um pore semipermeable membrane of the 24-well chamber was taken out. The BD matrix glue to 1% fetal bovine serum RPMI-1640 was removed, its concentration adjusted to 1:10, 50 µl was taken to place in the upper chamber. The cells were digested with conventional trypsin, centrifuged, adjusted to the concentration of 200 µl of 1% fetal bovine serum RPMI1640 medium, inoculated in the upper chamber. The cells were placed in methanol and glacial acetic acid (3:1) for 30 min. The cells were washed with 20% fetal bovine serum RPMI1640. Crystal violet staining, photography, the experiment was repeated three times.
Wound-healing assays
The logarithmic cells were selected for inoculation in 6-well plates with a cell density of 5 × 104 cells/ml. The cell density was 5 × 104 cells/ml. The disinfected 10 µL tip was used to evenly draw four horizontal lines after the cells were adhered to monolayer cells. The cells were washed with PBS and replaced with medium containing 1.5% fetal bovine serum. 3 wells were set and placed in a 5% CO2 incubator overnight. Samples were taken at 0, 24, 48, 72 h, and photographed under a microscope. The initial distance (0 time) of the scratch was measured. The distance of scratches was measured and photographed to calculate the cell mobility after 24, 48 and 72 hours. Each experiment was repeated three times.
Luciferase activity assays
The luciferase reporter vector was co-transfected with LV5-DAPK1 for pancreatic cancer cells. The transfected pRL-TK was used as standard internal control. The cells were harvested after transfection for 36 h. The luciferase activity of pancreatic cancer cells was detected by Promega’s luciferase activity assay kit. Calculate relative luciferase activity = firefly luciferase activity value/bloody luciferase activity value.
Pancreatic cancer xenografts
LV7-DAPK1 and LV5-DAPK1 + miR-182-mimic were transfected into 80-90% of the pancreatic cancer HS766T cells in logarithmic growth phase. A cell suspension having a cell concentration of 5 × 106/ml was prepared. Nude mice were selected from 4 to 6 weeks of age and randomly divided into 5 rats in each group and 0.2 ml of cell suspension was injected subcutaneously in the left forelimb armpit of each nude mice. The survival rate, body weight and survival status of the mice were monitored within 4 weeks after injection. The nude mice were sacrificed and the sterile ophthalmic scissors were taken out completely after 28 days. The tumor size and weight were measured.
Phalloidin assays
The cell climbing tablets were made, 4% paraformaldehyde fixed for 5-10 min after 24 h, 0.1% Triton X-100/PBS room temperature film breaking 3-5 min. 10 μl of FITC-Phalloidin stock solution was added to 150 μl of PBS to prepare a working solution (5 µg/ml) for cell staining for 30-60 min. Fluorescent seal liquid seal was added after cleaning to absorb excess water, fluorescence microscope observation.
Statistical analysis
SPSS 21.0 software was used for statistical analysis. The data were expressed as mean ± standard deviation. metrological data were analyzed by one-way ANOVA; variances were handled with the Karuskal-Wallis method. The difference was considered statistically significant at P < 0.05.
Results
Expression of DAPK1 and miR-182 in pancreatic cancer tissues, DAPK1 mRNA and miR-182 mRNA and mRNA in pancreatic cancer tissues
The expression of DAPK1 mRNA and miR-182 mRNA in pancreatic carcinoma and adjacent tissues were detected by qPCR method. As shown in Figure 1A, the expression of DAPK1 mRNA in the adjacent tissues was the highest, and the expression of DAPK1 mRNA in pancreatic cancer tissues decreased gradually. DAPK1 mRNA decreased significantly with the progress of tumor grade, the difference was statistically significant (P < 0.05), indicating that DAPK1 in the development of pancreatic cancer play a role in cancer. However, the expression of miR-182 in pancreatic cancer tissues and adjacent tissues was similar (Figure 1B). The expression of miR-182 mRNA was the highest and the lowest in adjacent tissues in pancreatic cancer stage III tumors. The expression of miR-182 mRNA was significantly increased with the increase of tumor grade, the difference was statistically significant (P < 0.05), indicating that miR-182 played a role in carcinogenesis during pancreatic cancer. The expression of DAPK1 in HS766T was significantly lower than that in PANC-1 cells In the pancreatic cancer cells PANC-1 and HS766T (Figure 1C), whereas miR-182 was significantly higher in HS766T than in PANC-1 cells, so we selected HS766T as a follow-up experimental cell lines.
Figure 1.
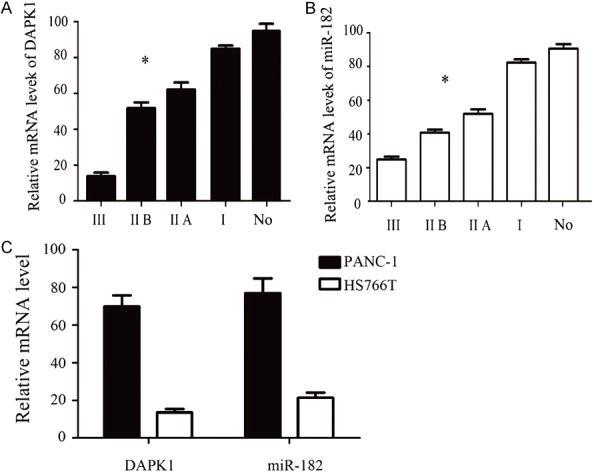
A. The mRNA expression of DAPK1 were detected by qPCR in different stage of pancreatic cancer tissue and normal tissue. B. The mRNA expression of miR-182 were detected by qPCR in different stage of pancreatic cancer tissue and normal tissue. C. The mRNA expression of DAPK1 and miR-182 were detected by qPCR in different pancreatic cancer cells. Error bars represent standard error. *P < 0.05.
Expression of DAPK1 and miR-182 in clinicopathological data of pancreatic
As shown in Table 1, statistical analysis showed that the expression level of DAPK1 in pancreatic cancer decreased gradually with the increase of pathological stage of pancreatic cancer. The expression of DAPK1 gradually decreased with the decrease of pancreatic cancer. The expression of miR-182 was significantly increased in the pancreatic cancer tissues with lymph node metastasis. The expression level of miR-182 increased with the increase of pathological stage of pancreatic cancer. The expression level of miR-182 increased gradually with the decrease of differentiation degree. The expression of miR-182 was significantly increased in pancreatic cancer tissues with lymph node metastasis. The expression levels of DAPK1 and miR-182 were not related to age and sex. The results showed that DAPK1 was negatively correlated with pathological stage of pancreatic cancer and lymph node metastasis, while miR-182 was positively correlated with sex, age and so on.
Table 1.
Relationship between DAPK1 and miR-182 expression and clinicopathological features of pancreatic cancer
| Clinicopathologic data | Amount | DAPK1 high expression | DAPK1 low expression | P value | MiR-182 high expression | MiR-182 low expression | P value |
|---|---|---|---|---|---|---|---|
| Sex | 0.108 | 0.628 | |||||
| Male | 38 | 22 | 16 | 18 | 20 | ||
| Female | 22 | 8 | 14 | 9 | 13 | ||
| Age (y) | 0.542 | 0.668 | |||||
| ≤ 60 | 46 | 22 | 24 | 20 | 26 | ||
| > 60 | 14 | 8 | 6 | 7 | 7 | ||
| Differentiation | 0.035 | 0.021 | |||||
| Well differentiated | 25 | 17 | 8 | 5 | 20 | ||
| Low differentiation | 35 | 13 | 22 | 28 | 7 | ||
| 30 | 25 | 5 | 27 | 3 | |||
| UICC period | 0.015 | 0.015 | |||||
| I + IIA | 39 | 24 | 15 | 17 | 22 | ||
| IIB + III | 21 | 6 | 15 | 16 | 5 | ||
| Lymph node metastasis | 0.016 | 0.017 | |||||
| N0 | 45 | 30 | 15 | 20 | 25 | ||
| N1 | 15 | 5 | 10 | 11 | 4 |
Changes of DAPK1 and miR-182 after transfection of LV5-DAPK1 in pancreatic cancer HS766T
As shown in Figure 2A, Western blot analysis showed that the expression level of DAPK1 was significantly higher than that of NC group after transfection of LV5-DAPK1. We used qPCR to detect the expression of miR-182 in pancreatic cancer cells transfected with LV5-DAPK1 to demonstrate whether overexpression of DAPK1 affects miR-182. The results were shown in Figure 2B. The expression of miR-182 in LV5-DAPK1 group was significantly lower than that in NC group (P < 0.05), indicating that overexpression of DAPK1 could inhibit the expression of miR-182.
Figure 2.
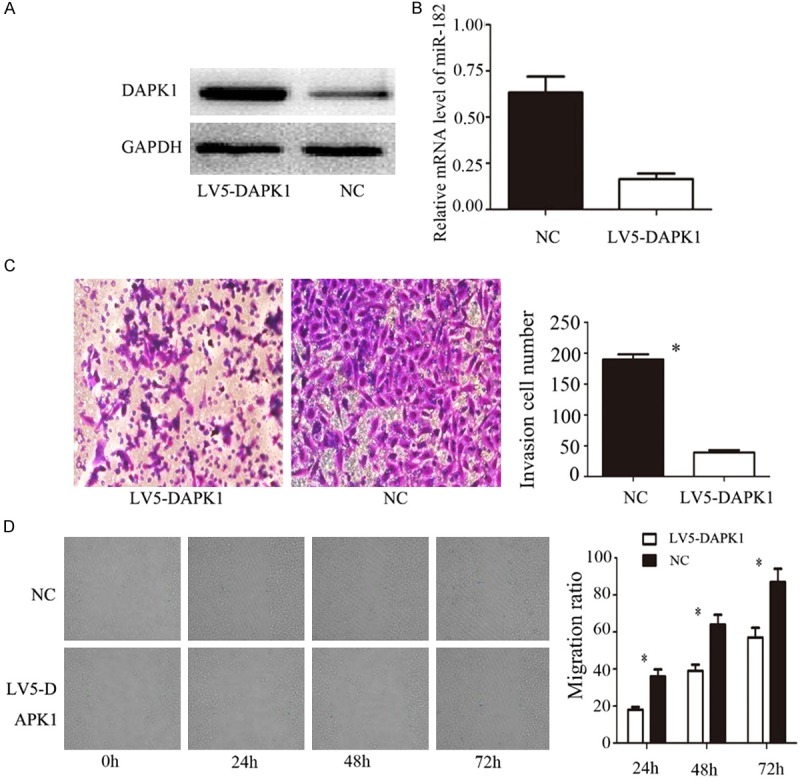
A. WB was used to detect the expression of DAPK1 after treated with LV5-DAPK1. B. qPCR was used to detected the expression of miR-182 after treated with LV5-DAPK1. C. Effect of LV5-DAPK1 on the invasion ability of HS766T cells were detected by Transwell matrigel invasion assays. D. Effect of LV5-DAPK1 on the HS766T cells migration ability were detected by wound healing assays. Error bars represent standard error. *P < 0.05.
Changes in the invasion and migration of pancreatic cancer cells after overexpression of DAPK1 examined by transwell and scratch tests
Transwell results showed that the invasive ability of pancreatic cancer cells in LV5-DAPK1 group was significantly lower than that in NC group; the difference was statistically significant (P < 0.05). The migration ability of pancreatic cancer cells in LV5-DAPK1 group was significantly weaker than that of NC group at different time points 24 h, 48 h and 72 h (P < 0.05), indicating that DAPK1 inhibits the invasion and migration of pancreatic cancer cells.
Expression of miR-182 detected by qPCR after miR-182-mimic was applied to pancreatic
MiR-182-mimic was used to detect the expression of miR-182 in pancreatic cancer cells. We used qPCR to detect the expression of the gene in order to confirm its effectiveness. The expression of miR-182 in the miR-182-mimic group was significantly higher than that in the NC group (Figure 3A), indicating that miR-182-mimic can significantly increase the expression of miR-182.
Figure 3.
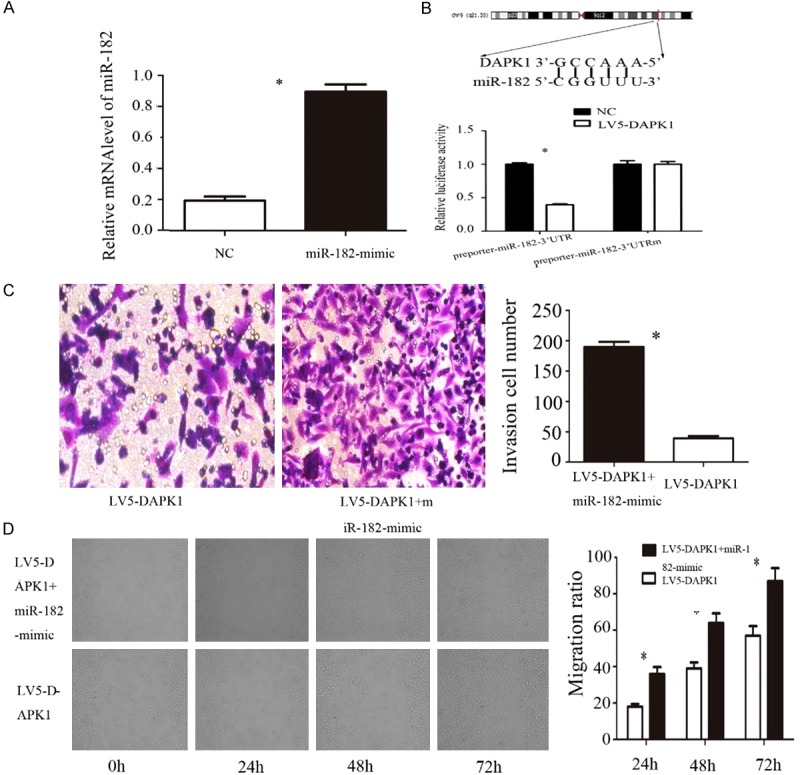
A. qPCR was used to detect the expression of miR-182 after treated with miR-182-mimic; B. Luciferase report gene detects miR-182 the direct target of DAPK1; C. Transwell matrigel invasion assays were used to detect whether the effect of invasion ability could recover after treated with LV5-DAPK1 and miR-182-mimic. D. Wound healing assays were used to detect whether the effect of migration ability could recover after treated with LV5-DAPK1 and miR-182-mimic. Error bars represent standard error. *P < 0.05.
Relationship between DAPK1 and miR-182 was detected by luciferase reporter gene
The results of previous experiments showed that the expression of miR-182 changes with DAPK1, and it was predicted that DAPK1 may interact directly with miR-182. In order to verify whether miR-182 target the 3’UTR of DAPK1, we stained DAPKl-siRNA with miR-182 into pancreatic cancer HS766T cells. Luciferase reporter gene results showed (Figure 3B) that DAPK1 significantly inhibited luciferase activity in miR-182. The results show that DAPK1 binds specifically to the 3’UTR of miR-182.
Transwell and scratch tests showed that miR-182 overexpression of DAPK1 could restore pancreatic cancer cell invasion and migration
Transwell results showed that the invasive ability of pancreatic cancer cells in LV5-DAPK1 + miR-182-mimic group was significantly higher than that in LV5-DAPK1 group; the difference was statistically significant (P < 0.05). The results of scratch test showed (Figure 3D) that the migration ability of pancreatic cancer cells in LV5-DAPK1 + miR-182-mimic group was significantly higher than that in LV5-DAPK1 group at different time points 24 h, 48 h and 72 h (P < 0.05). The overexpression of miR-182 after overexpression of DAPK1 could restore the ability of invasion and migration of pancreatic cancer cells.
Overexpression of DAPK1 and overexpression of miR-182 after cytoskeleton-associated protein changes
The results of previous experiments showed that DAPK1 and miR-182 played an important role in the invasion and migration of pancreatic cancer cells, and the invasion and migration of cells involve changes in cell adhesion, degradation of extracellular matrix and changes of cell morphology. The cytoskeleton was constantly changing to complete. It was speculated that DAPK1 may result in cytoskeleton remodeling and cell migration and invasion. WB test results showed that the expression of ROCK-1 and RhoA protein in LV5-DAPK1 group was significantly lower than that in NC group (P < 0.05). The protein expression of ROCK-1 and RhoA was significantly restored after adding miR-182-mimic (P < 0.05).
Cytoskeleton phalloidin staining showed (Figure 4B) that the expression of F-actin in LV5-DAPK1 group was significantly decreased compared with LV5-DAPK1 + miR-182-mimic group, the formation of cell membrane wrinkles was significantly reduced, and the formation of pseudopodia was significantly reduced.
Figure 4.
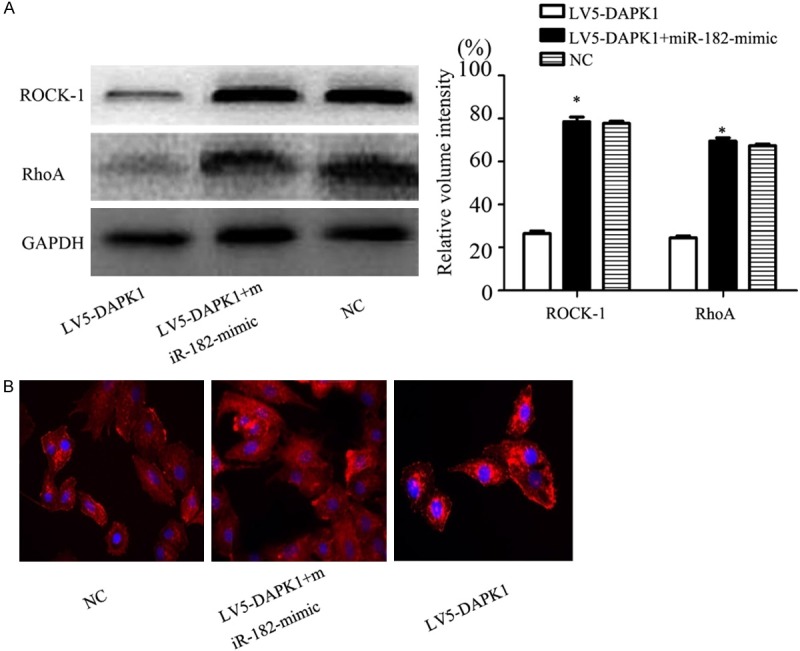
A. Expression of RhoA and ROCK-1 were detected by Western blot. B. Phalloidin was used to label the F-actin. Original magnification, 400 × . Error bars represent standard error. *P < 0.05.
Effect of DAPK1 and overexpression of miR-182 on tumor growth examined by subcutaneous tumor formation in nude mice
Tumor growth in nude mice (Figure 5A): the tumor size of LV5-DAPK1 + miR-182-mimic group was significantly increased compared with LV5-DAPK1 group, the difference was statistically significant (P < 0.05). Comparison of tumor weight and volume (Figure 5B, 5C): the tumor volume and weight of LV5-DAPK1 + miR-182-mimic group were significantly increased compared with LV5-DAPK1 group; the difference was statistically significant (P < 0.05).
Figure 5.
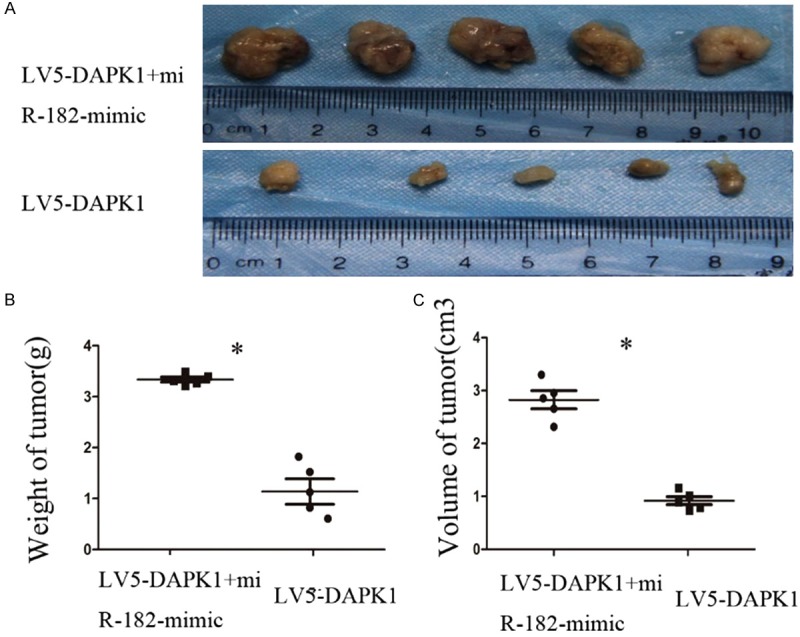
Effect on tumorigenesis after overexpression DAPK1 and miR-182 in vivo. A. Comparison of the tumor size of nude mice. B, C. Compare the volume and weight of the tumor in nude mice.
Discussion
Long-chain non-coding RNA is a widely-used functional transcript. With the development of genomic large-scale transcriptomics, 80% of the mammalian genome has been found to be regulated by lncRNA [12]. Previously, most people ignored the function of lncRNA, more and more investigations have shown that lncRNA and protein coding genes are closely related. LncRNA size is generally from 200 nt-100 kb, no obvious open reading frame [13]. It has been found that lncRNA HOTAIR (HOX antisense RNA) can affect the metastasis of breast cancer by changing the chromatin structure of transcript recently [14]. LncRNA SPRY4-IN1 can promote the invasion and metastasis of melanoma cells [15]. These results indicate that lncRNA has been gradually accepted as a transcript with specific function, and that the abnormal expression of lncRNA is closely related to the progression of the tumor. In this investigation, 60 cases of pancreatic cancer specimens were detected by PCR, and the expression of lncRNA DAPK1 was significantly increased in pancreatic cancer tissues. The expression of DAPK1 gradually decreased with the progression of the tumor. The expression of DAPK1 was negatively correlated with tumor differentiation and lymph node metastasis, suggesting that DAPK1 plays a role in tumor suppressor gene in pancreatic cancer. Furthermore, we detected the invasion and migration ability of pancreatic cancer cells after overexpression of DAPK1. It was found that the invasion and migration ability of pancreatic cancer cells were significantly decreased, indicating that DAPK1 also played an important role in the invasion and migration of pancreatic cancer cells.
In the early investigation of lncRNA, the investigators found that lncRNA regulation of gene expression may depend on the physical location of the lncRNA target gene and moderately act in a cis-acting manner [16]. Later researchers have found that lncRNA can also regulate protein coding genes and affect the biological function of related proteins by transcription, promotion, inhibition, reverse binding protein coding gene transcripts, etc. lncRNA can also be combined with miRNA at the same time, thus lifting the relevant miRNA on the downstream target gene negative regulation [17]. The expression of miR-182 was detected by overexpression of DAPK1 in this investigation. The expression of miR-182 was decreased after expression of DAPK. Further detection of luciferase reporter gene revealed that DAPK1 specifically binds to 3’UTR of miR-182, which affects the biological function of pancreatic cancer cells.
We added miR-182-mimic on the basis of overexpression of DAPK1 in order to clarify whether miR-182 can restore pancreatic cancer cell invasion and migration ability. It was found that the invasion and migration ability of pancreatic cancer cells were significantly restored after the addition of miR-182-mimic.
The Rho protein family is an important regulator of cytoskeletal actin [18]. Previous experimental results showed that DAPK1 and miR-182 could affect the invasion and migration of pancreatic cancer cells. The first step in the ability to metastasize tumor cells was to reduce tumor cell adhesion, enhanced cell motility, and then infiltrated the surrounding tissue and distant metastases. We speculate that DAPK1 and miR-182 may activate the signal pathway of the relevant cytoskeleton by regulating the ROCK-1/Rho family protein and promote the invasion and migration of pancreatic cancer cells. WB test results and phalloidin labeled cytoskeleton confirmed the above results.
In this investigation, the expression of DAPK1 and miR-182 in pancreatic and paracancerous tissues was detected by using qPCR, and the interaction between DAPK1 and miR-182 was further investigated, the role of DAPK1 and miR-182 in the process of invasion and migration of pancreatic cancer cells was further investigation. Researches have shown that DAPK1 is down-regulated in pancreatic cancer, whereas miR-182 is upregulated in pancreatic cancer, and DAPK1 interacts directly with miR-182. DAPK1 can target the invasion and migration of pancreatic cancer by miR-182. DAPK1 can regulate the expression of ROCK-1 and RhoA, and it can affect the change of cytoskeleton, which indirectly indicates that ROCK-1/RhoA signaling pathway plays an important role in the regulation of miR-182 on the biological function of pancreatic cancer cells by DAPK1. It suggests that DAPK1 and miR-182 may be involved in the invasion and migration of pancreatic cancer cells and may be a marker for predicting the progression, prognosis and monitoring of pancreatic cancer.
Acknowledgements
The Xinjiang Uygur Autonomous Region Natural Science Foundation (No.2014211C081).
Disclosure of conflict of interest
None.
References
- 1.Lin QJ, Yang F, Jinn C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988–8003. doi: 10.3748/wjg.v21.i26.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z, Zheng R, He Y, Sun X, Wang N, Chen T, Chen W. Risk factors for pancreatic cancer in China: a multicenter case-control study. J Epidemiol. 2016;26:64. doi: 10.2188/jea.JE20140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisman D, Gibson A, Patel M, Wang Y. Evidence for a role of a lncRNA encoded from the p53 tumor suppressor gene in maintaining the undifferentiated state of human myeloid leukemias. Gene Reports. 2016;5:45–50. [Google Scholar]
- 5.Gonzalez I, Munita R, Agirre E, Dittmer TA, Gysling K, Misteli T, Luco RF. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22:370–6. doi: 10.1038/nsmb.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhan A, Mandal SS. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahira AC, Kubrusly MS, Faria MF, Dazzani B, Fonseca RS, Maracaja-Coutinho V, Verjovski-Almeida S, Machado MC, Reis EM. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol Cancer. 2011;10:141. doi: 10.1186/1476-4598-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:1–6. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang L, Mao PL, Wu J, Huang J, Lin C, Yuan J, Qu L, Cheng SY, Li J. miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol. 2010;177:29–38. doi: 10.2353/ajpath.2010.090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, Wu J, Hu B, Cheng SY, Li M, Li J. TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J Clin Invest. 2012;122:3563–78. doi: 10.1172/JCI62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei R, Tang J, Zhuang X, Deng R, Li G, Yu J, Liang Y, Xiao J, Wang HY, Yang Q, Hu G. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene. 2014;33:1287–96. doi: 10.1038/onc.2013.65. [DOI] [PubMed] [Google Scholar]
- 12.Ballarino M, Cazzella V, D’Andrea D, Grassi L, Bisceglie L, Cipriano A, Santini T, Pinnarò C, Morlando M, Tramontano A, Bozzoni I. Novel long noncoding RNAs (lncRNAs) in myogenesis: a miR-31 overlapping lncRNA transcript controls myoblast differentiation. Mol Cell Biol. 2015;35:728–36. doi: 10.1128/MCB.01394-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng M, Blondeau JJ, Schmidt D, Perner S, Müller SC, Ellinger J. Identification of novel differentially expressed lncRNA and mRNA transcripts in clear cell renal cell carcinoma by expression profiling. Genom Data. 2015;5:173–175. doi: 10.1016/j.gdata.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SM, Fang DC, Yang JL, Liang GP, Lu R, Luo YH, Liu WW. Effect of antisense human telomerase RNA on malignant phenotypes of gastric carcinoma. J Gastroenterol Hepatol. 2002;17:1144–52. doi: 10.1046/j.1440-1746.2002.02857.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu ST, Chen L, Wang HJ, Tang XD, Fang DC, Yang SM. hTERT promotes the invasion of telomerase-negative tumor cells in vitro. Int J Oncol. 2009;35:329–336. [PubMed] [Google Scholar]
- 16.Tsai CC, Chen CL, Liu HC, Lee YT, Wang HW, Hou LT, Hung SC. Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J Biomed Sci. 2010;17:64. doi: 10.1186/1423-0127-17-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]


