Abstract
Endoplasmic reticulum (ER) stress has been increasingly recognized to have an important role in various liver diseases. Sepsis-induced multi-organ failure remains to have a high mortality rate, and the liver plays a central pathophysiological role. This study aims to explore whether ER stress is involved in liver injury in septic rats. Sepsis was induced via cecal ligation and puncture (CLP). Rats were randomly divided into five groups as follows: sham, CLP 2 h, CLP 6 h, CLP 12 h, and CLP 24 h. They were monitored to record body weight (BW) and liver weight changes for every time point after surgery. The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected via colorimetric activity assays. In addition, the morphological changes of the liver tissue were evaluated by staining the sections with hematoxylin and eosin and observing under light microscopy. The levels of glucose-regulated protein 78 (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), and cleaved caspase-12 were detected via Western blot analysis. Apoptosis was detected via terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method. The results showed that septic rat serums ALT and AST were increased, with the increase being more obvious in the CLP 24 h group. In addition, septic rats appeared to have histopathological abnormalities in the liver. The liver weight and index increased after CLP. No differences were noted in the BW between septic groups. The level of GRP78, CHOP, and cleaved caspase-12 were upregulated after CLP. However, CHOP and caspase-12 were induced later than GRP78. The density of TUNEL-positive apoptotic hepatocytes was significantly increased after 12 h and 24 h CLP. It indicates that the unfolded protein response occurred in the early stage of sepsis-induced liver damage. The ER stress-mediated apoptosis signal pathway is among the mechanisms of septic liver injury and may be a target in clinical prevention and therapy of sepsis-induced liver injury.
Keywords: Sepsis, liver injury, endoplasmic reticulum stress, apoptosis, caspase-12
Introduction
Sepsis is a serious public health issue with significant morbidity and mortality rates [1,2]. It can evolve to multiple organ dysfunction syndrome (MODS), whose severity accounts for a high mortality rate. During sepsis, liver injury is one of the MODS components and is usually associated with a poor prognosis [3,4]. Although significant progress on understanding the pathophysiology of sepsis has been made over the past decade, the mechanisms of sepsis-induced liver injury remain poorly understood. Thus, further exploring the mechanism of liver injury associated with sepsis is important.
Recently, endoplasmic reticulum (ER) stress has received growing attention because it causes pathologically relevant apoptosis and is associated with various diseases, including central nervous system, kidney, and cardiovascular diseases and diabetes [5-8]. ER stress also has been demonstrated in cecal ligation and puncture (CLP) model of sepsis. Ma et al. [9] found that ER stress contributes to abnormal lymphocyte apoptosis during sepsis in mice. Zhang et al. [10] have shown that the ER stress markers, namely, GRP94, CHOP, and caspase-12, were upregulated in the hearts of septic rats, also suggesting that inhibition of ER stress protected the myocardial function from ER stress-induced apoptosis in rats. However, whether ER stress-induced apoptosis affects the liver of septic rats is unknown. Thus, our study aimed to expound the role of ER stress pathway in CLP-induced liver injury.
Material and methods
Animals
The study was approved by the Animal Ethics Committee in Shihezi University, School of Medicine, Shihezi, China. All experimental procedures in this study complied with the National Institutes of Health guidelines. Adult Sprague-Dawley rats weighing 220-260 g were obtained from the Animal Center of Xinjiang Medical University (Urumqi). Rats were housed individually in cages under standard conditions at 22°C and a 12-h light/dark cycle with food and water available ad libitum.
Cecal ligation and puncture
The animals were subjected to CLP as previously described [11]. Rats were anesthetized with sodium pentobarbital (50 mg/kg) given intraperitoneally, and a 2 cm ventral midline abdominal incision was performed. Then, the cecum was exposed, ligated just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The incision was then closed in layers. Sham-operated animals (i.e. control animals) underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The rats were resuscitated with 30 ml/kg body weight (BW) normal saline subcutaneously immediately after surgery. They were then returned to their cages with free access to food and water.
Plasma and tissue collection
Blood was collected into EDTA-containing tubes (30 HL of 0.5 M EDTA) that were then placed on ice temporarily for at least 30 min and centrifuged at 4°C at 1 000 g for 10 min. The plasma supernatant was aliquoted for later analysis. Liver tissues were collected after brief portal vein perfusion and were either immediately frozen in dry ice and then stored at -80°C for further analysis or placed in 10% formalin overnight and then transferred to 70% ethanol for paraffin embedding and tissue slide preparation for histology and analysis.
Measurement of liver index and function
Liver weight/body weight ratios indicating fluid accumulation were measured as indexes of liver injury. Each group of rats was weighed before decapitation, and the liver was separated and weighed after decapitation. The liver indexes were then calculated.
Liver function was assessed by quantifying plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using colorimetric activity assays (Roche Diagnostic Ltd, Germany).
Histopathological observations
The liver tissue section was stained via hematoxylin eosin. Based on the liver injury scoring standard as previously described [12], the histological slides were blindly read by two experienced pathologists.
Western blotting
Approximately 40 mg of frozen liver tissue was homogenized in lysis buffer (Pierce, Rockford, IL, USA); the homogenate was centrifuged at 12,000 g for 30 min at -4°C, and the pellet was discarded. Protein concentration was determined using bicinchoninic acid method. Equal amounts of protein (30 µg) were separated using 10%-12% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Beyotime, Shanghai, China). The membranes were blocked in 5% nonfat milk for 2 h at room temperature and then incubated with rabbit Bip polyclonal antibodies (1:1000, #3183, Cell Signaling Technology, USA), rabbit caspase-12 polyclonal antibodies (1:1000, bs-1105R, BiOSS, Beijing), and mouse Anti-CHOP antibody (1:1000, ab114119, Abcam, USA) overnight at 4°C. The membranes were incubated with HRP-labeled goat anti-rabbit IgG (1:20000, ZB-2301, ZSGB-BIO, Beijing) or goat anti-mouse IgG (1:20000, ZB-2305, ZSGB-BIO, Beijing) for 1 h at room temperature. The membranes were then washed in TBST for 5×10 min. A chemiluminescent peroxidase substrate (Thermo Fisher Scientific, USA) was applied according to the manufacturer’s instruction, and the membranes were exposed to X-ray film. The densities of bands on Western blot were analyzed using the Image J software (National Institutes of Health, Bethesda, MD).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
TUNEL calorimetric assay (Roche, Mannheim Germany) was performed according to the product protocol. Eight fields at ×200 were randomly selected, and the number of TUNEL-positive cell was calculated.
Statistical analysis
All data were analyzed with SPSS 17.0 software package (SPSS, Chicago, IL). The results in our study are expressed as means ± SD and compared via one-way analysis of variance (ANOVA) and Student-Newman-Keuls test for multiple group analysis. Significance was accepted at P<0.05.
Results
Liver weight, body weight, and liver index changes
Figure 1 shows the liver weight, body weight, and liver index changes in the five groups. The liver weight and liver index were analyzed via one-way ANOVA and Student-Newman-Keuls method. The normal control group’s liver weight was (6.09 ± 0.40) g, compared with the control group, liver weight were significantly increased in the CLP 6 h, CLP 12 h, and CLP 24 h groups (CLP 6 h, 7.41 ± 0.55 g; CLP 12 h, 8.53 ± 0.56 g; CLP 24 h, 9.59 ± 1.08 g), the difference was statistically significant (P<0.05, Figure 1B). The sham group’s liver index was (2.57 ± 0.20)%, and the liver index of the rat was increased to (3.06 ± 0.19)% rapidly at CLP 6 h. it was (3.44 ± 0.28)% 12 hours later. And it reached the peak (3.92 ± 0.23)% 24 hours later, the difference was statistically significant (P<0.05, Figure 1C). However, no significant differences were observed in the body weights of the sham group and the CLP groups (Figure 1A).
Figure 1.
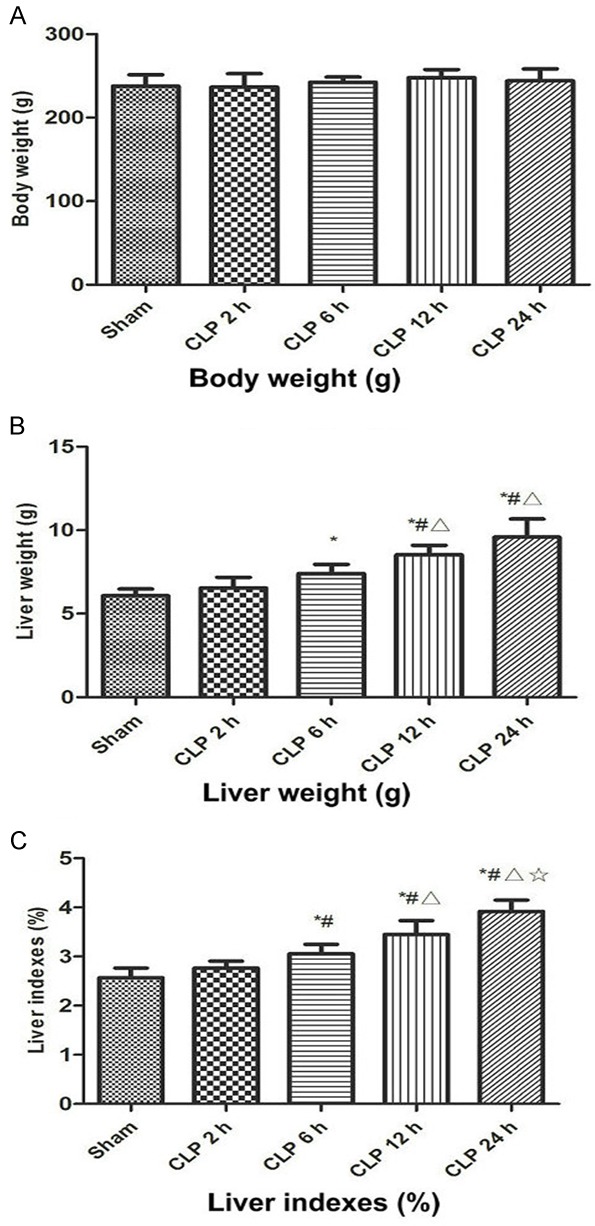
The change of rat weight (A), liver weight (B) and liver index (C) 2, 6, 12 and 24 h after CLP. Sham, sham-operated rats; CLP, rats subjected to cecal ligation and puncture. Data are presented as means ± SD (n=8) and compared by one-way ANOVA and Student-Newman-Keuls method: *P<0.05 VS. the control group; #P<0.05 VS. CLP 2 h group; ΔP<0.05 VS. CLP 6 h group; ☆P<0.05 VS. CLP 12 h group.
Liver injury in sepsis
Liver injury occurred after CLP (Figures 2 and 3). At CLP 6 h, CLP 12 h, and CLP 24 h groups, compare with sham group (ALT, 52.38 ± 3.38 U/l; AST, 253.75 ± 14.82 U/l), both serum ALT (CLP 6 h, 87.75 ± 8.96 U/l; CLP 12 h, 136.63 ± 16.92 U/l; CLP 24 h,161.50 ± 13.83 U/l) and AST (CLP 6 h, 445.5 ± 30.50 U/l; CLP 12 h, 650.75 ± 27.80 U/l; CLP 24 h, 930.38 ± 28.45 U/l) were elevated (P<0.05, Figure 2), and histological examination of the tissue revealed injury (Figure 3). The rat liver in the sham group was normal as well as glycogen distribution in hepatocytes, and no evidence suggested hepatocyte swelling or neutrophil and macrophage infiltration. At CLP 6 h, a small amount of swollen hepatocyte and minimal periportal neutrophil and macrophage infiltration were noted in the liver. At CLP 12 h, the liver showed lightly dyed nucleus or obscure nucleus partial hepatocytes, slight periportal neutrophil, and macrophage infiltration. At CLP 24 h, the liver showed vacuolar degeneration or ballooning degeneration of partial hepatocytes and severe periportal neutrophil and macrophage infiltration.
Figure 2.
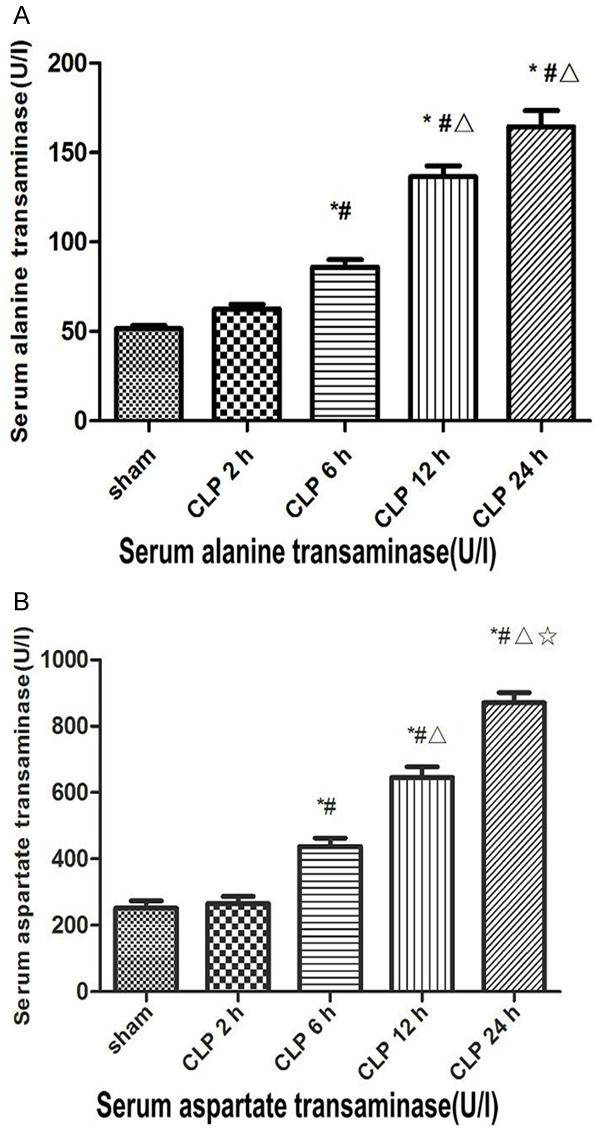
Serum transaminase level 2, 6, 12 and 24 h after CLP. Sham, sham-operated rats; CLP, rats subjected to cecal ligation and puncture. A. Serum alanine transaminase (ALT) concentration 2 h after sham surgery and 2, 6,12 and 24 h after CLP. B. Serum aspartate transaminase (AST) concentration 2 h after sham surgery and 2, 6, 12 and 24 h after CLP. Data are presented as means ± SD (n=8) and compared by one-way ANOVA and Student -Newman-Keuls method: *P<0.05 VS. the control group; #P<0.05 VS. CLP 2 h group; ΔP<0.05 VS. CLP 6 h group; ☆P<0.05 VS. CLP 12 h group.
Figure 3.
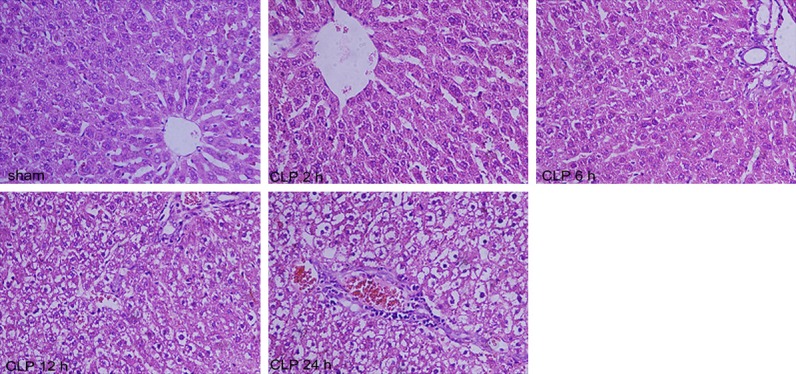
Histological liver injury after CLP. Sham, sham-operated rats; CLP, rats subjected to cecal ligation and puncture. Typical histology is presented 2 h post sham surgery, 2 h, 6 h, 12 and 24 h post CLP. The panels are ×20 original magnification. Visualization with a confocal microscope. (Scale bar =50 μm).
ER stress is triggered in the liver in progression in sepsis
Western Blot showed that the relative expression levels of the protein were normalized with β-actin. The expression level of GRP78 protein was significantly increased after CLP 2 h, and reached the peak at CLP 6 h, However, at CLP 12 h, the level of GRP78 protein decreased significantly, however, it was still significantly higher than the base value, and the difference was statistically significant (F=62.71, P<0.05, Figure 4A and 4B). As indicated in Figure 4C and 4D, the expression of CHOP protein was up-regulated after CLP 12 h, and increased with the time of injury, the difference was statistically significant (F=156.36, P<0.05). As shown in Figure 4E and 4F, the expression of cleaved caspase-12 protein was significantly increased at 12 h after CLP in vehicle-treated animals, and increased with the time of injury (F=48.95, P<0.05).
Figure 4.
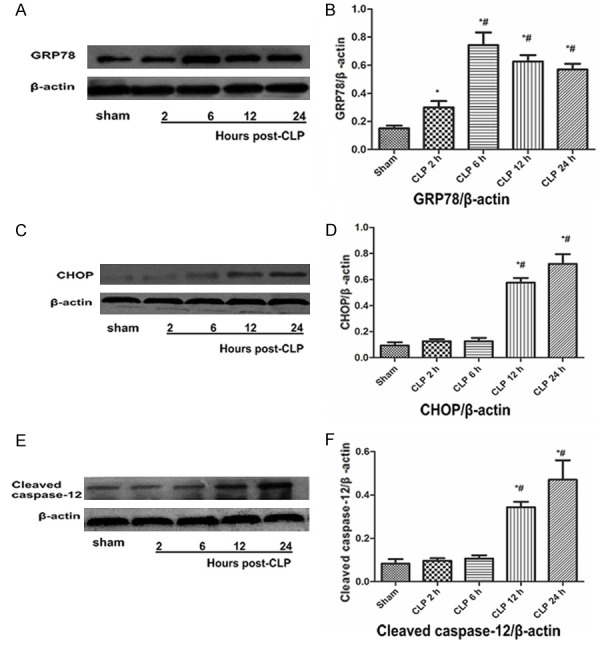
Endoplasmic reticulum (ER) stress occurs in liver after CLP. A representative Western blot from three experiments demonstrating that as the extension of the CLP molding time the protein levels of glucose-regulated protein 78 (GRP78) (A), CCAAT/enhancer binding protein homologous protein (CHOP) (C) and cleaved caspase-12 (E) were increased in liver. (B) GRP78 Protein level, (D) CHOP Protein level, (F) cleaved caspase-12 Protein level. Data are presented as means ± SD (n=3) and compared by one-way ANOVA and Student-Newman-Keuls method: *P<0.05 VS. the control group; #P<0.05 VS. CLP 2 h group.
Photomicrographs of TUNEL-stained sections in hepatic tissue
As shown in Figure 5, apoptotic cells with shrunken cytoplasm and pyknotic nuclei (arrows) were significantly increased after CLP 6 h, and increased with the time of injury, the difference was statistically significant (F=107.48, P<0.05). The results of semiquantitative scoring of TUNEL-positive cells were in line with the morphological changes.
Figure 5.
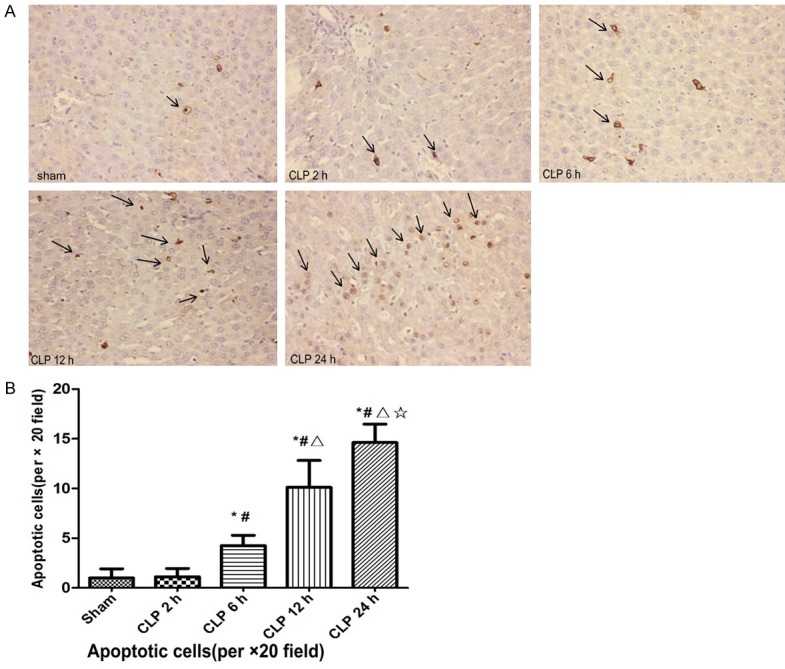
Photomicrographs of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-stained sections (A) and semiquantitative scoring of TUNEL-positive cells (B) in liver. Scale bar =50 μm. Arrows denoted characteristic morphology of apoptotic cells with pyknotic nuclei and shrunken cytoplasm. Data are presented means ± SD (n=8) and compared by one-way ANOVA and Student-Newman-Keuls method: *P<0.05 VS. the control group; #P<0.05 VS. CLP 2 h group; ΔP<0.05 VS. CLP 6 h group; ☆P<0.05 VS. CLP 12 h group.
Discussion
Sepsis complicated with MODS is the primary cause of death in intensive care units. The incidence rate of liver dysfunction in sepsis patients was 34.7% and is associated with poor prognosis [1,4]. Our study found that the rats had liver impairment following polymicrobial infection (CLP), which is consistent with the results of previous study [13]. During sepsis, several factors, such as bacterial toxins, inflammatory factors, and malnutrition, can lead to liver damage. However, clinical studies found that, in view of the above mechanism, existing treatment is not effective in relieving liver damage. Identifying the mechanisms involved in septic liver injury is important because it may provide new therapeutic targets and lead to implementation of effective treatment.
ER is among the most important organelles in mammalian cells. It is very sensitive to changes in the environment inside and outside the cell. ER stress is induced by a variety of factors, including ischemia, hypoxia, calcium ion erosion, and oxidative stress and is a result of the accumulation of unfolded or misfolded proteins in the ER. To respond to this stress, cells activate an adaptive pathway of unfolded protein response (UPR) to restore the normal ER function. However, if ER stress persists or is aggravated, adaptation starts to fail and apoptosis occurs [14]. Previous studies have demonstrated that ER stress is closely related to a variety of liver disease [15-17]. However, the mechanisms associated with septic liver injury still remain unclear.
The chaperone protein gluco-se-regulated protein 78 (GRP78), also known as binding immunoglobulin protein, is among the ER chaperone proteins. Under normal circumstances, it combines with three ER-localized protein sensors, namely, PERK, ATF6, and IRE-1. However, if unfolded proteins accumulate in the ER lumen, then the GPR78 dissociates from the ER stress transducers to combine unfolded proteins, leading to their activation and triggering the ER stress response (UPR). As such, the accumulation of unfolded or misfolded proteins in the ER is reduced, restoring the normal ER function. Therefore, it is a hallmark for ER stress [18-20]. In the present study, compared with sham-operated control rats, GRP78 expression in the liver of septic CLP rats was increased, indicating the occurrence of ER stress and UPR, which is aimed to recover ER homeostasis and increase hepatocyte survival.
ER stress is an adaptive response in regulating cellular responses, however, if the stress is persistent and cannot be resolved, then signaling switches from pro-survival to pro-apoptotic. CHOP is a key molecule involved in ER stress-mediated apoptosis [21]. In normal cells, CHOP expression is low, but it is markedly elevated under ER stress. Previous studies found that CHOP overexpression promotes apoptosis, whereas CHOP deficiency indicates resistance to ER stress-induced apoptosis [22-24]. CHOP induction indicates that ER began promoting apoptosis. In the current study, we found that the number of apoptotic hepatocytes was significantly increased in septic rats, and the level of CHOP was increased significantly in CLP groups. These results indicate that CHOP is crucial in mediating pathways of ER stress-induced apoptotic cell death in the liver of septic rats. In addition, murine caspase-12, which is a member of the caspase family, has been reported to play an important role in ER-stress mediated cell death [25,26]. Morishima et al. [27] found that caspase-12 activation is not dependent on mitochondrial or any death receptor activation, and its activation triggers the ER-specific caspase cascade that leads to apoptotic death. In the present study, our data show that the level of cleaved caspase-12 was significantly increased in the septic group, indicating that it plays an important role in ER stress-mediated cell death in liver from the CLP rats. Collectively, the induction of CHOP and caspase-12 strongly suggested that because of the insufficiency of the cellular protective mechanisms, the ER-mediated pro-apoptotic response drove the hepatocytes toward death. A previous study showed that CHOP deletion ameliorated liver injury and improved cellular function [28]. Another study found that inhibition of caspase-12 reduced the thapsigargin-induced cell death [29]. In the present study, results show a good correlation between apoptotic hepatocytes and liver injury. Given that CHOP and caspase-12 have important roles in ER-stress mediated cell death, we demonstrated that ER stress-induced hepatocyte apoptosis may accelerate septic liver injury.
Sepsis is an extremely complex disorder involving activation of numerous intersecting cascades. Thus, multiple pathways of cell death may be involved in liver injury of sepsis. The present findings indicate that activation of the ER-mediated apoptotic pathway is at least partially responsible for the extensive hepatocyte apoptosis in sepsis. However, whether a cross talk exists between these pathways and the relative importance of the ER stress-mediated pathway in sepsis-induced liver injury need to be elucidated in further studies. Our results are obtained from animal experiments, whereas the clinical context is considerably more complex. We cannot translate our result into humans, but we are interested in that to explore new therapeutic targets.
In conclusion, our result shows that ER stress pathway is involved in septic-induced liver injury. In addition, ER stress-induced cell apoptosis is involved in hepatocyte apoptosis in rats with CLP surgery. Further investigation of this mechanism may lead to effective intervention in this pathway to prevent liver injury induced by sepsis.
Disclosure of conflict of interest
None.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Dreiher J, Almog Y, Sprung CL, Codish S, Klein M, Einav S, Bar-Lavie Y, Singer PP, Nimrod A, Sachs J. Temporal trends in patient characteristics and survival of intensive care admissions with sepsis: a multicenter analysis*. Crit Care Med. 2012;40:855–860. doi: 10.1097/CCM.0b013e318236f7b8. [DOI] [PubMed] [Google Scholar]
- 3.Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P. Clinical review: the liver in sepsis. Critical Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobashi H, Toshimori J, Yamamoto K. Sepsis-associated liver injury: incidence, classification and the clinical significance. Hepatol Res. 2013;43:255. doi: 10.1111/j.1872-034X.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ, Lin L, Tan X, Ye LB, Xiao J. Endoplasmic reticulum stress: relevance and therapeutics in central nervous system diseases. Mol Neurobiol. 2015;51:1343–1352. doi: 10.1007/s12035-014-8813-7. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Cui J, He Q, Chen Z, Arvan P, Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol Aspects Med. 2015;42:105–118. doi: 10.1016/j.mam.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong B, Zhou H, Han C, Yao J, Xu L, Zhang M, Fu Y, Xia Q. Ischemia/reperfusion-induced CHOP expression promotes apoptosis and impairs renal function recovery: the role of acidosis and GPR4. PLoS One. 2014;9:e110944. doi: 10.1371/journal.pone.0110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sozen E, Karademir B, Ozer NK. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic Biol Med. 2015;78:30. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Han L, Gao Y, Li L, Shang X, Hu W, Xue C. The endoplasmic reticulum stress-mediated apoptosis signal pathway is involved in sepsis-induced abnormal lymphocyte apoptosis. Eur Surg Res. 2008;41:219. doi: 10.1159/000135631. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Liu Y, Zhang JS, Zhang XH, Chen WJ, Yin XH, Qi YF. Cortistatin protects myocardium from endoplasmic reticulum stress induced apoptosis during sepsis. Mol Cell Endocrinol. 2015;406:40–48. doi: 10.1016/j.mce.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Rittirsch D, Huberlang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L, Xie K, Qin M, Peng D, Ma S, Shang L, Li N, Li S, Ji G, Lu Y. Effects of reactive oxygen species scavenger on the protective action of 100% oxygen treatment against sterile inflammation in mice. Shock. 2010;33:646–654. doi: 10.1097/SHK.0b013e3181c1b5d4. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Li J, Bao Y, Yuan D, Huang Z. Xuebijing injection alleviates cytokine-induced inflammatory liver injury in CLP-induced septic rats through induction of suppressor of cytokine signaling 1. Exp Ther Med. 2016;12:3. doi: 10.3892/etm.2016.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151:217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 15.Lebeaupin C, Proics E, Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Thuc OL. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HY, Du YJ, Zhao L, Gastroenterology DO. Endoplasmic reticulum stress in liver disease. World Chinese Journal of Digestology. 2012;54:795–809. [Google Scholar]
- 17.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 18.Lin P, Liu H, Chang C, Chen Y, Chang C, Shih W. Avian reovirus S1133-induced apoptosis is associated with Bip/GRP79-mediated Bim translocation to the endoplasmic reticulum. Apoptosis. 2015;20:481–490. doi: 10.1007/s10495-015-1085-5. [DOI] [PubMed] [Google Scholar]
- 19.Rao RV, Hermel E, Castro-Obregon S, Rio GD, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. J Biol Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- 20.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 21.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 22.Tajiri S, Yano S, Morioka M, Kuratsu J, Mori M, Gotoh T. CHOP is involved in neuronal apoptosis induced by neurotrophic factor deprivation. FEBS Lett. 2006;580:3462–3468. doi: 10.1016/j.febslet.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Ji C, Mehrianshai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in Hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 26.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 28.Rao J, Zhang C, Wang P, Lu L, Qian X, Qin J, Pan X, Li G, Wang X, Zhang F. C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1α signaling in acute liver failure. Biochem J. 2015;466:369–378. doi: 10.1042/BJ20140412. [DOI] [PubMed] [Google Scholar]
- 29.Rao RV, Peel A, Logvinova A, Del RG, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


