Abstract
microRNA-128 (miR-128), a kind of short, noncoding RNAs, functioned as a tumor marker. However, the underlying function and mechanism of miR-128 in human thyroid cancer were uncertain. Therefore, in the present study, the effects of miR-128 on the proliferation and apoptosis of cultured human thyroid cancer cells were investigated. After slicing miR-128 in human thyroid cancer cells, the proliferation was measured by methyl thiazolyl tetrazolium (MTT) method, the expression of miR-128, CCAAT/enhancer binding protein-α (C/EBPα), peroxisome proliferator-activated receptor-γ (PPARγ), Caspase-3 and Caspase-9 was determined by RT-PCR, and protein expression of chemokine receptor 4 (CXCR4) and Ras homolog gene family, member A (RhoA) was analyzed by Western blot. It was found that knockdown of miR-128 promoted the optical density (OD) value of cells, enhanced mRNA expression of PPARγ and C/EBPα, while inhibited cell apoptotic rate, and Caspase-3, Caspase-9 expression. Furthermore, higher protein expression of CXCR4 and RhoA was found in the absence of miR-128. Notably, miRNA-128 over-expression-inhibited proliferation and induced-apoptosis of human thyroid cancer cells were partially changed following the block of CXCR4/RhoA signaling pathway by the CXCR4 inhibitor (AMD3100). It was indicated that miR-128 down-regulated proliferation while promoted apoptosis of human thyroid cancer cells through suppression of CXCR4/RhoA signaling pathway.
Keywords: CXCR4/RhoA, function, human thyroid cancer cells, mechanism, miR-128
Introduction
Thyroid cancer is the most common endocrine malignancy and its frequency is increasing [1,2]. Thyroid cancer is originated from follicular and parafollicular cells and classified into differentiated papillary carcinoma (PTC), follicular carcinomas (FTC) and undifferentiated anaplastic carcinoma (ATC) [3,4]. Consequently, there is a need for exploration of the mechanism involved in thyroid cancer initiation and progression to improve our understanding of thyroid tumorigenesis and develop effective therapeutic strategies.
microRNAs (miRNAs) are a class of endogenous, small, non-coding single-stranded RNAs which are also known to play important roles in various types of cancers [5]. Previous evidence proved that miRNAs had the distinctive ability to function as tumor suppressors or oncogenes [6]. miR-128 is one of the miRNAs, which has been shown to be down-expressed in several types of cancer including prostate cancer [7], bladder cancer [8], ovarian cancer [9] and lung cancer [10]. Increasing evidence suggested that constitutively expressed miR-128 inhibited cancer cell proliferation and invasion by targeting RhoE [11], VEGF-C [8], ZEB1 [7], p70S6K1 [12], Bmi-1 and ABCC5 [13], ITGA2 and ITGA5 [14]. By contrast, miR-128 was reported to act as an onco-miR in primary osteosarcoma [15]. However, there are still no data available for the expression and function of miR-128 in human thyroid cancer. Therefore, in the present study, we analyzed the function of miR-128 and its putative targets using human thyroid cancer cells. Our results showed that miR-128 expression was down-regulated in human thyroid cancer cells. Enhanced expression of miR-128 inhibited the proliferation and promoted the apoptosis in human thyroid cancer cells by targeting CXCR4/RhoA signaling pathway, suggesting that miR-128 might act as a suppressor of thyroid cancer.
Materials and methods
Cell culture
The human thyroid cancer cells purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, and 10 mg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was changed every three days and cells were subcultured before forming confluent monolayer.
Treatments
The experiments were randomly divided into the following groups: Control, miR-128 siRNA control, miR-128 mimic control, miR-128 siRNA treatment, miR-128 mimic treatment, AMD3100 treatment, and miR-128 mimic plus AMD3100 treatment. AMD3100 was purchased from Santa Cruz Biotechnology (2145 Delaware Ave Santa Cruz, CA, USA). miR-128 mimic (sense: UAGCUUAUCAGACUGAUGUUGA; anti-sense: AACAUCAGUCUGAUAAGCUAUU) and miR-128 siRNA were purchased from Genepharma (Shanghai, China).
Cell viability assay
MTT method was used to determine cell viability of human thyroid cancer cells. Cells were seeded in 96-well plates at a density of 2.0×103/mL in RPMI-1640 medium containing 10% FBS for 24 h, then the medium was changed and the cells were cultured in a serum-free medium. After incubation for 24 h, cells were incubated with 3 µg miR-128 in fresh RPMI-1640 medium using HiPerFect (Qiagen, Valencia, CA, USA). At 48 and 72 h, cell proliferation was evaluated. The 490 nm absorbance was measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Flow cytometry
A total of 2.0×103 human thyroid cancer cells were seeded onto a 96-well plate and 24 h later treated with miR-128 siRNA control, miR-128 mimic control, miR-128 siRNA, miR-128 mimic, AMD3100, and miR-128 mimic plus AMD3100, and cell apoptotic rate was identified by Flow cytometry according to the manufacturer instructions. Cell apoptotic rate was analyzed using a FACScan Flow Cytometry apparatus (BD Biosciences, San Jose, CA, USA).
Western blot analysis
The examination of the protein expression levels of CXCR4 and RhoA was performed separately using Western blot analysis. Total protein was extracted and the protein concentration was measured according to the published report [16]. Antibodies were purchased from Santa Cruz Biotechnology, CA, USA. Band density was quantitated using Image J software.
Gene expression analysis
Expression of miR-128, PPARγ, Caspase-3, Caspase-9 and C/EBPα in human thyroid cancer cells was determined at indicated times by RNA preparation and quantitative reverse transcription polymerase chain reaction (RT-PCR). Briefly, total cellular RNA was isolated from cells on 6-well plates using TriZOL reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Real time RT-PCR analysis was carried out using the QuantiTect SYBR Green RTPCR Kit (Qiagen, Valencia, CA) under the ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA, USA). β-actin expression was used as an internal control. Specific primer sequences were synthesized in BIOSUNE Biological Technology Corp (Shanghai, China), and the sequences of the primers were shown in Table 1.
Table 1.
Primers used in this study
| Gene | Serial number | Primers |
|---|---|---|
| β-actin | M28424.1 | Sense: 5’-CTTAGCACCCCTGGCCAAG-3’ |
| Antisense: 5’-GATGTTCTGGAGAGCCCCG-3’ | ||
| Caspase-3 | AY219866.1 | Sense: 5’-TTTGTTTGTGTGCTTCTGAGCC-3’ |
| Antisense: 5’-GATGTTCTGGAGAGCCCCG-3’ | ||
| C/EBPα | NM_001287424.1 | Sense: 5’-TGCGCAAGAGCCGAGATAAAG-3’ |
| Antisense: 5’-TCACGGCTCAGCTGTTCCAC-3’ | ||
| miR-128 | NR_029672.1 | Sense: 5’-ACACTCCAGCTGGGTCACAGTGAACCGGTC-3’ |
| Antisense: 5’-TGGTGTCGTGGAGTCG-3’ | ||
| PPARγ | NM_013261.3 | Sense: 5’-TGAACGACCAAGTAACTCTCCTC-3’ |
| Antisense: 5’-GCTTTCGCAGGCTCATTTAG-3’ |
Stable transfection of miR-128
For stable transfections, human thyroid cancer cells in the exponential growth phase were grown to 65% confluence and then transfected with 3 μg of miR-128 specific siRNA construct, miR-128 mimic, miR-128 mimic control or non-targeting siRNA using HiPerFect (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocols. After cells were cultured in medium for 48 h, the efficiency of miR-128 siRNA was determined by RT-PCR. miR-128 specific siRNA was as follows: UCAACAUCAGUCUGAUAAGCUA; miR-128 siRNA control: CAGUACUUUUGUGUAGUACAA.
Statistical analysis
All results were analyzed using SPSS 17.0 statistical software (IBM, Armonk, NY, USA). Data are presented as the mean ± standard deviation (SD). Student’s two-tailed t-test was used to determine the statistical differences between the treatment and control groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Down-regulation of miR-128 expression was found in human thyroid cancer cells
In order to investigate the possible roles of miR-128 in thyroid cancer, we first examined the expression of miR-128 in human thyroid cancer cells by real time RT-PCR. As shown in Figure 1, at 48 and 72 h, the expression levels of miR-128 were obviously lowered in human thyroid cancer cells compared with normal human thyroid cells (P<0.05). These results indicated that miR-128 might play a suppressing miRNA in the development of human thyroid cancer.
Figure 1.
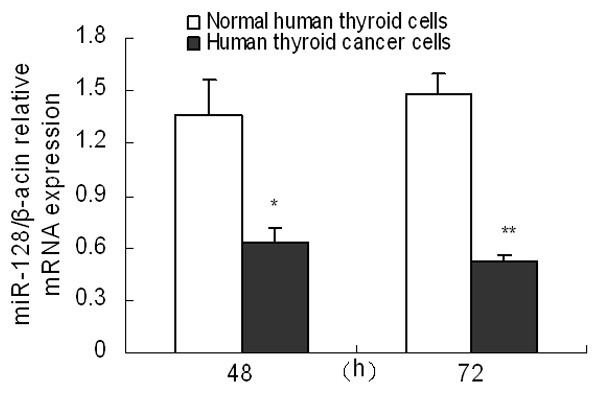
miR-128 expression was down-regulated in human thyroid cancer cells. Expression of miR-128 was measured by real time RT-PCR in human thyroid cancer cells and normal human thyroid cells at 48 and 72 h. Three individual experiments were performed and each treatment had five replicates. Results were presented as mean ± SD. *P<0.05 or **P<0.01 compared with control.
Down-regulated miR-128 expression promoted the proliferation of human thyroid cancer cells
The effects of miR-128 on the regulation of cell viability were evaluated using MTT assay in cultured human thyroid cancer cells. After cells were transfected with miR-128 siRNA for 48 and 72 h, the expression of miR-128 was significantly down-regulated (P<0.05), indicating that miR-128 siRNA construct could be used in the following experiments (Figure 2A). The results from MTT assay showed that down-regulated miR-128 expression in human thyroid cancer cells led to approximately 26.06% to 32.13% promotion of cell viability at 48 and 72 h (P<0.05) (Figure 2B). It was indicated that miR-128 down-regulation promoted the proliferation of human thyroid cancer cells.
Figure 2.
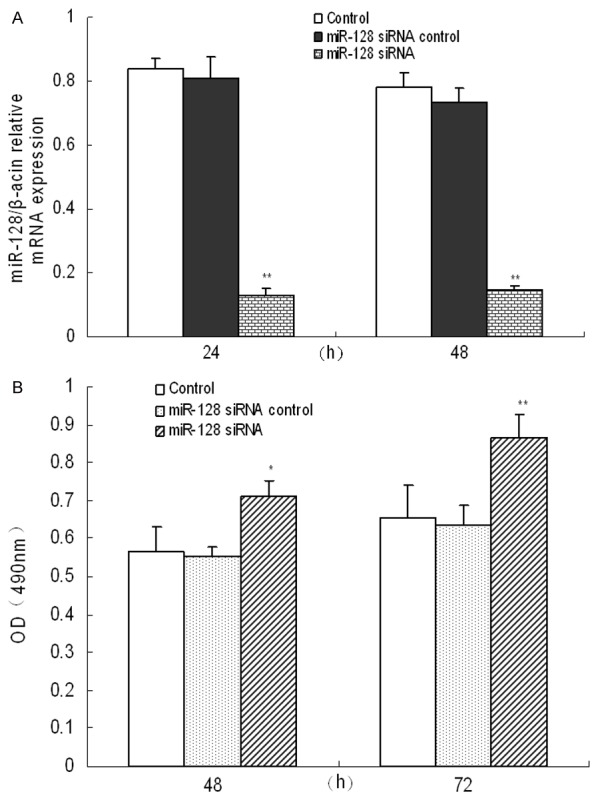
miR-128 down-expression promoted the proliferation of human thyroid cancer cells. Expression of miR-128 in human thyroid cancer cells was measured after miR-218 slicing (A). Treated with siRNA control or miR-128 siRNA for 48 and 72 h, cell proliferation of human thyroid cancer cells was determined by MTT assay (B). Three individual experiments were performed and each treatment had five replicates. Results were presented as mean ± SD. *P<0.05 or **P<0.01 compared with control.
Down-regulated miR-128 expression inhibited the apoptosis of human thyroid cancer cells
In order to further explore the effect of miR-128 on apoptosis of human thyroid cancer cells, we applied Flow Cytometry to test the level of apoptosis. Results in Figure 3 showed that slicing of miR-128 could obviously decrease the apoptotic rates of human thyroid cancer cells at 48 and 72 h in comparison with the control (P<0.05), and the results at 72 h presented most significant.
Figure 3.
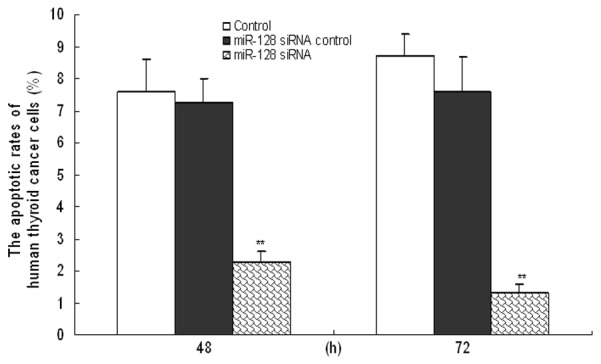
Apoptosis of human thyroid cancer cells was reduced by miR-128 down-expression. Following transfection with siRNA control or miR-128 siRNA for 48 and 72 h, the apoptotic rate of human thyroid cancer cells was measured by Flow Cytometry. Three individual experiments were performed and each treatment had five replicates. Results were presented as means ± SD. *P<0.05 or **P<0.01 compared with control.
Down-regulated miRNA-128 expression regulated the levels of cell growth and apoptotic mediators
The expression profiles of cell proliferation and apoptotic mediators were analyzed by RT-PCR. It was suggested that, compared to the control groups, the cell proliferation indicators including PPARγ and C/EBPα, were significantly promoted in miR-128 down-regulated human thyroid cancer cells (P<0.05). In contrast, the expression of pro-apoptotic regulator Caspase-3 and Caspase-9 in miR-128 slicing human thyroid cancer cells were significantly down-regulated compared with the control cells (P<0.05) (Figure 4).
Figure 4.
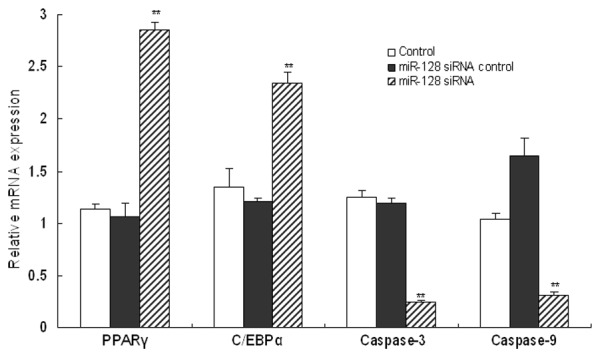
miR-128 changed the expression levels of cell growth and apoptotic mediators. The expression profiles of cell proliferation and apoptotic mediators including PPARγ, C/EBPα, Caspase-3 and Caspase-9 in human thyroid cancer cells were analyzed using RT-PCR. Three individual experiments were performed and each treatment had five replicates. Results were presented as means ± SD. *P<0.05 or **P<0.01 compared with control.
Down-regulated miR-128 expression activated CXCR4/RhoA signaling pathway
To evaluate the effects of miR-218 on CXCR4/RhoA signaling pathway in human thyroid cancer cells, protein expression of CXCR4 and RhoA was determined separately by Western blot analysis (Figure 5A). Results of band ratio analysis showed that knockdown of miR-218 markedly promoted the protein expression level of CXCR4 and RhoA (P<0.01, Figure 5B), indicating that miR-218 down-regulation activated CXCR4/RhoA signaling pathway in human thyroid cancer cells.
Figure 5.
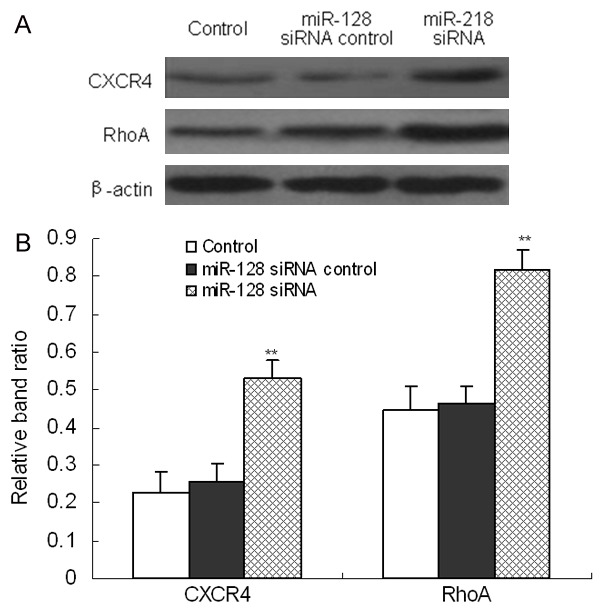
miR-128 down-expression activated CXCR4/RhoA signaling pathway in human thyroid cancer cells. After cells were treated with siRNA control or miR-128 siRNA for 48 h, protein expression of CXCR4 and RhoA was determined by Western blot (A). Furthermore, band relative ratio was analyzed using Image J software (B). Three individual experiments were performed and each treatment had three replicates. Results were presented as means ± SD. *P<0.05 or **P<0.01 compared with control.
CXCR4/RhoA signaling pathway was the target of miR-128
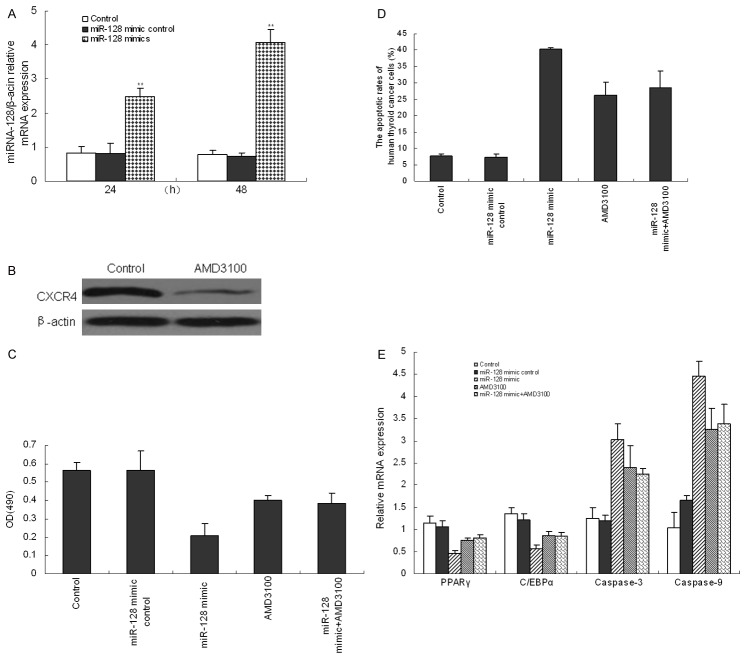
To determine whether CXCR4/RhoA signaling pathway was required for cell proliferation and apoptosis following stimulation of miR-128, we used miR-128 mimics to up-regulate miR-128 expression and ADM3100 to block CXCR4/RhoA signaling pathway in cultured human thyroid cancer cells. Each mimic enhanced expression of its target gene by 2.5 times at 24 h and 4.2 times at 48 h (Figure 6A). After blocking CXCR4/RhoA signaling pathway with AMD3100 for 48 h, CXCR4 expression was significantly decreased (Figure 6B). It was observed that, compared to the control cells without miR-128 mimic stimulation, there was a significant inhibition in cell proliferation (Figure 6C) and the expression of PPARγ and C/EBPα (Figure 6E) in miR-128 mimic-treated cells (P<0.01). Moreover, miRNA-128 over-expression promoted the apoptotic rate (Figure 6D), and mRNA expression of Caspase-3 and Caspase-9 in cells significantly (Figure 6E). In addition, AMD3100 alone decreased cell proliferation while promoted cell apoptosis of human thyroid cancer cells (P<0.01). However, there was no difference between AMD3100-treated cells and AMD3100 plus miR-128 mimic-treated cells (P>0.05). All results above indicated that the CXCR4/RhoA signaling pathway was involved in miRNA-128-meditated proliferation and apoptosis of human thyroid cancer cells.
Figure 6.
miR-128 over-expression inhibited cell proliferation and induced cell apoptosis in human thyroid cancer cells through CXCR4/RhoA signaling pathway. After cells were transfected with miR-128 mimics for 24 and 48 h, miR-218 expression was measured by RT-PCR (B). After CXCR4/RhoA signaling pathway was blocked using AMD-3100, protein expression of CXCR4 was tested by Western blot (A). After cells were treated with miR-128 mimics, AMD-3100 or miR-128 mimic plus AMD-3100, cell proliferation (C), the apoptotic rate (D) and mRNA expression of PPARγ, C/EBPα, Caspase-3 and Caspase-9 (E) were demonstrated. Three individual experiments were performed and each treatment had five replicates. Results were presented as means ± SD. *P<0.05 or **P<0.01 compared with control.
Discussion
The current study demonstrated that miR-128 expression was significantly reduced in human thyroid cancer cells. Moreover, over-expression of miR-128 led to the suppression ofCXCR4/RhoA signaling pathway, which subsequently inhibit thyroid cancer cell proliferation and promoted cell apoptosis in vitro. Our results underlined a fundamental role of miR-128 as a tumor suppressor in thyroid cancer.
Numerous evidences suggested that miR-128 was abnormally expressed in many kinds of malignant cancers, acting as oncogenes or tumor repressors. In the majority of cases, miR-128 functioned as a tumor inhibitor and its expression was frequently changed in tumor cells [17]. Recent studies have found the differential expression of miR-128 in many type of cancers, such as lung cancer [18], glioma [19,20], prostate cancer [21], ovarian cancer [22], and breast cancer [13]. miR-128 levels displayed a significant reduction in prostate cancer [21]. After investigating 57 breast cancer patients, Zhu et al. (2011) demonstrated that the level of miR-128 expression in breast cancer tissues was reduced and correlated with the response of breast tumor patients to novel adjuvant chemotherapy and the survival of patients. However, the levels of miR-128 expression in the other tumor tissues were obviously different [13]. Zhu et al. (2012) detected the acute leukemia (AL)-related miRNAs by qRT-PCR, and found that miR-128 was highly expressed in acute lymphoblastic leukemia (ALL) patients [23]. Volinia et al. (2006) measured 540 different kinds of malignant tumor samples using a large-scale miRnome analysis and found that miR-128 expression was significantly up-regulated in tumor tissues of colon, lung and pancreatic cancer [24]. In addition, the expression level of miR-128a was up-regulated in undifferentiated gastric cancer tissues while mir-128b was down-regulated [25]. In this study, we proved that the miR-128 expression level was significantly reduced in human thyroid cancer cells.
Recent studies demonstrated that miR-128 served as an important role in regulating growth, invasiveness, differentiation and apoptosis of different types of tumor cells. For example, up-regulated miR-128 expression significantly inhibited cell viability approximately 20 to 40% in head and neck squamous cell carcinoma lines JHU-13 miR-128 and JHU-22 [26]. miR-128 over-expression inhibited glioma cells proliferation by targeting transcription factor E2F3a [27] or p70S6K1 [12]. It has been proposed that up-regulated miR-128 reduced cell motility and invasiveness in neuroblastoma [28] and prostate cancer [21]. miR-128 over-expression mediated cell apoptosis via inhibition of NTRK3 and Bax expression and up-regulation of BCL2 in neuroblastoma and embryonic kidney cells [29,30]. Over-expression of miR-128 significantly inhibited hepatocellular carcinoma cell proliferation and metastasis via ITGA2 and ITGA5 [14]. Furthermore, over-expression of miR-128 increased cell death in programmed acute myeloid leukemia but had no effect on cell cycle profile, apoptosis or autophagy [31]. In contrast, knockdown of miR-128 was shown to promote lung cancer cell growth and colony formation by inhibiting AKT phosphorylation [10]. Zhou et al. (2015) further indicated that over-expression of miR-128 inhibited cell proliferation, migration and invasion of bladder cancer cells. However, knockdown of miR-128 exerted the opposite functions [8]. Our study also suggested that down-regulated miR-128 promoted cell proliferation while inhibited cell apoptosis of human thyroid cancer cells, and up-regulated miR-128 had exactly the reverse effects.
Exerting control in the major cellular pathways has shown to play a crucial role in mediating tumor cell proliferation, differentiation and survival. In ovarian cancer, glioblastoma and breast tumor-initiating cells, over-expression of miR-128 might promote chemosensitivity via different signaling pathways [9,13,32]. Ectopic High expression of miR-128 significantly promoted while suppression of miR-128 inhibited the proliferation of MG63 and U2OS cells by PTEN/AKT signaling [33]. Over-expression of miR-128 could inhibit proliferation and induce apoptosis in U251 cells, and those effects could be restored by miR-128 knockdown by directly targeting RhoE [11]. Chemokine receptors, such as CXCR4, are G-protein-coupled receptors. CXCR4 is frequently over-expressed in malignant tumors of the breast and other tissues [34,35]. Thus, CXCR4 is considered as an important diagnostic and therapeutic target for human malignancies, and CXCR4 signaling has demonstrated to be involved in the regulation of growth, motility and metastatic homing of primary tumor cells, as well as modification of the tumor microenvironment [34-36]. Previous reviews focused on CXCR4 signaling events and demonstrated that CXCR4 could activate RhoA signaling to regulate tumor cell viability and migration [37,38]. Our results suggested that miR-128 played an important role in the proliferation and apoptosis of human thyroid cancer cells by directly regulateing CXCR4/ RhoA signaling.
In conclusion, miR-128 acted as a tumor suppressor to inhibit the proliferation of human thyroid cancer cells by directly targeting CXCR4/RhoA signaling pathway. Our results brought a better understanding in function and its potential molecular mechanism of miR-128, and further provided valuable information for developing novel diagnostic markers and targeted therapy in human thyroid cancer.
Disclosure of conflict of interest
None.
References
- 1.Likhterov I, Tuttle RM, Haser GC, Su HK, Bergman D, Alon EE, Bernet V, Brett E, Cobin R, Dewey EH, Doherty G, Dos Reis LL, Klopper J, Lee SL, Lupo MA, Machac J, Mechanick J, Milas M, Orloff L, Randolph G, Ross DS, Rowe ME, Smallridge R, Terris D, Tufano RP, Urken ML. Improving the adoption of thyroid cancer clinical practice guidelines. Laryngoscope. 2016;126:2640–2645. doi: 10.1002/lary.25986. [DOI] [PubMed] [Google Scholar]
- 2.Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C, Sabini E, Passannati P, Puleo L, Matrone A, Pontillo-Contillo B, Battaglia V, Mazzeo S, Vitti P, Elisei R. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23:R185–R205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- 3.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 4.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seven M, Karatas OF, Duz MB, Ozen M. The role of miRNAs in cancer: from pathogenesis to therapeutic implications. Future Oncol. 2014;10:1027–1048. doi: 10.2217/fon.13.259. [DOI] [PubMed] [Google Scholar]
- 6.Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi: 10.3402/jev.v5.31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Li Y, Yu J, Pei H, Luo P, Zhang J. miR-128 modulates chemosensitivity and invasion of prostate cancer cells through targeting ZEB1. Jpn J Clin Oncol. 2015;45:474–482. doi: 10.1093/jjco/hyv027. [DOI] [PubMed] [Google Scholar]
- 8.Zhou XU, Qi L, Tong S, Cui YU, Chen J, Huang T, Chen Z, Zu XB. miR-128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF-C upregulation. Oncol Lett. 2015;10:3183–3190. doi: 10.3892/ol.2015.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Chen H, Wu N, Zhang WJ, Shang LX. Deregulation of miR-128 in ovarian cancer promotes cisplatin resistance. Int J Gynecol Cancer. 2014;24:1381–1388. doi: 10.1097/IGC.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Li J, Le Y, Zhou C, Zhang S, Gong Z. PFKL/miR-128 axis regulates glycolysis by inhibiting AKT phosphorylation and predicts poor survival in lung cancer. Am J Cancer Res. 2016;6:473–485. [PMC free article] [PubMed] [Google Scholar]
- 11.Shang C, Hong Y, Guo Y, Liu YH, Xue YX. miR-128 regulates the apoptosis and proliferation of glioma cells by targeting RhoE. Oncol Lett. 2016;11:904–908. doi: 10.3892/ol.2015.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi ZM, Wang J, Yan Z, You YP, Li CY, Qian X, Yin Y, Zhao P, Wang YY, Wang XF, Li MN, Liu LZ, Liu N, Jiang BH. MiR-128 inhibits tumor growth and angiogenesis by targeting p70S6K1. PLoS One. 2012;7:e32709. doi: 10.1371/journal.pone.0032709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao H, Gong C, Chen J, Su F, Zhang Y, Song E. Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res. 2011;17:7105–7115. doi: 10.1158/1078-0432.CCR-11-0071. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Wu Y, Lv Z. miR-128 modulates hepatocellular carcinoma by inhibition of ITGA2 and ITGA5 expression. Am J Transl Res. 2015;7:1564–1573. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Tian Z, Guo B, Yu M, Wang C, Zhang H, Liang Q, Jiang K, Cao L. Upregulation of micro-ribonucleic acid-128 cooperating with downregulation of PTEN confers metastatic potential and unfavorable prognosis in patients with primary osteosarcoma. Onco Targets Ther. 2014;7:1601–1608. doi: 10.2147/OTT.S67217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong XY, Tang SQ, Zhang W, Gao W, Chen Y. GPR39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the PI3K/AKT cell signaling pathway. J Recept Signal Transduct Res. 2016;36:130–138. doi: 10.3109/10799893.2015.1056308. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Iwaya T, Sawada G, Kurashige J, Matsumura T, Uchi R, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann Surg Oncol. 2014;21:205–212. doi: 10.1245/s10434-013-3264-3. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G, Fu S, Zhang Y, Feng K, Feng Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur J Cancer. 2014;50:2336–2350. doi: 10.1016/j.ejca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Dong Q, Cai N, Tao T, Zhang R, Yan W, Li R, Zhang J, Luo H, Shi Y, Luan W, Zhang Y, You Y, Wang Y, Liu N. An axis involving SNAI1, microRNA-128 and SP1 modulates glioma progression. PLoS One. 2014;9:e98651. doi: 10.1371/journal.pone.0098651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Chen X, Peng X, Zhou J, Kung HF, Lin MC, Jiang S. MicroRNA-128 promotes cell-cell adhesion in U87 glioma cells via regulation of EphB2. Oncol Rep. 2013;30:1239–1248. doi: 10.3892/or.2013.2596. [DOI] [PubMed] [Google Scholar]
- 21.Khan AP, Poisson LM, Bhat VB, Fermin D, Zhao R, Kalyana-Sundaram S, Michailidis G, Nesvizhskii AI, Omenn GS, Chinnaiyan AM, Sreekumar A. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol Cell Proteomics. 2010;9:298–312. doi: 10.1074/mcp.M900159-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo HH, László CF, Greco S, Chambers SK. Regulation of colony stimulating factor-1 expression and ovarian cancer cell behavior in vitro by miR-128 and miR-152. Mol Cancer. 2012;11:58. doi: 10.1186/1476-4598-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu YD, Wang L, Sun C, Fan L, Zhu DX, Fang C, Wang YH, Zou ZJ, Zhang SJ, Li JY, Xu W. Distinctive microRNA signature is associated with the diagnosis and prognosis of acute leukemia. Med Oncol. 2012;29:2323–2331. doi: 10.1007/s12032-011-0140-5. [DOI] [PubMed] [Google Scholar]
- 24.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katada T, Ishiguro H, Kuwabara Y, Kimura M, Mitui A, Mori Y, Ogawa R, Harata K, Fujii Y. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–542. [PubMed] [Google Scholar]
- 26.Hauser B, Zhao Y, Pang X, Ling Z, Myers E, Wang P, Califano J, Gu X. Functions of MiRNA-128 on the regulation of head and neck squamous cell carcinoma growth and apoptosis. PLoS One. 2015;10:e0116321. doi: 10.1371/journal.pone.0116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch DH, Barres BA, Verma IM, Kosik KS. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31:1884–1895. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, Galardi S, Buè MC, Massalini S, McDowell HP, Messi E, Gulino A, Farace MG, Ciafrè SA. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 2009;23:4276–4287. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 29.Adlakha YK, Saini N. miR-128 exerts proapoptotic effect in a p53 transcription-dependent and -independent manner via PUMA-Bak axis. Cell Death Dis. 2013;4:e542. doi: 10.1038/cddis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y. Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol. 2010;11:95. doi: 10.1186/1471-2199-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seca H, Lima RT, Almeida GM, Sobrinho-Simoes M, Bergantim R, Guimaraes JE, Vasconcelos MH. Effect of miR-128 in DNA damage of HL-60 acute myeloid leukemia cells. Curr Pharm Biotechnol. 2014;15:492–502. doi: 10.2174/1389201015666140519122524. [DOI] [PubMed] [Google Scholar]
- 32.She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G, Xiang J, Wu M, Li G. MiR-128 and miR-149 enhance the chemosensitivity of temozolomide by Rap1B-mediated cytoskeletal remodeling in glioblastoma. Oncol Rep. 2014;32:957–964. doi: 10.3892/or.2014.3318. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Chen XD, Zhang YH. MicroRNA-128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069–2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 34.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 37.Struckhoff AP, Vitko JR, Rana MK, Davis CT, Foderingham KE, Liu CH, Vanhoy-Rhodes L, Elliot S, Zhu Y, Burow M, Worthylake RA. Dynamic regulation of ROCK in tumor cells controls CXCR4-driven adhesion events. J Cell Sci. 2010;123:401–412. doi: 10.1242/jcs.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicente-Manzanares M, Cabrero JR, Rey M, Pérez-Martínez M, Ursa A, Itoh K, Sánchez-Madrid F. A role for the Rho-p160 Rho coiledcoil kinase axis in the chemokine stromal cellderived factor-1alpha-induced lymphocyte actomyosin and microtubular organization and chemotaxis. J Immunol. 2002;168:400–410. doi: 10.4049/jimmunol.168.1.400. [DOI] [PubMed] [Google Scholar]