Abstract
Recent evidence has suggested that microRNAs (miRNAs) may play a significant role in the pathogenesis of inflammatory skin conditions such as atopic dermatitis (AD). The aim of the present study was to evaluate the potential functions of miRNAs in AD and to identify the underlying mechanisms. We firstly analyzed miRNA expression in the skin lesions of patients with AD using microarray analysis. Validation analysis was performed in skin biopsy specimens and in serum using quantitative reverse transcription PCR (qRT-PCR). The relationship between microRNA-29b (miR-29b) and development of AD was further explored. Subsequently, gain- and loss-of-function studies were performed to determine the functions of miR-29b in interferon-γ (IFN-d)-induced keratinocytes (KCs) apoptosis. Further bioinformatics analysis and luciferase reporter assays were performed to predict its target genes, then the effects of miR-29b on the expression of BCL2-like2 (Bcl2L2) were investigated using qRT-PCR and western blot analysis. Finally, KCs were transfected with miR-29b mimics or Bcl2L2 siRNA (si-Bcl2L2) to explore the mechanism by which miR-29b plays in the pro-apoptotic roles in IFN-γ-treated keratinocytes. The miR-29b was found to be one of the most significantly up-regulated miRNAs in the skin lesions of patients with AD, as compared with healthy control and its expression was statistically associated with the development of AD. We, therefore, selected miR-29b as a candidate miRNA and investigated its function. Our in vitro data showed that the keratinocytes apoptosis induced by IFN-duced by IFN-ptosis induced by IFN-vestigated its function. Our stically associated with the deve particular, we identified Bcl2L2 as a direct target of miR-29b. More importantly, siRNA-induced knockdown of Bcl2L2 attenuated the protective effects of miR-29b inhibition on keratinocytes apoptosis. These results suggested that miR-29b knockdown may play an important role in preventing cell apoptosis in IFN-cktreated keratinocytes, and these effects might be partially through regulation of Bcl2L2. These findings revealed that the miR-29b/Bcl2L2 regulatory axis was involved in the pathogenesis of AD and suggested that knockdown of miR-29b might be a novel therapeutic target for AD.
Keywords: Atopic dermatitis, microRNA-29b, keratinocyte, apoptosis, IFN-optosis
Introduction
Atopic dermatitis (AD) is a chronic and relapsing skin disease that is characterized by the epidermal barrier dysfunction, skin inflammation and apoptosis of keratinocytes [1,2]. Despite the great efforts that have been made to improve the functional outcome of patients with AD, advances in therapy for AD have, thus far, been limited. Several studies reported that keratinocytes apoptosis induced by activated T cells is one of the major mechanisms in the pathogenesis of AD [2,3]. IFN--LINK \l “_ENREF_3” \o “Rebane, 2012 #4”#1578”r mechanisms in the panced and disease-related apoptosis of keratinocytes [2]. Monitoring keratinocyte apoptosis in the treatment of atopic dermatitis is important for successfully managing AD [4]. However, the precise mechanisms of such apoptosis are not currently fully understood.
MicroRNAs (miRNAs) are small non-coding RNAs about 18~22 nucleotides in length that regulate gene expression at the transcriptional and/or post-transcriptional levels by binding to the 3’UTR of the target gene mRNA [5]. The alteration of miRNAs expression plays important roles in various cellular processes, such as proliferation, apoptosis, differentiation and metabolism [6]. Several miRNAs, including miR-21, miR-146, miR-155 and miR-223, have been found to be differentially expressed in skin samples of AD patients [7]. In addition, some miRNAs have also been shown to have favorable diagnostic and prognostic determinations for AD patients [8,9]. A number of microRNAs have been shown to functionally participate in the keratinocyte apoptosis. MiRNAs such as miR-130 [10] and miR-21 [11] inhibited keratinocyte apoptosis, whereas miR-125b [12] and miR-330-5p [13] promoted keratinocyte apoptosis. Like many other microRNAs, miR-29b, a member of miR-29 family, has been shown to be involved in the apoptosis in various types of cells [14-16]. Most interestingly, miR-29b has been shown to be one of the most up-regulated miRNAs in melanoma cells after IFN-r IFN-een sh [17]. A similar change pattern was observed in colorectalcancer (CRC) treated with IFN-r [18]. However, little is known whether miR-29b is also involved in the apoptosis of keratinocytes induced by IFN-dand how it works.
In the present study, we identified miR29b was one of the most significantly up-regulated miRNAs in the skin lesions of patients with AD, as compared with healthy control and its expression was associated with the development of AD. Furthermore, knockdown of miR-29b was found to inhibit IFN-γ-induced keratinocyte apoptosis, which might be attributed to the up-regulation of its target gene, Bcl2L2.
Materials and methods
Specimens collection
Lesional skin biopsy specimens (diameter, 4 mm) were obtained from 12 healthy control subjects (7 women, 5 men, age, 19-42 years), 21 patients with AD (10 men, 11 women, age 18-41 years). Patients with atopic dermatitis were identified according to the criteria defined by Hanifin and Rajka [19]. Blood samples were obtained from 30 healthy control subjects (17 women, 13 men, age, 18-40 years), 30 patients with AD (14 women, 16 men, age, 19-39 years).The specimens were immediately frozen in liquid nitrogen and stored at -80°0 for further use. The study was approved by the Ethical Review Committees on Human Research of the University of Tartu and the University of Szeged. All participants signed a written informed consent form. None of the patients had been treated with systemic antihistamines and/or topical corticosteroids for at least 1 week before study participation.
Cell culture
Primary human keratinocytes were isolated from healthy skin as described previously [20,21] and cultured to 80% of confluence in keratinocyte serum-free medium supplemented with keratinocyte growth supplement (Invitrogen, Carlsbad, CA, USA). Human 293T embryonic kidney cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen) and 1/100 streptomycin-penicillin mix (Sigma-Aldrich, St. Louis, MO, USA). All cells were cultured at 37° in a humidified atmosphere containing 5% CO2.
MicroRNA expression profiling assay
MiRNA profiling of lesional skin biopsy specimens was performed with the 7th generation of miRCURYTM LNA Array (v.18.0, Exiqon) as described previously [22]. Expressed data were normalized using the median normalization. After normalization, significant differentially expressed miRNAs were identified through Volcano plot filtering. Hierarchical clustering was performed using MEV software (v4.6, TIGR). The miRNAs were considered to be significantly differentially expressed between the two groups (AD patient versus healthy control) if the fold changes (FC) was > 2.0 and the P value was <0.05.
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was isolated from the lesional skin biopsy specimens, serums and keratinocytes using the TRIzol reagents (Invitrogen, CA, USA) following the manufacturer’s instructions, and first-strand cDNA was generated using Script™ cDNA Synthesis Kit (Bio-Rad Laboratories). Real-time PCR was then performed on an Applied Biosystems 7900HT cycler using Super Real PreMix Plus (Tiangen, China) or the miRcute miRNA qPCR Detection Kit (Tiangen, China) according to the manufacturer’s guidelines. U6 and GAPDH functioned as normalization control in the expression analysis of miR-29b and BCL2L2, respectively. The relative expression of RNAs was calculated using the 2-ΔΔCt method. Each reaction was conducted in triplicate.
Transfection
The miR-29b mimics, miR-29b inhibitor and negative control (NC) were synthesized by GenePharma (Shanghai, China). The Bcl2L12 siRNA and NC siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary human keratinocytes transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Apoptosis analysis
Flow cytometry was used to detect the effect of miR-29b on cell apoptosis. 24 h after transfection, cells were harvested, washed with ice-cold PBS, and re-suspended in 500 μl of binding buffer. After the density of the cells was adjusted to 5 × 105/ml, cells were incubated with 5 μl Annexin V and 5 μl propidium iodide (PI) (BD Biosciences, San Jose, CA) at room temperature in the dark for 15 min. Apoptotic keratinocytes were quantified by BD FACSCalibur Flow Cytometer (BD Biosciences).
Cell viability assay
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed to detect the effect of miR-29b on cell proliferation. Briefly, cells were seeded in 96-well plates at a density of 5 × 103 cells/well/100 olium bromitransfection, cells were treated with 10 μ MTT solution for 4 h. Then, the absorbance at a wavelength of 570 nm was detected by a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Western blot assay
Western blot was performed as previously described [23]. The membranes were blotted with rabbit primary antibody against Bcl2L2, cleaved-caspase 3 and total caspase-3 (1:1000, Santa Cruz, CA, USA) or β-actin (1:2000, Santa Cruz, CA, USA) overnight at 4 at 4 or β-y against Bcl2L2, cleaved- by a micropIgG-HRP secondary antibody (1:2000, Santa Cruz, CA, USA) at room temperature for 1 h, and then visualized by using an enhanced chemiluminescence detection method (Thermo Fisher Scientific, Waltham, MA, USA). Relative intensities were determined by Quantity One 4.6.2 software (Bio-Rad, CA, USA).
Luciferase assays
A cDNA fragment of the Bcl2L2 3’-UTR mRNA containing the seed sequence of the miR-29b-binding site or a mutated binding site was cloned into the pmirGLO dual-luciferase vector (Promega, Madison, WI, USA). The constructed dual-luciferase vector was co-transfected with miR-29b mimics, miR-29b inhibitor or NC into 293T cells. The cells were harvested and lysed 24 h later, and the luciferase activity was measured by the Dual-Luciferase Assay System (Promega) in accordance to the manufacturer’s instructions.
Caspase-3 activity assay
Activity of caspase-3 was performed as previously described [24]. Briefly, after transfection, keratinocytes were lysed and re-suspended in 50 μa of ice-cold cell lysis buffer for 30 min. Caspase-3 activity was determined using a Caspase-3 activity kit (Beyotime, China). Caspase-3 activity was quantified in the samples with a microplate spectrophotometer (Biotek) at an absorbance of 405 nm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences were analyzed with the Student’s t-test between two groups or with one-way ANOVA among four groups. Correlation analyses were made with Spearman’s correlation analysis test. A P-value of less than 0.05 was considered statistically significant.
Results
MiR-29b was up-regulated in the lesional skin and serum of AD patients
To elucidate the expression of miRNAs associated with AD, we performed a miRNA microarray on skin samples from AD patients and healthy individuals. Using the screening criteria of FC > 2 and P<0.05, we identified 37 differentially expressed miRNAs in AD patients versus healthy individuals (Figure 1A). Among the 37 miRNAs, 11 were up-regulated and 26 were down-regulated. Based on several studies that miR-29b could induce cell apoptosis in many types of cells [14-16], we tested if miR-29b was also involved in the progression of AD. In the first step, we examined the expression of miR-29b in 21 lesional skin biopsy specimens derived from patients with AD and 12 skin biopsy specimens derived from healthy donors using quantitative real-time PCR (qRT-PCR). According to qRT-PCR analysis, miR-29b expression was markedly increased in skin biopsy specimens from AD patients, as compared to healthy control group (Figure 1B). qRT-PCR analysis was also applied to detect miR-29b expression in 30 sera from AD patients and 30 sera from healthy controls. The expression of miR-29b was significantly increased in sera from AD patients, as compared with that in healthy control group. In addition, the miR-29b expression levels were positively correlated with the AD disease severity score, as measured by the SCORAD system (Spearman r=0.8006, P<0.001; Figure 1C). These results suggest that miR-29b may play important roles in AD progression and may serve as potential biomarkers for the early detection of AD.
Figure 1.
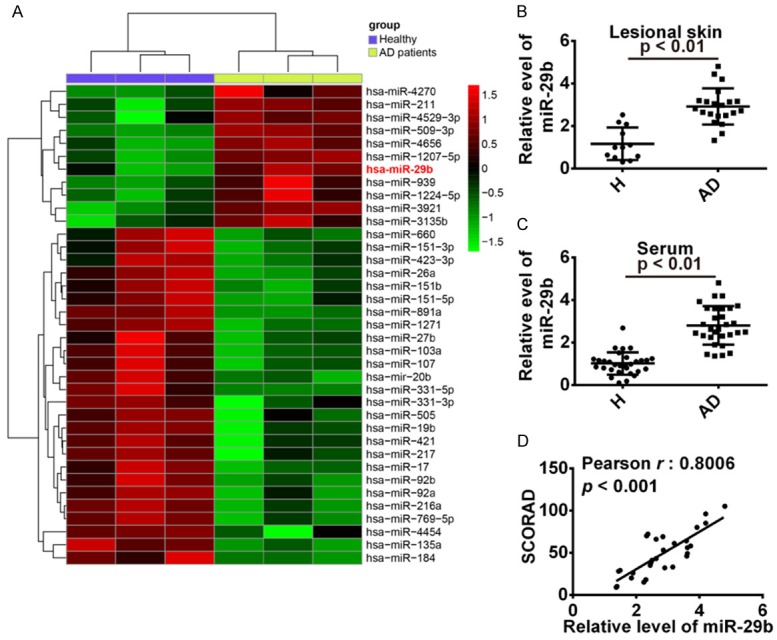
MiR-29b was up-regulated in lesional skin and sera from AD patients. A. Heat map of miRNA array data of skin from healthy subjects (H) and lesional skin from patients with atopic dermatitis AD. B. Quantitative real-time PCR analysis of miR-29b in healthy (n=12) and atopic dermatitis lesional (n=21) skin. P<0.01 vs. H group. C. Quantitative real-time PCR analysis of miR-29b in healthy (n=30) and atopic dermatitis (n=30) serum P<0.01 vs. H group. D. miR-29b serum levels of patients with AD were correlated withthe SCORAD values (r=0.8006, P<0.001).
Knockdown of miR-29b suppressed INF-γ induced apoptosis in keratinocyte
It is reported that INF-γ increased the expression of miR-29b in melanoma cells and colon cancer cells after IFN-γ treatment [17,18] and miR-29b plays an important role in FN-3 “1579” of miR-29b in [18]. To examine whether IFN-γ affected miR-29b in keratinocytes, keratinocytes were treated with IFN-γ (10 ng/ml for 12 h, 24 h and 48 h), and then miR-29b was measured by qRT-PCR. As shown in Figure 2A, miR-29b was strongly up-regulated by IFN-γ in a time-dependent manner. Subsequently, miR-29b was over-expressed or knocked down in IFN-a stimulated keratinocytes prior to the assessment of cell viability and cell apoptosis. As shown in Figure 2B, MTT assay revealed that the cell viability of keratinocytes was inhibited at 24 h and 48 h after IFN-h after IFN-ocytes was inhibited at 2429bmimics aggravated the inhibitory effect of IFN-γ on the cell viability of keratinocytes, whereas miR-29b inhibitor reversed the inhibitory effect of IFN-f (Figure 2B). Western blot assay showed that the expression of cleaved caspase 3 was markedly increased in miR-29b mimics group, while the expression of cleaved caspase 3 was significantly decreased in miR-29b inhibitor group (Figure 2C). In addition, the level of pro caspase 3 has no difference in both groups. In response to this finding, we assessed the role of miR-29b in IFN-γ 3 inducedd apoptosis and found that over-expression of miR-29b aggravated the apoptosis induced by IFN-r, whereas inhibition of miR-29b could attenuate the apoptosis mediated by IFN-e (Figure 2D, 2E). These data indicated that the knockdown of miR-29b could protect keratinocytes from apoptosis induced by IFN-F.
Figure 2.
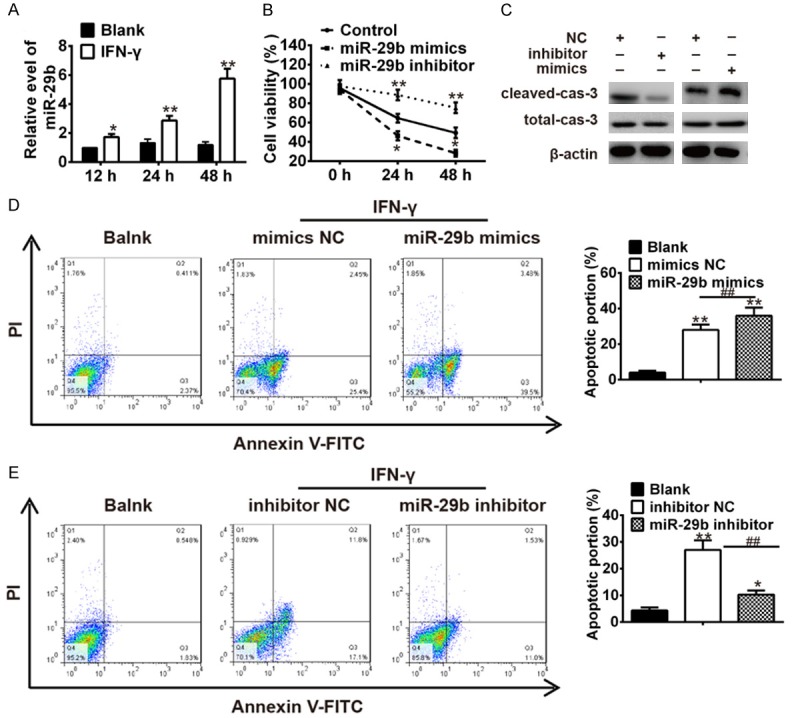
IFN-γ stimulated keratinocytes apoptosis by up-regulating miR-29b. A. Keratinocytes were treated with IFN-γ (10 ng/ml for 12 h, 24 h and 48 h), and then miR-29b was measured by qRT-PCR. *P<0.05, **P<0.01 vs. Blank group. IFN-γ treated keratinocytes were transfected with miR-29b mimics, miR-29b inhibitor and miRNA negative control. B. Cell viability was measured by MTT assay. *P<0.05, **P<0.01 vs. Control group. C. The expressions of cleaved-caspase-3 and total caspase-3 were analyzed by Western Blot. D and E. The apoptosis was measured by flow cytometer. *P<0.05, **P<0.01 vs. Blank group, ##P<0.01 vs. inhibitor NC or mimic NC. Data are presented as mean ± SD from three independent experiments.
MiR-29b targets and inhibits BCL2L2 gene expression
To assess the role of miR-29b in regulating the apoptosis in keratinocytes, bioinformatics tools were used to search for the potential targets of miR-29b. According to the results of these analyses, we focused on BCL2L2, an important anti-apoptotic protein since it has recently been found to be a target gene of miR-29b in neuronal cells [16] and human glioblastoma cells [25]. Thus, BCL2L2 was selected for further investigation. The binding sites between BCL2L and miR-29b are illustrated in Figure 3A. Dual-luciferase reporter assay was performed to test whether miR-29b targets BCL2L2. The results showed that over-expression of miR-29b significantly decreased the luciferase activity of wt-BCL2L2-3’UTR, whereas knockdown of miR-29b increased the luciferase activity of wt-BCL2L2-3’UTR. Likewise, cells co-transfected with miR-29b mimics, miR-29b inhibitor, and BCL2L2-mut-3’UTR, showed no obvious change in their luciferase activity (Figure 3B). In addition, we explored whether miR-29b could modulate the expression of BCL2L2. As shown in Figure 3C and 3D, the mRNA and protein levels of BBCL2L2 was decreased after over-expression of miR-29b, whereas it increased after inhibition of miR-29b, supporting that miR-29b can regulate BCL2L2 expression in vitro. These results demonstrated that BCL2L2 is a potential target of miR-29b in keratinocytes.
Figure 3.
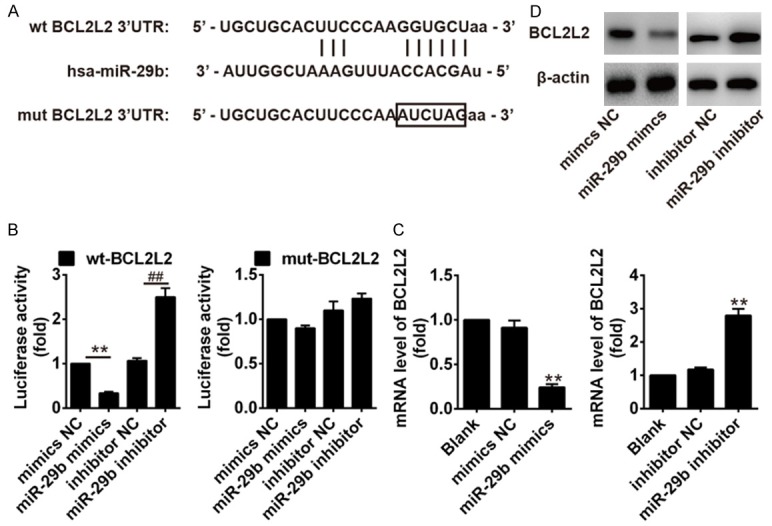
MiR-29b targeted and negatively regulated BCL2L2. A. Conservation of miR-29b in the binding site of BCL2L2 was snapshotted. B. Relative luciferase activity was performed by dual-luciferase reporter assay. **P<0.01 vs. BCL2L2 wt + mimics NC group; ##P<0.01 vs. BCL2L2 wt + inhibitor NC group. C and D. The effects of miR-29b on the mRNA and protein levels of BCL2L2. **P<0.01 vs. mimics NC group or inhibitor NC group. Data are presented as mean ± SD from three independent experiments.
MiR-29b mediated the expression of BCL2L2 is involved in the IFN-n-regulated cell proliferation and apoptosis of keratinocytes
Following on from the above findings, we sought to further explore whether miR-29b/BCL2L2 axis was involved in pro-apoptotic effect of IFN-a in keratinocytes. First, miR-29b inhibitor and si-BCL2L2 were co-transfected into IFN-d into IFNkeratinocytes, and the cell viability of keratinocytes was compared with that in the IFN-t in the IFN-mpared with that IFN-compared with that in the IFN-t in the IFN-mpared with that in the IFN-t in the IFN-sting effects of miR-29b inhibitor on cell viability so that, when administered together, cell viability was significantly decreased than in the miR-29b inhibitor + IFN-γ group (Figure 4A). The caspase-3 activity was decreased in the miR-29b inhibitor + IFN-γ group, as compared with the IFN-γ group, and that this inhibitory effect was removed by the co-administration of si-BCL2L2 (Figure 4B). Similarly, we also observed that si-BCL2L2 could reverse the inhibitory effect of miR-29b inhibitor on the apoptosis and the expression of cleaved caspase 3 (Figure 4C, 4D). Collectively, we concluded that knockdown of miR-29b could inhibit the apoptosis in IFN-llecti vely keratinocytes by up-regulating BCL2L2.
Figure 4.
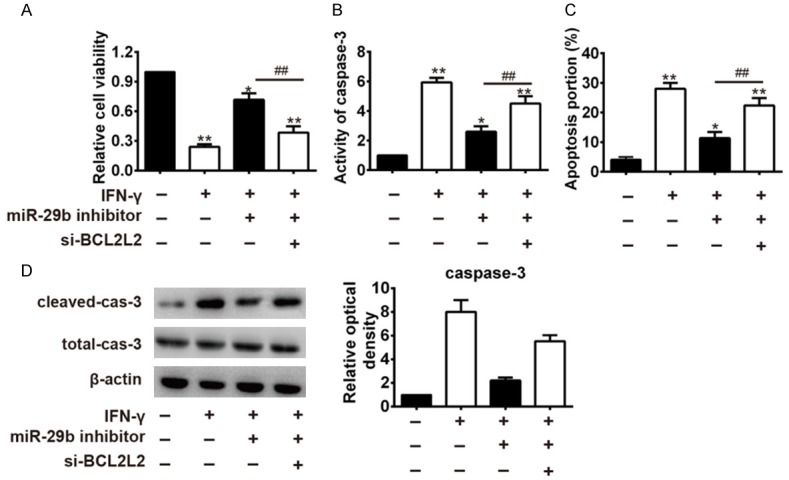
Knockdown of miR-29b attenuated IFN-γ-mediated keratinocytes apoptosisby targeting BCL2L2. IFN-γ treated keratinocytes were co-transfected with miR-29b inhibitor and si-BCL2L2. A. Cell viability was measured by MTT assay. B. Activity of caspase-3 was measured by a Caspase-3 activity kit. C. Apoptosis was measured by flow cytometer. D. The expressions of cleaved-caspase-3 and total caspase-3 were analyzed by Western Blot. *P<0.05, **P<0.01 vs. Control group; ##P<0.01 vs. IFN-γ + miR-29b inhibitor group. Data are presented as mean ± SD from three independent experiments.
Discussion
In the present study, we found that miR-29b was up-regulated in lesional skin and sera from AD patients, and miR-29b up-regulation was positively correlated with AD severity. Moreover, inhibition of miR-29b could protect keratinocytes from INF-tes from INF-patients, and miR-29b up-regulation important anti-apoptotic gene. These findings suggest that miR-29b plays a important role in AD progression, providing the future research direction and therapeutic options of treatment for patients with AD.
Increasing evidence emerging to suggest that microRNAs play a crucial role in the pathogenesis of many immune-mediated diseases including AD [9]. For example, it has been shown that miR-143 suppressed IL13-induced down-regulation of epidermal barrier-related proteins (filaggrin, loricrin and others) and inflammation through targeting the IL13 receptor, IL-13R, IL-13R3R keratinocytes [26]. Furthermore, miR-151a may be involved in the pathogenesis of AD by regulating IL12RB2 [27]. However, the biological roles of miRNAs in AD remain largely unknown until now. In this study, we analyzed miRNA expression in healthy and lesional skin of patients with AD by using microRNA arrays, and listed a catalogue of miRNAs potentially involved in the regulation and progression of AD. One of the miRNAs with highest over-expression in patients with AD was miR-29b, which is one member of miR-29 family. It has been reported that miR-29 was dysregulated in several disorders including skin diseases [28-30]. For example, miR-29b was up-regulation in E16 and E19 fetal mouse skin and might be thought to mediate several extracellular matrixc (ECM) proteins, which contributes to skin scarless phenotype and healing [31]. In this study, we found that miR-29b was up-regulated in the lesional skin and sera of AD patients and miR-29b levels of AD patients were positively correlated with the disease severity score. These results indicate that miR-29b may be involved in the development of AD. However, the underlying mechanism needs to be further delineated.
Recent investigations have greatly increased our understanding of the immunological mechanisms that are involved in the pathogenesis of AD. Although in appropriate immune response, especially excessive Th2 response, is mainly responsible for the development and progress of AD, Th1 cells are also involved in chronic states of the disease [32,33]. IFN-R, as representative Th1 cytokines, is dominant in the skin of patients with AD, which leads to enhanced and disease-related apoptosis of keratinocytes in the eczematous lesions of patients with AD through up-regulating Fas receptors on keratinocytes [2]. A recent study from Li et al. reported that miR-29b was markedly increased after IFN-e treatment in colorectal cancer cell [18], suggesting that IFN-N-8” \o “Yuan, 2015 #1583”miR-29b was mupregulating the expression of miR-29b. In this study, we first investigated whether IFN-regulatedd the expression of miR-29b in keratinocytes and we observed that miR-29b was up-regulated after IFN- we observe in a time-dependent manner. Thus, we hypothesized that the presence of IFN-a time-dlead to keratinocytes apoptosis through upregulating the expression of miR-29b. As expected, overexpression of miR-29b aggravated the apoptosis induced by IFN-m, whereas inhibition of miR-29b could attenuate the apoptosis induced by IFN-e. These data suggested that knockdown of miR-29b might be a novel therapeutic target to block AD progression.
BCL2L2, as a member of the Bcl-2 family, is one of the key regulator of apoptosis [34,35]. Zhang et al. found that down-regulation of miR-133b suppressed apoptosis of lens epithelial cell by up-regulating BCL2L2 in age-related cataracts [36]. Moreover, a recent study reported that miR-29b directly targeted and inhibited Bcl2L2 gene expression, and then increased neuronal cell death after ischemic brain injury [16]. However, whether BCL2L2 is involved in miR-29b-mediated IFN-v-induced keratinocytes apoptosis remains unknown. Consistent with these findings, our data showed that BCL2L2 was identified as a target gene of miR-29b in keratinocytes. Our data demonstrated that knockdown of miR-29b resulted in increased expression of BCL2L2 at both the protein and the mRNA level, whereas over-expression of miR-29b had an opposite result, which indicated that miR-29b negatively regulated the expression of BCL2L2. Furthermore, we knocked down the expression of BCL2L2 in keratinocytes with si-BCL2L2. We found that si-BCL2L2 reversed the inhibitory effect of miR-29b inhibitor on the apoptosis in IFN-treated keratinocytes. These results suggest that IFN-est thaed apoptosis of keratinocytes might be partially through miR-29b/BCL2L2 axis, which contributes to the development of AD.
In summary, we found the expression of miR-29b in keratinocytes was upregulated under IFN-under IFN-IFN-ed under IFN-R-29b in keratithat miR-29b acts as a pro-apoptotic miRNA involved in IFN-IFN-IFN-keratinocytes apoptosis through directly targeting BCL2L2 gene.Based on our findings, we propose that targeting miR-29b/BCL2L2 pathway can be considered as a potential target in the therapy for AD in the future.
Disclosure of conflict of interest
None.
References
- 1.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, Abram K, Metsalu T, Pihlap M, Meyer N, Folster-Holst R, Nagy N, Kemeny L, Kingo K, Vilo J, Illig T, Akdis M, Franke A, Novak N, Weidinger S, Akdis CA. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:1297–1306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K, Akdis CA. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001;108:839–846. doi: 10.1067/mai.2001.118796. [DOI] [PubMed] [Google Scholar]
- 5.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Rozalski M, Rudnicka L, Samochocki Z. MiRNA in atopic dermatitis. Postepy Dermatol Alergol. 2016;33:157–162. doi: 10.5114/ada.2016.60606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12:52. doi: 10.1186/s13223-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebane A. microRNA and Allergy. Adv Exp Med Biol. 2015;888:331–352. doi: 10.1007/978-3-319-22671-2_17. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Chen H, Liu L, Lu L, Wang Z, Tian F, Zhao Y. microRNA-130a promotes human keratinocyte viability and migration and inhibits apoptosis through direct regulation of STK40-mediated NF-kappaB pathway and indirect regulation of SOX9-meditated JNK/MAPK pathway: a potential role in psoriasis. DNA Cell Biol. 2017;36:219–226. doi: 10.1089/dna.2016.3517. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Li X, Qin Z. MicroRNA-21 promotes the proliferation and invasion of cholesteatoma keratinocytes. Acta Otolaryngol. 2016;136:1261–1266. doi: 10.1080/00016489.2016.1202447. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Luo H, Xiao Y, Wang L. miR-125b inhibits keratinocyte proliferation and promotes keratinocyte apoptosis in oral lichen planus by targeting MMP-2 expression through PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2016;80:373–380. doi: 10.1016/j.biopha.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Kim BK, Yoo HI, Choi K, Yoon SK. miR-330-5p inhibits proliferation and migration of keratinocytes by targeting Pdia3 expression. FEBS J. 2015;282:4692–4702. doi: 10.1111/febs.13523. [DOI] [PubMed] [Google Scholar]
- 14.Xue T, Wei L, Zha DJ, Qiu JH, Chen FQ, Qiao L, Qiu Y. miR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1alpha signaling: Implications for age-related hearing loss. Int J Mol Med. 2016;38:1387–1394. doi: 10.3892/ijmm.2016.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Pan Q, Sun B, Yang R, Fang X, Liu X, Yu X, Zhao Z. miR-29b Targets LPL and TDG Genes and Regulates Apoptosis and Triglyceride Production in MECs. DNA Cell Biol. 2016;35:758–765. doi: 10.1089/dna.2016.3443. [DOI] [PubMed] [Google Scholar]
- 16.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 17.Reinsbach S, Nazarov PV, Philippidou D, Schmitt M, Wienecke-Baldacchino A, Muller A, Vallar L, Behrmann I, Kreis S. Dynamic regulation of microRNA expression following interferon-gamma-induced gene transcription. RNA Biol. 2012;9:978–989. doi: 10.4161/rna.20494. [DOI] [PubMed] [Google Scholar]
- 18.Yuan L, Zhou C, Lu Y, Hong M, Zhang Z, Zhang Z, Chang Y, Zhang C, Li X. IFN-gamma-mediated IRF1/miR-29b feedback loop suppresses colorectal cancer cell growth and metastasis by repressing IGF1. Cancer Lett. 2015;359:136–147. doi: 10.1016/j.canlet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Thulin H, Hanifin JM, Bryant R. Leukocyte adherence in atopic dermatitis: diminished responses to histamine and isoproterenol. Acta Derm Venereol. 1980;60:235–238. [PubMed] [Google Scholar]
- 20.Sonkoly E, Wei T, Janson PC, Saaf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, Stahle M, Pivarcsi A. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, Beer Z, Bata-Csorgoo Z, Magocsi M, Rajnavolgyi E, Dobozy A, Kemeny L. Expression and function of toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Du GQ, Zhu ZT, Zhang C, Sun XW, Liu JJ, Li X, Wang YS, Du WJ. Identification of apoptosis-related microRNAs and their target genes in myocardial infarction post-transplantation with skeletal myoblasts. J Transl Med. 2015;13:270. doi: 10.1186/s12967-015-0603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, An J, Hua Y, Li Z, Yan N, Fan W, Su C. MicroRNA-194 regulates keratinocyte proliferation and differentiation by targeting Grainyhead-like 2 in psoriasis. Pathol Res Pract. 2017;213:89–97. doi: 10.1016/j.prp.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Jia R, Wang C, Hu T, Wang F. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines. Biochem Biophys Res Commun. 2014;452:775–781. doi: 10.1016/j.bbrc.2014.08.150. [DOI] [PubMed] [Google Scholar]
- 25.Chung HJ, Choi YE, Kim ES, Han YH, Park MJ, Bae IH. miR-29b attenuates tumorigenicity and stemness maintenance in human glioblastoma multiforme by directly targeting BCL2L2. Oncotarget. 2015;6:18429–18444. doi: 10.18632/oncotarget.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng YP, Nguyen GH, Jin HZ. MicroRNA-143 inhibits IL-13-induced dysregulation of the epidermal barrier-related proteins in skin keratinocytes via targeting to IL-13Ralpha1. Mol Cell Biochem. 2016;416:63–70. doi: 10.1007/s11010-016-2696-z. [DOI] [PubMed] [Google Scholar]
- 27.Chen XF, Zhang LJ, Zhang J, Dou X, Shao Y, Jia XJ, Zhang W, Yu B. MiR-151a is involved in the pathogenesis of atopic dermatitis by regulating interleukin-12 receptor beta2. Exp Dermatol. 2016 doi: 10.1111/exd.13276. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Li Z, Wang Y, Li L, Wang D, Zhang W, Liu L, Jiang H, Yang J, Cheng J. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl Res. 2016;178:38–53. e36. doi: 10.1016/j.trsl.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Wang Y, Wang D, Wu Y. Identification of collagen 1 as a post-transcriptional target of miR-29b in skin fibroblasts: therapeutic implication for scar reduction. Am J Med Sci. 2013;346:98–103. doi: 10.1097/MAJ.0b013e318267680d. [DOI] [PubMed] [Google Scholar]
- 31.Cheng J, Yu H, Deng S, Shen G. MicroRNA profiling in mid- and late-gestational fetal skin: implication for scarless wound healing. Tohoku J Exp Med. 2010;221:203–209. doi: 10.1620/tjem.221.203. [DOI] [PubMed] [Google Scholar]
- 32.Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy. 2009;39:310–316. doi: 10.1111/j.1365-2222.2008.03146.x. [DOI] [PubMed] [Google Scholar]
- 33.Hyung KE, Kim SJ, Jang YW, Lee DK, Hyun KH, Moon BS, Kim B, Ahn H, Park SY, Sohn UD, Park ES, Hwang KW. Therapeutic effects of orally administered CJLP55 for atopic dermatitis via the regulation of immune response. Korean J Physiol Pharmacol. 2017;21:335–343. doi: 10.4196/kjpp.2017.21.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Meng W, Tong B. Down-regulation of MicroRNA-133b suppresses apoptosis of lens epithelial cell by up-regulating BCL2L2 in age-related cataracts. Med Sci Monit. 2016;22:4139–4145. doi: 10.12659/MSM.896975. [DOI] [PMC free article] [PubMed] [Google Scholar]


