Abstract
Objective: This study is to investigate the effect of β-adrenergic receptor kinase inhibitor (Adeno-βARKct) on heart failure (HF) rat model. Methods: Male SD rats weighted 250 g undertaken ligation of the left anterior descending branch of coronary artery to establish HF model. Survival rats were randomly divided into experimental treated group (EXP) and control treated group (CONT). Additionally, pseudo-operated rats were taken as sham-ligated group (SHAM). Adeno-βARKct particles or Adeno empty vector was injected to the rats. Multiple indicators including left ventricular ejection fraction (LVEF), index of hemodynamics, plasma and myocardial tissue catecholamine, NT-ProBNP, mRNA levels of β1AR, β2AR and βARK1, and protein level of βARK1 were measured 4 weeks later. Results: Compared with rats in SHAM group, levels of LVEF, ±dp/dt max, and catecholamine in myocardial tissue were lower while plasma NT-ProBNP and plasma catecholamine were higher in rats of EXP and CONT. Additionally, β1AR and β2AR mRNA expressions were downregulated whereas βARK1 mRNA and βARK1 protein levels were upregulated in EXP and CONT. Compared with CONT, the levels of LVEF, -dp/dt max, and catecholamine in myocardial tissue were higher, while plasma NT-ProBNP and plasma catecholamine were lower in EXP. β1AR and β2AR mRNA expressions were upregulated, whereas βARK1 mRNA expression and βARK1 protein levels were downregulated in EXP. Conclusion: In vivo delivery of Adeno-βARKct by caudal vein is feasible and can improve cardiac function in rats with HF after myocardial infarction.
Keywords: Heart failure, βARKct, myocardial infarction, rat model
Introduction
Heart failure (HF), as the terminal period of several cardiovascular diseases, has become the most common and hazardous disease. The 5-year incidence of HF is about 50%, which is comparable to that of several malignant diseases [1]. Tremendous efforts on researches of chronic HF have been executed and an important understanding of the pathophysiologic abnormalities in the catecholamine-stimulated beta adrenergic receptor (βAR) signaling cascade has been described in the setting of HF [2]. The βAR signaling pathway is one of the most powerful and complicated regulators of cardiovascular function and is tightly regulated by a family of G protein-coupled receptor kinases [3,4]. During HF, the kinase βARK1 (also known as GRK2, G protein-coupled receptor kinase-2) expressing in myocardium is one of the most important kinases and plays vital roles in the phosphorylation of activated G protein-coupled receptors, thus causing desensitization and impaired βAR responsiveness [2,5].
Notwithstanding tremendous advances in drug therapies have been devoted to the treatment of myocardial infarction, traditional pharmacotherapy is still unable to obtain satisfactory results. With the extensive study of genetics and the advancement of gene vector technology in recent years, gene therapy has become an intriguing potential treatment for HF. One mechanism for administering gene therapy is adenovirus, which is a linear, double-stranded, and non-enveloped DNA virus. Currently, types 2 and 5 adenoviruses are most commonly used for human gene therapy. Recombinant adenovirus carrying the gene of interest adheres to the host cell surface via the coxsackie/adenovirus receptor, following which viral particles are endocytosed and trafficked to the nucleus through the nuclear pore [6]. Once released into the nucleus, the target gene is transcribed and translated via normal gene expression pathways [7].
It is shown that βARKct, the inhibitor of βARK1, may hold promise as a therapy for post-infarction HF [5,8]. βARKct is a polypeptide with a structure identical to the 194 c-terminal residues of βARK1 and can competitively inhibit the binding of βARK1 with Gγβ, thus reducing ARK1 activation and reducing βAR desensitization and internalization [9]. The adenovirus could be also used in the mediation of transfer of βARKct. Adeno-βARKct has been shown to exhibit favorable physiologic and pathological effects in different laboratory HF models [2].
The levels of several indicators such as hemodynamic parameters, cardiac catecholamine, and brain natriuretic peptide (BNP) can be used to measure heart function, providing links between molecular pathways and their physiological effects. In this study, we compared the levels of these indicators statistically and investigated the effect and mechanism of adenovirus-mediated delivery of Adeno-βARKct through intravenous administration on a rat model of HF following myocardial infarction.
Materials and methods
Animals and reagents
This study was carried out in accordance with the ethical recommendations in the guidelines for the use of animals in scientific research. Virus antibody, free/specific pathogen, and free male Sprague Dawley rats weighing ~250 g for each were purchased from Vital River (Beijing, China). Animals were fed at labium with general feed and bottled tap water. Nembutal powder and benzyl penicillin sodium were obtained from Beijing Chemical Works (Beijing, China) and North China pharmaceutical Group Corporation (Hebei, China). Adeno-βARKct and Adeno empty vector were purchased from Vector Gene Technology Company Ltd (Beijing, China).
Preparation of post-myocardial infarction HF model
After fasted for 6 hrs, the rats were weighed and anesthetized by 1% sodium pentobarbital (40 mg/kg) administered intraperitoneally, followed by immobilized on the operating table with their chests shaved and disinfected with Anerdian. Using a 16 g trocar, orotracheal intubation was conducted to a depth of about 4 cm past the incisor teeth, and then a Taimeng HX-100E small animal ventilator was attached. The respiratory rate was set to 60 cycles/min, with a tidal volume of 4 ml and a respiratory ratio of 2:3. A ~3 cm long incision was made through the skin along the left sternal border, and the subcutaneous, pectoralis major, and serratus anterior muscle were bluntly dissected to expose the ribs and intercostal muscles. The intercostal muscles in the fifth intercostal space were then bluntly dissected. Following insertion of a speculum to spread the ribs, forceps were used to tear the pericardium. For animals in the myocardial infarction group, a 5-0 suture was used to ligate the left anterior descending artery approximately 3 mm below the left atrial appendage and pulmonary cone junction. In contrast, a slipknot was used around the coronal anterior descending artery for rats in the SHAM group. The site was observed to confirm the absence of active bleeding and the speculum was removed. Subsequently, the major pectoralis and serratus anterior muscles were sutured closed, followed by suture of the skin and treatment with penicillin around the surgical site. Mechanical ventilation was continued postoperatively until the rats regained consciousness. After surgery, penicillin was administered 100,000 U/day via intramuscular injection for 3 days. A 12-lead ECG was used to monitor for acute myocardial infarction and elevation of the ST segment with a characteristic upward arch, seen via the I, avL, and V1-V6 leads indicated successful induction of infarction. Sham-ligated rats showing signs of acute myocardial infarction by ECG were excluded from further analysis. HF was allowed to progress for 4 weeks during left ventricular remodeling.
Animal grouping and treatment
Rats from the ligated cohort with HF arose from myocardial infarction were randomly divided into an experimental treated group (n = 8) and a control treated group (n = 8). Sham-ligated rats were maintained as a separate group (n = 8). Treatment was administered 4 weeks after surgery by way of tail vein injection. Recombinant type 5 adenovirus vector was used in this study and the sham-ligated group was treated with 5×1010 pfu empty adenovirus vector, while 5×1010 pfu Adeno-βARKct particles were injected into each of the experimental treated rats.
Measurement of left ventricular function
Four weeks after administration of injection treatment, the rats were anesthetized and immobilized in order to assess cardiac function. Echocardiography was carried out using a 20-45 MHz ultrasound probe with a VeVo770 high-resolution small animal ultrasound system. The left ventricular long axis was displayed by the probe, while the results of M-mode measurements revealed many other parameters including left ventricular end-diastolic diameter (LVEDD), left ventricular systolic diameter (LVESD), and thickness of left ventricular posterior wall (LVPW). Calculation of left ventricular ejection fraction (LVEF) and other analysis were carried out by using onboard ultrasound analysis software. Measurements were repeated 3 times and averaged.
Hemodynamic measurements
Hemodynamic parameters for rats in each group were measured by echocardiography 4 weeks after administration of injection treatment. Anesthesia and immobilization were performed as above, followed by neck skin cutting longitudinally along the right side. The right carotid artery was separated from the inside of the sternocleidomastoid muscle and a segment of approximately 1.5 cm was ligated at the distal end, filled with heparin saline and a 20 G sheath connected with Taimeng BL-420S-type biological and functional experimental system was inserted and threaded into the left ventricle. Parameters measured included left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and maximum rate change of left ventricular pressure (±dp/dt max).
Collection of plasma catecholamine and N-terminal pro-B-type natriuretic peptide (NT-ProBNP)
Prior to sacrifice, 5 ml of arterial blood was extracted from each rat through the right carotid using a pre-cooled syringe with heparin tube. Blood was then centrifuged for 15 min at 3000 r/min to separate plasma, which was then split into several pre-cooled tubes and stored at -80°C. NT-ProBNP and catecholamine levels were measured by ELISA later.
Collection of myocardial tissue catecholamine, β-adrenergic receptor and βARK1
The heart was removed immediately after sacrifice of rats, followed by 100 mg of left ventricle tissue taken from the non-infarcted myocardium and stored at -80°C. Afterwards, catecholamine levels were measured by ELISA, β-adrenergic receptor and βARK1 mRNA expression were quantified by RT-PCR, and βARK1 protein expression was determined by Western blot.
Statistical methods
SPSS 18.0 statistical software (Chicago, IL, USA) was used for data analysis. Data is presented as mean ± standard deviation, and comparisons between the two groups were calculated using one-way ANOVA. P<0.05 was considered as statistically significant.
Results
Observed mortality from HF after myocardial infarction
Of the 46 rats used for modeling, endotracheal intubation failure led to 8 deaths with the mortality rate of 17.4%. Among successfully intubated rats, 14 animals in the myocardial infarction group died with the mortality rate of 46.7%. All 8 sham-ligated rats survived.
Pathological observation of rat myocardial infarction
To assess the fundamental features of the rats in each group, histological examination was performed. Compared with the SHAM group, the left ventricular anterior wall of heart tissue from both the control treated and Adeno-βARKct treated myocardial infarction groups was visibly more yellow-white in color. Furthermore, tissues around the infarct zone were notably collapsed. Longitudinal incision of the left ventricle revealed that the wall at the infarction zone was significantly thinner, with features of necrosis involving the full thickness of the wall.
Analysis of left ventricular ejection fraction and hemodynamic parameters
In order to learn the heart state and cardiac function in each group, left ventricular eviction fraction and hemodynamic parameters were examined. As shown in Table 1, compared with the SHAM group, ±dp/dt in both subgroups of the myocardial infarction cohort was significantly decreased (P<0.05). At the same time, -dp/dt in the experimental treated group was significantly higher (P<0.05) in contract with the control treated group. LVEF levels in both control and experimental treated groups were significantly decreased in comparison with the SHAM group (P<0.05), and between these two myocardial infarction groups the LVEF level in the experimental treated group was significantly higher (P<0.05). These differences of LVEF, +dp/dt and -dp/dt levels indicated distinct cardiac states.
Table 1.
Left ventricular ejection fraction and hemodynamic parameters 4 weeks after administration of treatment
| Group | n | LVSP (mmHg) | LVEDP (mmHg) | +dp/dt max | -dp/dt max | LVEF |
|---|---|---|---|---|---|---|
| SHAM | 8 | 139.4±8.0 | 6.6±2.3 | 4131±387 | 3458±314 | 0.63 |
| CONT | 8 | 135.9±8.1 | 6.8±3.1 | 3041±345* | 2558±284* | 0.26* |
| EXP | 8 | 131.0±9.2 | 6.1±2.7 | 3243±437* | 2911±231*,# | 0.37*,# |
| F-number | 1.986 | 0.118 | 17.56 | 21.245 | 257.492 |
P<0.05 vs SHAM group;
P<0.05 vs control treated group.
Plasma NT-ProBNP levels
As to unravel the effect of treatment on the levels of brain natriuretic peptide (BNP), levels of plasma NT-ProBNP in each group were measured. As shown in Table 2, compared with the SHAM group, plasma levels of NT-ProBNP in the experimental treated group and control treated group were significantly higher (P<0.05). Meanwhile, for the two subgroups of the myocardial infarction cohort, plasma levels of NT-ProBNP in the Adeno-βARKct treated group were significantly lower than that in the control treated group (P<0.05). Taken together, the results suggested that NT-ProBNP could be taken as an indicator of cardiac function, while βARKct treatment might help to recover the NT-ProBNP level.
Table 2.
Plasma NT-ProBNP levels 4 weeks after administration of treatment
P<0.05 vs SHAM group;
P<0.05 vs control group.
Plasma and myocardial tissue catecholamine levels
To understand the extents of HF in different groups, levels of catecholamine in plasma and myocardial tissues were determined. As shown in Table 3, compared with the SHAM group, plasma norepinephrine (NE) levels of the Ad-βARKct treated myocardial infarction group and control treated group were significantly higher (P<0.05). The plasma NE level in Ad-βARKct treated group was significantly lower than that in the control treated group (P<0.05). The norepinephrine levels in myocardial tissue were just the opposite to plasma norepinephrine levels, with the values in control treated group reduced significantly compared with the SHAM group (P<0.05) and level in Adeno-βARKct treated group was much higher than that of the control treated group (P<0.05). Levels of epinephrine in both plasma and myocardial tissue were quite similar to that of norepinephrine, with myocardial epinephrine significantly lower in both control and Ad-βARKct treated groups (P<0.05) and plasma epinephrine significantly higher in control treated group (P<0.05). Between these two treated groups, plasma epinephrine and myocardial epinephrine in Ad-βARKct treated group showed much closer levels to the SHAM group than that in control treated group. In line with this, it is possible to draw a conclusion that the catecholamine levels varied greatly in HF rats while Ad-βARKct treatment might contribute to recover the HF to certain content.
Table 3.
Plasma and myocardial tissue catecholamine levels
| Group | n | Plasma NE (pg/ml) | Plasma E (pg/ml) | Myocardial NE (μg/g) | Myocardial E (μg/g) |
|---|---|---|---|---|---|
| SHAM | 8 | 324.3±99.7 | 291.3±58.2 | 6.58±1.11 | 4.90±1.21 |
| CONT | 8 | 921.3±158.5* | 500.6±104.7* | 3.32±1.11* | 1.05±0.55* |
| EXP | 8 | 600.8±75.1*,# | 358.7±59.4# | 5.54±1.22# | 3.38±1.24*,# |
P<0.05 vs Sham-operated group;
P<0.05 vs control group.
Real-time PCR of βARK1 from myocardial tissue
To study the mRNA levels of βARK1 from myocardial tissue in each group, real-time PCR was performed. As shown in Figure 1, compared with the sham-ligated group, mRNA levels of βARK1 in myocardial tissue from the Adeno-βARKct treated group and control treated group were significantly higher (P<0.05). At the same time, compared with the control treated group, βARK1 mRNA expression in myocardial tissue from the Adeno-βARKct treated group was significantly lower (P<0.05). In summary, these results indicated that level of βARK1 mRNA from myocardial tissue would increase significantly in HF rats while Ad-βARKct treatment could resist this increase.
Figure 1.
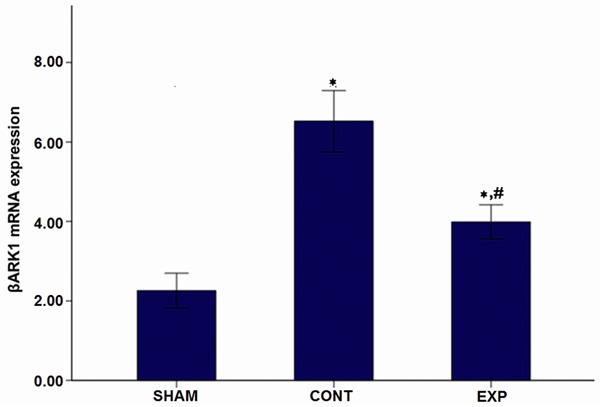
Myocardial tissue βARK1 mRNA levels after 4 weeks of administration. Note: *P<0.05 vs Sham-operated group; #P<0.05 vs control group.
Western blot analysis
To study the βARK1 protein levels from myocardial tissue in each group, western blot was performed. As shown in Figure 2A and 2B, the result showed significantly increased protein expression levels in myocardial tissue from the control and Adeno-βARKct treated myocardial infarction groups in comparison with that in the SHAM group (P<0.05 for both). At the same time, βARK1 protein levels in myocardial tissue from the Adeno-βARKct treated rats were significantly lower than that in control treated rats (p<0.05). Taken together, the result indicated that βARK1 protein levels from myocardial tissues in each group was in consistent with βARK1 mRNA levels and also presented increase in both treated groups, and Ad-βARKct treatment could recover this situation significantly.
Figure 2.
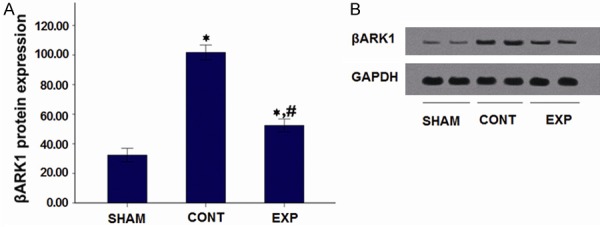
Myocardial tissue βARK1 protein levels after 4 weeks of administration. A. Western Blot results; B. βARK1 protein levels. Note: *P<0.05 vs Sham-operated group; #P<0.05 vs control group.
Myocardial βAR mRNA levels
To understand the effect of Adeno-βARKct treatment on rats in each group more clearly, mRNA levels of β1AR and β2AR were determined. As shown in Figure 3A and 3B, the results demonstrated that compared with the SHAM group, mRNA levels of myocardial β1AR in the two myocardial infarction subgroups were significantly decreased (P<0.05 for both). Furthermore, myocardial tissue β2AR mRNA levels of both the two treated groups were significantly decreased relative to the sham-ligated group (P<0.05). Meanwhile, compared with the control treated group, mRNA levels of myocardial β1AR and β2AR were both significantly higher in the Adeno-βARKct treated group (P<0.05 for both). In summary, the mRNA levels of both β1AR and β2AR were both reduced significantly in myocardial infarction groups while Ad-βARKct treatment could recover this level significantly.
Figure 3.
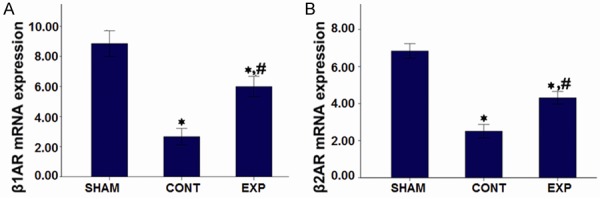
Myocardial β-adrenergic receptor mRNA levels of each group after 4 weeks of administration. A. β1-adrenergic receptor mRNA levels; B. β2-adrenergic receptor mRNA levels. Note: *P<0.05 vs Sham-operated group; #P<0.05 vs control group.
Discussion
HF represents one of the most common causes of mortality worldwide nowadays and the number of patients suffering from this disease would continue to increase [3]. Previous studies have indicated that cardiac βAR signaling pathway turbulence by βARK1 activation occurred in cardiac abnormalities and that inhibition of βARK1 via βARKct could be a potential therapy for chronic HF [2,3,5]. In this study the function of βARKct was studied via a rat HF model and multiple cardiac indicators including levels of LVEF, ±dp/dt max, catecholamine, NT-ProBNP, βARK1 mRNA expression and βARK1 protein levels were measured to enlarge our knowledge and provide molecular and pathological basis for βARKct gene therapy of HF.
As an important participator in HF, βARK1 was found in previous reports to be over expressed during this process. βARK1 is activated mainly through binding with the βγ subunit (Gβγ) of G protein. This kinase phosphorylates carboxyl terminus of the activated β-adrenergic receptor, hence, mediating the binding of the receptor and the β inhibition protein [10]. This step leads to uncoupling of the receptor from the G protein, thereby blocking the catalytic G protein and resulting in β adrenergic receptor desensitization along with a decrease in receptor density [10,11]. Increased activity of myocardial βARK1 and subsequent β-adrenergic receptor desensitization in the early stages of HF have a protective effect on the heart, however, eventually aggravate myocardial injury [12]. In these situations, β-adrenergic receptor signaling is impaired and myocardial adrenergic receptor expression is significantly decreased. This in turn causes excitation of the sympathetic nervous system, decreases myocardial catecholamine reserves, increases plasma catecholamine levels, and ultimately results in a vicious cycle. βARK1 also directly regulates downstream GPCR kinases in the cytoplasm such as PI3K, Akt, p38, and GIT1 [9]. Furthermore, it can alter endothelial and immune cell migration, which is involved in ischemia-reperfusion injury.
Measurement of LVEF is a noninvasive assessment of left ventricular systolic function which is commonly used in clinical settings to indicate the severity left ventricular dysfunction. Therefore, after Adeno-βARKct gene transfer, we measured the LVEF and the results presented here demonstrate that compared with the SHAM group, LVEF was significantly reduced in control treated rats 8 weeks after acute myocardial infarction (4 weeks post-infarction to induce HF followed by administration of control Adeno vector and an additional 4 weeks). LVEF of the sham-ligated and Adeno-βARKct treated experimental group was significantly higher than that of the control treated group, suggesting βARKct could significantly improve left ventricular systolic function. ±dp/dt max refers to the maximum rate of left ventricular pressure increase during the isovolumetric contraction phase. During the isovolumetric relaxation phase, the rate of left ventricular pressure drops and the difference between these two numbers reflects the maximum rate of change in left ventricular pressure. A greater absolute value indicates better compliance of the left ventricular wall [13]. Our results suggest that ±dp/dt max in rats with HF after myocardial infarction was significantly decreased, while both +dp/dt max and -dp/dt max of myocardial infarction rats treated with Adeno-βARKct tended to increase, suggesting that βARKct could improve the compliance of left ventricular wall. This result was consistent with previous studies [5].
Reserves of cardiac catecholamine in rats with HF are decreased, while plasma catecholamine levels were significantly increased. Decreased cardiac catecholamine suggests a reduction in neurotransmitters from the adrenergic nerve endings, resulting in a slow response to sympathetic triggers. High plasma catecholamine levels are directly correlated with left ventricular insufficiency and mortality [14]. In this study, following exogenous βARKct over-expression in rats, myocardial catecholamine levels were significantly elevated, while plasma catecholamine levels were significantly reduced. This result indicated that βARKct over-expression could ameliorate damage to the myocardium and help gradually restore the tissue from a chronic cardiac sympathetic storm, supporting a role for this molecule in the treatment of HF following infarction.
Brain natriuretic peptide (BNP) helps to promote natriuresis, diuresis, and dilation of blood vessels, and antagonizes biological activity of the renin-angiotensin-aldosterone system. Plasma BNP levels increase in congestive HF patients, which may reflect the extent of the pressure load of myocardial cells. NT-ProBNP, which itself has no physical activity, is secreted into the blood flow with BNP at a 1:1 ratio. This protein has a long half-life, strong in vitro stability, and is less vulnerable to degradation than exogenous BNP, making it a sensitive indicator of the degree of cardiac dysfunction [15]. The results presented here show that NT-ProBNP in βARKct treated rats was significantly decreased compared with the control treated group, suggesting βARKct can be used to help ameliorate ventricular dysfunction.
In this study, using peripheral injection of adenovirus mediated meridians of βARKct, we obtained similar experimental results as those seen in studies with coronary injection and direct myocardial injection [5,16]. Our work further demonstrated that that βARK1 is a valid target for the treatment of HF, and showed the feasibility of myocardial transfection through peripheral intravenous injection of Adeno-βARKct. Additionally, our work indicated that Adeno-βARKct ameliorated abnormalities in the myocardial β-adrenergic receptor signaling system, and therefore improving cardiac function during HF that follows myocardial infarction in rats. Peripheral administration of therapy is especially attractive and the risks associated with intravenous injection are significantly lower than those involved in opening the chest for cardiac injection or administration via cardiac catheterization. Together, the work presented here provided some methodological basis for clinical gene therapy for HF patients.
Acknowledgements
This study was supported by Beijing Funding Project for Talents (PYZZ090415001662).
Disclosure of conflict of interest
None.
References
- 1.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Molina EJ, Gupta D, Palma J, Gaughan JP, Macha M. Right ventricular beneficial effects of beta adrenergic receptor kinase inhibitor (betaARKct) gene transfer in a rat model of severe pressure overload. Biomed Pharmacother. 2009;63:331–336. doi: 10.1016/j.biopha.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 3.Brinks H, Koch WJ. betaARKct: a therapeutic approach for improved adrenergic signaling and function in heart disease. J Cardiovasc Transl Res. 2010;3:499–506. doi: 10.1007/s12265-010-9206-6. [DOI] [PubMed] [Google Scholar]
- 4.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 5.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams ML, Koch WJ. Viral-based myocardial gene therapy approaches to alter cardiac function. Annu Rev Physiol. 2004;66:49–75. doi: 10.1146/annurev.physiol.66.032102.141555. [DOI] [PubMed] [Google Scholar]
- 7.Nadeau I, Kamen A. Production of adenovirus vector for gene therapy. Biotechnol Adv. 2003;20:475–489. doi: 10.1016/s0734-9750(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–1194. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 9.Penela P, Murga C, Ribas C, Lafarga V, Mayor F Jr. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penela P, Murga C, Ribas C, Tutor AS, Peregrín S, Mayor F Jr. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Petrofski JA, Koch WJ. The beta-adrenergic receptor kinase in heart failure. J Mol Cell Cardiol. 2003;35:1167–1174. doi: 10.1016/s0022-2828(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 12.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 13.Tartière JM, Tabet JY, Logeart D, Tartière-Kesri L, Beauvais F, Chavelas C, Cohen Solal A. Noninvasively determined radial dP/dt is a predictor of mortality in patients with heart failure. Am Heart J. 2008;155:758–763. doi: 10.1016/j.ahj.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI40–48. [PubMed] [Google Scholar]
- 15.Peacock WF. Heart failure management in the emergency department observation unit. Prog Cardiovasc Dis. 2004;46:465–485. doi: 10.1016/j.pcad.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Müller OJ. AAV6. betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]


