Abstract
Objectives: Toll-like receptors (TLRs) are related to human papillomavirus (HPV) infections including condyloma acuminatum (CA). This study was designed to investigate the mechanism of TLR9 and nuclear factor κB (NF-κB) in CA. Methods: Expression of TLR9 protein in CA patients was detected and compared with those in CA relapse-free (CaRF) patients and normal control. HaCaT cells were transfected with HPV11 genome and NF-κB p65 siRNA or IκB kinase inhibitor BMS345541. Expression of NF-κB and TLR9 were detected using both PCR and Western blot methods. Results: TLR9 was downregulated in CA specimens as compared to CaRF and normal controls. HPV11 transfection into HaCaT (HPV11.HaCaT) cells reduced TLR9 expression and activated NF-κB p65 expression. However, administration of NF-κB p65 siRNA or IκB kinase inhibitor BMS345541 significantly inhibited NF-κB p65 expression and rescued the expression of TLR9. Conclusion: Inhibition of NF-κB activation could rescue TLR9 expression in HPV11.HaCaT cells. TLR9/NF-κB mechanism may provide new target for clinical treatment of CA.
Keywords: Condyloma acuminatum, HPV11, NF-κB, HaCaT
Introduction
Human papilloma viruses (HPVs) infection was related to the progression of diseases including cervical cancer, cutaneous benign lesions, and other tumors [1,2]. The development of cervical cancer had been reported to link to the persistent infection by high-risk mucosal HPVs types, especially HPV16 [2-4].
Toll-like receptors (TLRs) are specific pattern recognition molecules that have been identified to take great part in initiating host antiviral immune responses [1,5,6]. Generally, TLRs bind to microbial components to trigger innate immunity and direct adaptive immunity. Interestingly, the polymorphisms of TLR9 gene are associated with HPV16 infection susceptibility in cervical cancer patients [3,7,8]. Presence of HPV infection with TLR9 SNP (rs352140) increased the risk of cervical cancer [7].
Condyloma acuminatum (CA) is one of the epidermal infective diseases caused by HPVs and is regarded as a benign tumor [9]. Most CA cases (~90%) are caused by HPV type 6 (HPV6) and HPV11, both are low risk HPVs [9-11]. CA progression involves in host immune response to HPVs and subsequent local inflammatory responses induced by HPVs infection, which are still poorly understood. As reported that the suppression of TLR9 in HPV16 induced cervical cancer was mediated by nuclear factor κB (NF-κB) p65 activation [2,12,13], which might demonstrate the TLR9/NF-κB signaling in HPVs induced immune responses.
To our best knowledge, up to now there are no international studies reporting role of TLR9 in CA patients and the possible mechanisms. In order to investigate the association of TLR9 and NF-κB in HPV11 induced CAs, we established the HPV11.HaCaT cells using HPV11 genome transfection and detected the expression of TLR9 and NF-κB in HPV11.HaCaT cells. These results may provide us new insights into TLR9/NF-κB mechanism in HPV11 induced CAs.
Methods and materials
Samples collection and processing of tissues
Fresh skin tissues were obtained from CA patients (n = 15) and CA relapse-free (CaRF) patients (n = 12) from Department of Dermatology, Shanghai General Hospital, Shanghai Jiao Tong University, China. Normal (NC, n = 8) specimens cut from patients in the Department of Plastic Surgery were collected and used as control. Each specimen was cut into 0.5 cm3 pieces. The second piece was fixed in 4% paraformaldehyde solution for immunohistochemistry analysis. All experiment protocols were approved by the Ethics Committee of Shanghai General Hospital. Written consent was obtained from all participants before surgeries.
Cell line and culture conditions
Human HaCAT cells (ATCC, Rockville, Md., USA) were maintained in DMEM (Sigma Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma, USA) and 100 U/ml penicillin/streptomycin (Sigma, USA) at 37°C in 5% CO2.
HPV11.HaCAT cell recombination and monolayer culture
The recombinant HPV11.HaCaT cells, which had stable HPV 11 genomic expression, was established as described elsewhere [14,15]. Cells transfected with pTK-neo DNA (Invitrogen, Carlsbad, CA, USA) were considered as control. Lipofectamine™ 2000 reagent (Invitrogen, USA) was used for cell transfection. HPV11.HaCaT cells were selected using G418 (Sigma, USA). HPV11.HaCaT cells were expanded, cultured in DMEM supplemented with 10% FBS, calcium chloride at 37°C in 5% CO2. Identification of HPV11.HaCaT cells was performed by HPV 11 E1^E4 transcript detection, and immunofluorescence analysis of HPV11 E7 in cells.
NF-κB siRNA plasmid recombination and inhibitor administration
NF-κB expression silencing was performed using transfection of recombinant NF-κB p65 siRNA plasmids, which was synthesized by (Shanghai Sangon Biotechnology Co., Ltd., (Shanghai, China). Six hours after p65 siRNA transfection, HPV11.HaCaT cells were treated with TNF-α (10 ng/mL) for 24 h in DMEM at 37°C in 5% CO2. For the inhibition of NF-κB signaling, HPV11.HaCaT cells were treated with IκB kinase inhibitor BMS345541 (1 μM, Sigma, USA) for one hour, and then cultured in DMEM supplemented with TNF-α administration (10 ng/mL) for 24 h at 37°C in 5% CO2. Each treatment was performed in 5 repetitions. HaCaT cells and HPV11.HaCaT cells transfected with siRNA negative controls (siRNA-NC) was considered as normal or negative control, respectively.
Nested RT-PCR and qRT-PCR
Nested RT-PCR was used for the detection of HPV11 E1^E4 transcript in HPV11.HaCaT cells as previously described [14,15]. Total RNA was isolated from HaCaT cells for the reverse transcription of the first-strand cDNA (TaKaRa Reverse Transcriptase Kit, TaKaRa, China). The mRNA relative expression levels were determined using SYBR ExScript qRT-PCR Kit (TaKaRa, China) on an Applied Biosystems (ABI) 7500 PCR System (ABI, USA). Primers for nested RT-PCR were synthesized according to the previous sequences (Table 1). Blank control was normal HaCaT cells without HPV11 DNA. The primers were synthesized and listed in Table 1. The internal reference gene was β-actin. All reactions were run in triplicate.
Table 1.
Oligonucleotide primer used for PCR in this study
| Gene name | Primer | Sequence (5’-3’) |
|---|---|---|
| HPV11 E1^E4 | First PCR forward | 5’-TACAAGACCTTTTCGTGGGCACA-3’ |
| First PCR reverse | 5’-AAAGGCAGGAAAATAGCACACAC-3’ | |
| Second PCR forward | 5’-ATATTGTGTGTCCCATCTGCG-3’ | |
| Second PCR reverse | 5’-CAGCAATTTGTACAGGCACTA-3’ | |
| β-actin | First PCR forward | 5’-GATGACCCAGATCATGTTTG-3’ |
| First PCR reverse | 5’-GGAGCAATGATCTTGATCTTC-3’ | |
| Second PCR forward | 5’-AACACCCCAGCCATGTACGTTG-3’ | |
| Second PCR reverse | 5’-ACTCCATGCCCAGGAAGGAAGG-3’ | |
| TLR9 | PCR forward | 5’-CGTCTTGAAGGCCTGGTGTTGA-3’ |
| PCR reverse | 5’-CTGGAAGGCCTTGGTTTTAGTGA-3’ | |
| NF-κb p65 | PCR forward | 5’-GTCTTCGTGCTCGGTGATG-3’ |
| PCR reverse | 5’-AGGACCTCTGACCCAAATG-3’ | |
| β-actin | PCR forward | 5’-AGCGAGCATCCCCCAAAGTT-3’ |
| PCR reverse | 5’-GGGCACGAAGGCTCATCATT-3’ |
Immunohistochemical analysis
Immunohistochemistry were performed on the skin tissues. Tissues were fixed in 4% paraformaldehyde solution, transparent by dimethylbenzene (Beyotime, Shanghai, China) and then embedded with Paraplast Plus medium (Sigma, USA). Sections of 6 µm were dewaxed and then blocked by normal goat serum. Then the slides were incubated with specific primary antibody against TLR9 (1:500 dilution, Cell Signaling Technology, CST, Denvers, MA, USA) at 4°C overnight, followed by additional incubation with HRP-secondary antibody conjugate at 37°C for 4 h. The signal visualization for protein expression was performed with diaminobenzidine (DAB) staining. Double blind scoring method was used for histological scoring of TLR9 expression [16]. Cells with brown granules were considered as TLR9 positive cells. Percentage of moderately positive (TLR9++) cells and strongly positive cells (TLR9+++) were calculated as: averaged positive cells number/total cell numbers of five random fields.
Immunofluorescence assay
Immunofluorescence analysis was performed to identify the recombinant HPV11.HaCaT cells (Sun et al., 2016). HaCaT cells that received HPV11 genome transfection were cultured on glass slides in petri dishes for 12 h, at 37°C in 5% CO2. The slides were the rinsed with PBS, fixed in 4% paraformaldehyde solution. Triton-X-100 (1:299 v/v, TBS) was added for compound scrubbing. Next, the slides were washed with PBS, immersed in goat serum for 1 h, and incubated with anti-HPV11 E7 antibody (1:300, CST, USA) overnight at 4°C, followed by additional dark incubation with goat anti-mouse IgG-conjugated with Alex Fluor 488 (1:400; Beyotime, China) for another 1 h at 37°C. Nuclear staining was achieved with 3 µg/mL DAPI solution (Beyotime, China). For immunofluorescence assay, slides were observed using a FV300-SU laser scanning confocal microscope (Olympus, Tokyo, Japan). Normal HaCaT cells were used as control.
Western blotting analysis
Harvested cells at 24 h post-transfection were washed with PBS, and lysed in RIPA buffer (Thermo Scientific, Rockford, IL, USA). Protein concentrations detection was then performed using a BCA protein assay kit (Thermo, USA). Equal 35 µg protein solutions were separated by 10% SDS-PAGE gels. Separated proteins were then transferred to PVDF membranes (Invitrogen Corp., Carlsbad, CA, USA), and blocked with 5% skimmed milk (BD Biosciences, Franklin Lakes, NJ, USA). Next, PVDF membranes were incubated with primary antibody against TLR9 (dilution 1:1500, CST, USA), NF-κB p65 (dilution 1:1000, CST, USA), and β-actin (1:1500 dilution, CST) at 4°C overnight. At last, membranes were incubated with secondary antibodies for 1 h in dark at 37°C. Enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL, USA) were used and polypeptide bands were quantified by AlphaEase software (Alpha, USA).
Statistical analysis
Each experiment was performed in triplicate. Data were expressed as mean ± SD. Statistical analysis was performed using Student’s t test and a one-way analysis of variance (ANOVA) test for difference between two groups and among groups more than three, respectively. P < 0.05 was considered to be statistically significant.
Results
TLR9 shows relative lower expression level in CA specimens
TLR9 expression was observed on the surface of epithelial cell membranes (Figure 1). Immunohistochemistry analysis showed that the expression of TLR9 protein in CA and CaRF patients were significantly different (P < 0.05), and both were significantly lower than that of the normal healthy control (P < 0.05, Figure 1).
Figure 1.
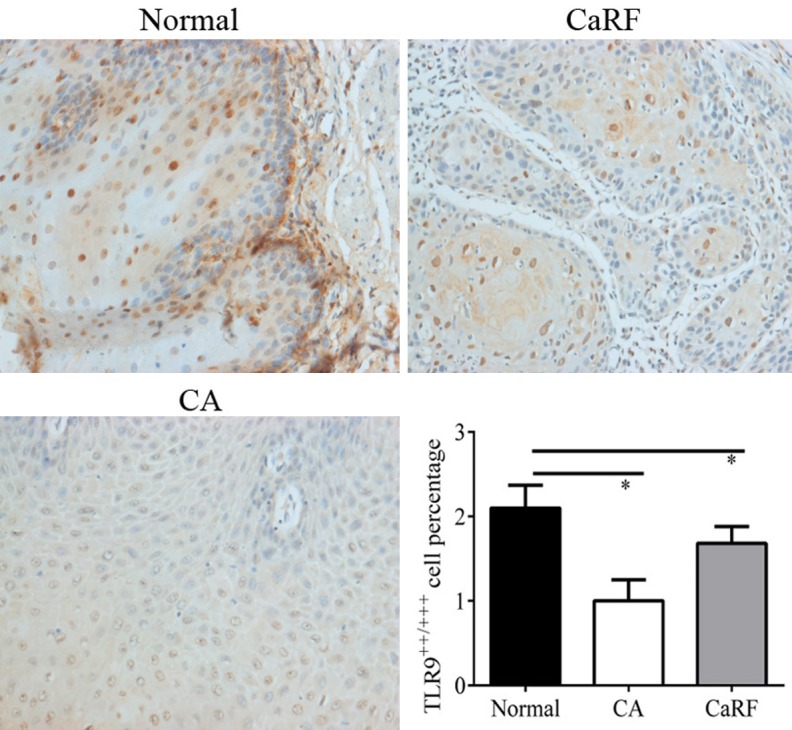
Immunohistochemistry analysis for TLR9 in CA patients. Percentage of moderately TLR9 positive (TLR9++) cells and strongly TLR9 positive cells (TLR9+++) were considered as TLR9 positive cells. *represents significant level at P < 0.05, vs. control. Magnification × 400.
Identification of recombinant HPV11.HaCaT cells
PBR322.HPV11 plasmid was identified using agarose gel (Figure 2A). Identification of HPV11 mRNA transcript in recombinant HPV11.HaCaT cells were performed using nested PCR. The results of nested PCR only showed HPV11 E1^E4 transcript in HPV11.HaCaT cells, while β-actin expressed in both HaCaT cells and HPV11.HaCaT cells (Figure 2B). In addition, results of immunofluorescence assay showed the fluorescence of HPV11 E7 protein in HPV11.HaCaT cells (Figure 2C). On the contrary, no HPV11 mRNA and HPV11 E7 protein fluorescence was detected in HPV11.HaCaT cells. These demonstrated the successful recombinant of stable HPV11 E7 cells.
Figure 2.
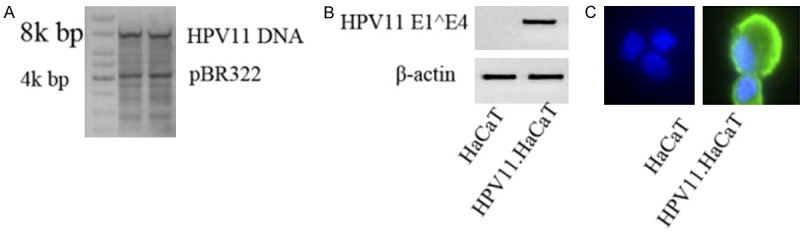
Identification of recombinant HPV11.HaCaT cells. A: Digested products of pBR322.HPV11 DNA using BamHI enzyme. B: HPV11 early genes (E1^E4) only expressed in HPV11.HaCaT cells. C: HPV11.HaCaT cells showed fluorescence of E7 protein. Blue fluorescence indicated cell nucleus. Magnification × 400.
HPV11 infection increases NF-κB expression and inhibits TLR9 expression in HaCAT cells
After HPV11 infection, the expression of NF-κB and TLR9 mRNA and protein were significantly changed compared with the controls (Figure 3). Results showed that the mRNA and protein expression of NF-κB p65 in HPV11.HaCaT cells were significantly higher than those in the HaCaT (control) cells or cells transfected with control plasmids (P < 0.05). On the contrary, the mRNA and protein expression of TLR9 were significantly reduced in HPV11.HaCaT cells as compared to HaCaT (control) cells (P < 0.05). This demonstrated that NF-κB and TLR9 expression was significantly activated and inhibited by HPV11 infection, respectively.
Figure 3.
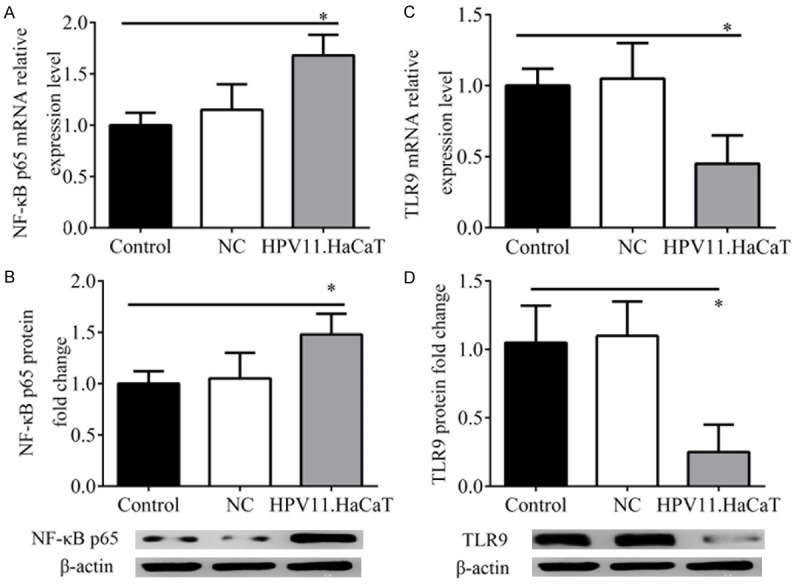
Effect of HPV11 infection on NF-κB and TLR9 expression. A: mRNA expression of NF-κB p65 by qRT-PCR. B: Protein expression of NF-κB p65 by western blotting analysis. C: mRNA expression of TLR9 by qRT-PCR. D: Protein expression of TLR9 by western blotting analysis. *represents significant level at P < 0.05, vs. control (HaCaT).
NF-κB inhibition activates TLR9 expression
According to the above results we found there might be negative correlation between expression of NF-κB and TLR9. In order to investigate whether TLR9 expression in HPV11.HaCaT cells is inhibited by NF-κB activation, we treated HPV11.HaCaT cells with NF-κB inhibitor BMS345541 or NF-κB p65 siRNA plasmids. Cellular mRNA and protein were harvested after 24 h for the detection of NF-κB p65 and TLR9 expression. The results showed that both BMS345541 and NF-κB p65 siRNA plasmid administration could significantly inhibit expression of NF-κB p65, vs. HPV11.HaCaT cells not treated by BMS345541 and NF-κB p65 siRNA plasmid (P < 0.05, Figure 4A and 4B). Moreover, the expression of NF-κB p65 and TLR9 in HPV11.HaCaT cells were significantly activated by either BMS345541 administration or NF-κB p65 siRNA transfection (P < 0.05, Figure 4C and 4D). This confirmed that there NF-κB p65 siRNA and rescued TLR9 expression in HPV11.HaCaT cells.
Figure 4.
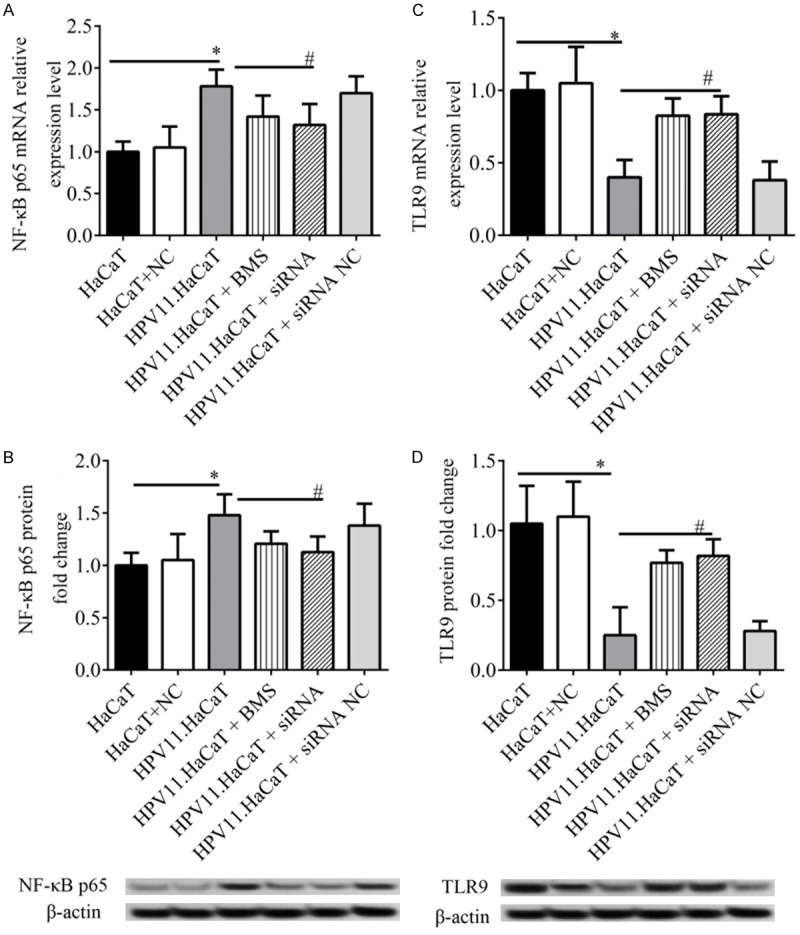
Correlation of NF-κB and TLR9 expression in HPV11.HaCaT cells. A: mRNA expression of NF-κB p65 by qRTPCR. B: Protein expression of NF-κB p65 by western blotting analysis. C: mRNA expression of TLR9 by qRT-PCR. D: Protein expression of TLR9 by western blotting analysis. *, and # represents significant level at P < 0.05, vs. HaCaT and HPV11.HaCaT, respectively. BMS, BMS345541.
Discussion
In the present study, we confirmed that HPV11 infection in HPV11.HaCaT cells significantly reduced TLR9 expression and inversely activated NF-κB. On the contrary, the inhibition of NF-κB signaling by BMS345541 and NF-κB p65 siRNA plasmid significantly canceled the suppression of TLR9 by HPV11 infection. We demonstrated that TLR9 had a negative correlation with NF-κB signaling in HPV11.HaCaT cells.
TLR9 downregulation in HPV16 induced cervical cancer had been reported to be associated with NF-κB activation [2]. In this study, we demonstrated the HPV11 infection could inhibit expression of TLR9, but improve NF-κB activation, in accordance with the results from Hasan et al. This suggested the downregulation of TLR9 in HPV11.HaCaT cells was mediated by the NF-κB activation.
Expression of TLR9 in endothelial and epithelial cells had been reported [17]. TLR9 upstreams NF-κB signaling [2,17]. Human keratinocytes produces proinflammatory cytokines including interleukin (IL)-6, IL-8, MIP3α, interferon (IFN) to defense infections [17]. The infection of HPV16 E7 protein induced loss of TLR9 expression and prevented production of IL-8, IFN, and MIP3α [2]. Hasan et al. treated cervical cancer cell lines with NF-κB inhibitor or siRNA for IKKα or IKKβ and determined the recovery of TLR9 mRNA and protein. Thus they demonstrated the transcriptional inhibition of TLR9 depended on NF-κB activation [17]. In our study, we silenced expression of NF-κB p65 by siRNA and a chemical inhibitor of IκB kinase BMS345541 in HPV11.HaCaT cells. We found the similar results to Hasan et al. that expression of TLR9 mRNA and protein was rescued by siRNA or BMS345541 administration in HPV11.HaCaT cells. This showed that the mechanism of TLR9/NF-κB signaling in HPV11 induced CA procession might similar to that in HPV16+ cervical cancer. Nonetheless, we did not explore which HPV oncoprotein in charge of this recovery of TLR9 expression. We also did not investigate whether TLR9 recovery could influence cellular behavior of HPV11.HaCaT cells or HaCaT cells, such as cell proliferation and cell cycle. All those deletions would be carried out in the future studies to further demonstrate the role of TLR9 in CA.
Conclusion
In conclusion, in the present study we firstly confirmed the TLR9 downregulation in CAs tissues, and the mechanism of TLR9/NF-κB signaling in HPV11 induced CAs procession. Inhibition of NF-κB activation by p65 siRNA or IκB kinase inhibitor BMS345541 could rescue TLR9 expression in HPV11.HaCaT cells. These results can provide experimental and clinical basic for treatment of CA.
Disclosure of conflict of interest
None.
References
- 1.Zhou Q, Zhu K, Cheng H. Toll-like receptors in human papillomavirus infection. Arch Immunol Ther Exp (Warsz) 2013;61:203–215. doi: 10.1007/s00005-013-0220-7. [DOI] [PubMed] [Google Scholar]
- 2.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol (Baltimore, Md.: 1950) 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 3.Lai ZZ, Ni-Zhang , Pan XL, Song L. Toll-like receptor 9 (TLR9) gene polymorphisms associated with increased susceptibility of human papillomavirus-16 infection in patients with cervical cancer. J Int Med Res. 2013;41:1027–36. doi: 10.1177/0300060513483398. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, et al. Excess transmission of TP53 Arg72 variant in women with HPV16 and 18 related invasive cervical cancer. Cancer Research. 2008:68. [Google Scholar]
- 5.Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426:1246–1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Z, Zhang E, Yang D, Lu M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cell Mol Immunol. 2015;12:273–282. doi: 10.1038/cmi.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai ZZ, Ni-Zhang , Pan XL, Song L. Toll-like receptor 9 (TLR9) gene polymorphisms associated with increased susceptibility of human papillomavirus-16 infection in patients with cervical cancer. J Int Med Res. 2013;41:1027–1036. doi: 10.1177/0300060513483398. [DOI] [PubMed] [Google Scholar]
- 8.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 9.Weng H, Liu H, Deng Y, Xie Y, Shen G. Effects of high mobility group protein box 1 and toll like receptor 4 pathway on warts caused by human papillomavirus. Mol Med Rep. 2014;10:1765–71. doi: 10.3892/mmr.2014.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huimin S, et al. A comparative study of HPV infection in condyloma acuminate tissues of vulva, vagina and cervix. International Journal of Laboratory Medicine. 2016:2239–2241. [Google Scholar]
- 11.Hawkins MG, Winder DM, Ball SL, Vaughan K, Sonnex C, Stanley MA, Sterling JC, Goon PK. Detection of specific HPV subtypes responsible for the pathogenesis of condylomata acuminata. Virol J. 2013;10:137. doi: 10.1186/1743-422X-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacini L, Savini C, Ghittoni R, Saidj D, Lamartine J, Hasan UA, Accardi R, Tommasino M. Down-regulation of toll-like receptor 9 expression by beta human papillomavirus type 38 and implications for cell cycle control. J Virol. 2015;89:11396–405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhavathy D, Subramanian CK, Karunagaran D. Re-expression of HPV16 E2 in SiHa (human cervical cancer) cells potentiates NF-κB activation induced by TNF-α concurrently increasing senescence and survival. Biosci Rep. 2015:35. doi: 10.1042/BSR20140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Li X, Song S, Wang Y, Gu H. Integrity of a HPV11 infection cell model and identification of (-)-Epigallocatechin-3-gallate as a potential HPV11 inhibitor. Oncotarget. 2016;7:37092–37102. doi: 10.18632/oncotarget.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Li X, Song S, Wu J. Development of basal-like HaCaT keratinocytes containing the genome of human papillomavirus (HPV) type 11 for screening of anti-HPV effects. J Biomol Screen. 2014;19:1154–63. doi: 10.1177/1087057114536987. [DOI] [PubMed] [Google Scholar]
- 16.Weng H, Liu H, Deng Y, Xie Y, Shen G. Effects of high mobility group protein box 1 and toll like receptor 4 pathway on warts caused by human papillomavirus. Mol Med Rep. 2014;10:1765–1771. doi: 10.3892/mmr.2014.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M, Taylor-Papadimitriou J, Picard D, Sylla BS, Trinchieri G, Medzhitov R, Tommasino M. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J Exp Med. 2013;210:1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]


