Abstract
Acute pancreatitis (AP), a common disease, causes significant morbidity and mortality in clinical practice. Our objective of this study was to establish an experimental mouse AP model with cerulein treatment and to explore the susceptibility of mouse strains on the severity of pancreatic injury and the subsequent repair and regeneration. C57BL/6 and FVB/N mouse strains were used in this study. AP model was induced by six hourly intraperitoneal (i.p.) injections of cerulein dissolved in saline (100 μg/kg) administered on four consecutive days. Animals were sacrificed on 1, 3 and 7 days after last cerulein treatment, and then pancreas tissues were harvested and subjected to various histological, cellular and molecular analysis. Analyses of pancreatic injury and pancreatic amylase expression indicated that this cerulein-induced AP model was established successfully and that FVB/N mice showed more severe pancreatic injury and poor recovery compared to C57BL/6 strain. Analyses of myeloperoxidase (MPO), IL-1β and NF-κB showed that FVB/N strain exhibited more severe inflammation in the pancreas compared to C57BL/6 mice. Immunofluorescence analysis of activated caspase-3 and TUNEL assay indicated that the pancreas of FVB/N strain had more apoptosis compared to C57BL/6 mice. Analysis of Ki67 indicated FVB/N mice experienced more active proliferation compared to C57BL/6 strain. Collectively, these results demonstrated that there exists differential susceptibility on pancreatic injury and regeneration between FVB/N and C57BL/6 mice in the cerulein-induced AP.
Keywords: Apoptosis, cerulein-induced acute pancreatitis, inflammation, NF-κB, proliferation, strain susceptibility
Introduction
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases for hospitalization around the worldwide. Gallstones and alcohol are the two leading causes of AP [1]. The pathogenesis of AP is the inappropriate activation of trypsin inside pancreas, and thus inducing the self-digestion, edema, bleeding and even necrosis of pancreas [2]. In the past decades, we have witnessed the increasing prevalence of AP with the overall mortality about 5% [3,4]. According to the Atlanta Classification, the severity of AP is classified as mild, moderately severe and severe [5]. For severe AP, it is able to lead to multiple organ injuries, even death of individual [6,7], therefore, AP has been attended from both basic mechanisms and clinical therapy in the world.
Animal model is an ideal complement to study the mechanisms of human diseases. Several animal models of AP have been developed for various research purposes. These models include cerulein-induced AP, L-Arginine-induced AP, sodium taurocholate-induced AP, biliopancreatic duct ligation induced AP [8]. Cerulein-induced AP is a widely accepted AP model [9-12] due to its relatively easy performance. Another advantage of this model is that it not only allows us to study the pancreatic injury, but also facilitates the investigation of repair and regeneration after pancreatic injury [13]. Recent reports have adopted cerulein-induced pancreatitis model to examine the regeneration of pancreas [14,15].
Various animal models may be suitable for different research purposes. However, the results sometime appeared to be inconsistent for certain disease models due to the use of different animal background/strains. Differential effects of different mouse strains have been observed in a variety of animal models in several previous studies. For instance, Fox GB [16] found that, in traumatic brain injury model, three background mouse strains (C57BL/6, FVB/N, and 129/SvEMS) showed significantly different behavioral responses. In Moran’s study [17], it has been found that, four common inbred mouse strains (FVB/N, C57BL/6, BALB/c and C3H/He) exhibited different bone-regeneration ability following bone marrow ablation. Li et al. [18] studied platelets from five mouse strains (C57BL/6, FVB/N, BALB/c, C3H/He, and 129Sv) and found that platelets from FVB/N mice only expressed about half the amount of integrin2 as platelets from other mouse strains.
In this study, we established an optimal cerulein-induced AP mouse model using two different common mouse strains -- FVB/N and C57BL/6. The aim was to explore the effect of strain difference on severity of pancreatitis and the subsequent regeneration, which will provide critical and useful information for further mechanistic studies of AP.
Materials and methods
Animals
Wild type (WT) C57BL/6 and FVB/N mice were purchased from the Jackson laboratory and maintained in the animal core facility at the SUNY Upstate Medical University. Male and female mice (8-10 weeks old, weighing 20-25 g) were used in this study and were housed in the pathogen-free conditions. The protocol for this study (IACUC#410) has been approved by Institutional Animal Care and Use Committee at SUNY Upstate Medical University and meets the National Institutes of Health and ARRIVE guidelines on the use of laboratory animals.
Cerulein-induced acute pancreatitis (AP) model
In this study, AP was induced in 8-10 weeks old, sex-matched WT C57BL/6 and FVB/N mice, via six hourly i.p. injections of cerulein (Sigma-Aldrich, C9026) dissolved in 0.9% saline (100 μg/kg) administered on four consecutive days. Mice were sacrificed on different time points after last cerulein treatment, e.g. 1, 3 and 7 days, 6 mice per group, and then pancreas tissues were harvested for further analysis.
Histological analysis and pathological scoring method
Pancreas tissues were fixed in 10% formalin for 24 hours, then embedded in paraffin. About 4-μm sections from six mice for each group were stained with hematoxylin and eosin (H&E). These sections were evaluated under light microscope (Nikon ECLIPSE TE2000-U) by two experienced investigators who were blinded to the experiments. Pancreatic pathological score was based on edema, inflammation, vacuolization, and necrosis according to the method described by Schmidt et al. [19].
Immunofluorescence analysis
Paraffin-embedded slides were incubated at 60°C for 1 hour, de-paraffined with Xylene twice, then subjected to grade series of ethanol [100%, 95%, 90%, 80%, 70% and 50% ethanol]. Then slides were performed antigen retrieval with Tris-EDTA buffer (PH 9.0) at boiling point for 10 minutes. Permeabilization was performed with 0.02% Triton X-100 (Sigma-Aldrich, T9284). Slides were subsequently blocked with 10% donkey serum (Abcam, ab7475) for 1 hour at room temperature. Slides were incubated overnight with primary antibodies listed below and then were incubated with Alexa conjugated secondary antibodies at room temperature for 1 hour. Slides were mounted with DAPI containing mounting media (Abcam, ab104139) and stored at 4°C to protect from light. Incubation time of different primary antibodies was subjected to slight adjustment.
Antibodies used in immunofluorescence analysis include: Anti-Pancreatic alpha amylase antibody (1:200, Abcam, ab21156), Anti-Cleaved Caspase-3 antibody (1:200, Cell Signaling, #9661), Anti-Myeloperoxidase antibody (1:25, Abcam, ab9535), Donkey Anti-Rabbit IgG H&L Alexa Fluor 488 (1:200, Abcam, ab150073).
Immunohistochemical analysis
In brief, slides were de-paraffined, after antigen unmasking, blocked endogenous peroxidase activity in 3% H2O2 for 10 minutes as described previously [20]. After incubating with 10% donkey serum (Abcam, ab7475) for 1 hour at room temperature, the sections were incubated with anti-NF-κB antibody (1:200, Santa Cruz Biotechnology, SC-109), or anti-IL-1β antibody (1:100, Abcam, ab9722), or anti-Ki67 antibody (1:50, Santa Cruz Biotechnology, sc-7856) at 4°C overnight. Subsequently, biotinylated secondary antibody Goat Anti-Rabbit IgG (H+L)-HRP Conjugate (1:200, Bio-Rad Laboratories, #170-6515) or Rabbit Anti-Goat IgG (H+L)-HRP Conjugate (1:200, Bio-Rad Laboratories, #172-1034) was used for hybridization at room temperature for 1 hour. DAB was used to develop color in the sections as a chromogenic substrate. Then, the sections were counterstained with hematoxylin (Thermo Scientific, TA-125-MH). Finally, slides were dehydrated in a graded series of ethanol [100%, 80%, 70%] and twice in xylene.
TUNEL assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) is a method for detecting DNA fragmentation by labeling the 3’ - OH termini in the double-strand DNA breaks generated in the process of apoptosis. We used Click-iT Plus TUNEL Assay kit (Invitrogen, C10618) following the manufacturer’s instructions. In brief, slides were incubated at 60°C for 1 hour, then de-paraffined slides in Xylene and a graded series of ethanol [100%, 95%, 90%, 80%, 70% and 50%]. After fixing slides with 4% paraformaldehyde for 15 minutes, we performed permebilization with proteinase K. Then slides were incubated with TdT reaction buffer for 10 minutes at 37°C. Then the slides were incubated with TdT reaction mixture for 1 hour at 37°C. After washing slides with 3% BSA and 0.1% Triton X-100 in PBS, the slides were incubated with Click-It Plus TUNEL reaction cocktail for 30 minutes at 37°C and protected from light.
Statistical analysis
All the data were expressed as mean ± standard deviation. Statistical analysis was performed using the SPSS software (SPSS 17.0, Chicago, Ill). Statistical differences were assessed using student’s T tests. P value less than 0.05 was considered statistically significant.
Results
Characterization of cerulein-induced AP
Both strains showed acute pancreatic injury after cerulein treatment as described in the section of materials and methods. Compared to C57BL/6 strain, FVB/N mice displayed more severe injury on AP 1 d, 3 d, 7 d, respectively. There were severe lobules damage, neutrophil infiltration, acinar cell necrosis and fat cell necrosis in FVB/N mice on AP 1 d after 4-days cerulein treatment (Figure 1F), whereas C57BL/6 mice demonstrated milder inflammation at the time point (Figure 1B). C57BL/6 mice recovered quickly after cerulein treatment, and on AP 7 d, only mild inflammation could be observed in the pancreas of treated C57BL/6 mice (Figure 1D). But in FVB/N strain, there still existed injury of acinar cells on AP 7 d, interstitial leukocyte infiltration remained at this time point (Figure 1H). Analysis of pathological scores at each time point further demonstrated the statistical difference of pancreatic injury between C57BL/6 and FVB/V strains (Figure 1Q).
Figure 1.
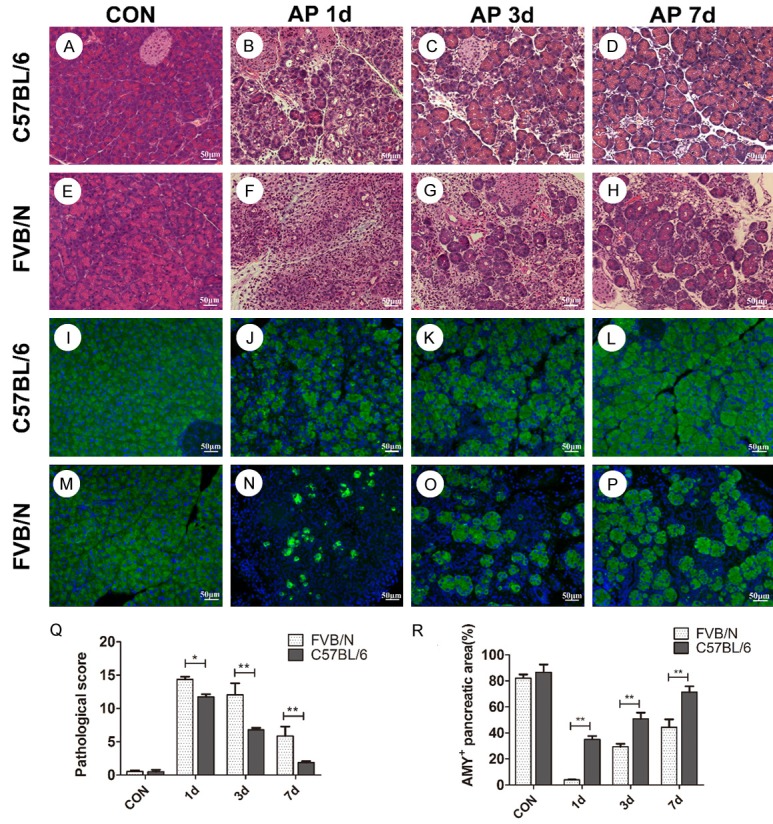
FVB/N mice showed more severe pancreatic injury compared to C57BL/6 mice. A-H. Pancreatic histology of control group and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; I-P. The expression of amylase of pancreatic tissues in control group and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; Q. Pancreas pathological scores of C57BL/6 and FVB/N strains; R. Statistical analysis of AMY+ pancreatic area (%) of C57BL/6 and FVB/N strains. **P<0.01, *P<0.05.
To assess acinar cell injury and recovery, the expression of amylase was examined using immunofluorescence assay. As shown in Figure 1I, 1M, both control groups showed normal expression of amylase. But treated FVB/N mice showed much less expression of amylase in pancreatic tissues compared to treated C57BL/6 mice on AP 1 d (Figure 1J, 1N, P<0.01). On AP 3 d and AP 7 d, there were still significant differences of the expression of amylase between the two strains (Figure 1K, 1L, 1O, 1P, P<0.01).
C57BL/6 mice showed less neutrophil infiltration than FVB/N strain
To investigate whether there was difference of neutrophil infiltration after pancreatitis between the two strains, we performed immunofluorescence to examine the expression of myeloperoxidase (MPO), which is a biomarker of neutrophil infiltration. As shown in Figure 2A, 2E, both C57BL/6 and FVB/N control groups showed rare expression of MPO. However, on AP 1 d, FVB/N mice showed intense expression of MPO in infiltrated neutrophils, which was higher than that of C57BL/6 strain (Figure 2B, 2F, P<0.01). The MPO+ cells decreased on AP 3 d in both strains, but there was significant difference of MPO+ cell counts between the two strains (Figure 2C, 2G, P<0.01). Both strains showed decreased MPO+ cells on AP 7 d, but there was significant difference between the two strains (Figure 2D, 2H, P<0.05).
Figure 2.
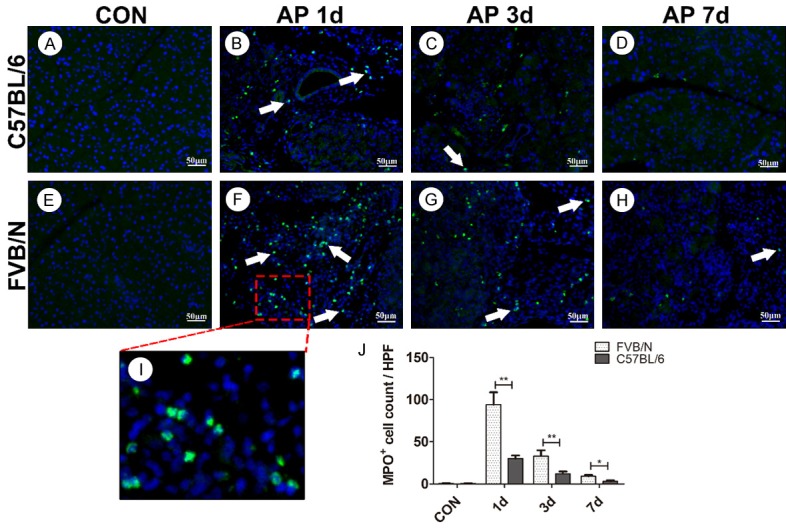
FVB/N mice showed more infiltration of neutrophils than C57BL/6 mice. A-H. Myeloperoxidase (MPO) expression in control group and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; I. Magnification of AP 1 d MPO expression of FVB/N strain; J. Statistical analysis of MPO+ cells of C57BL/6 and FVB/N strains. **P<0.01, *P<0.05.
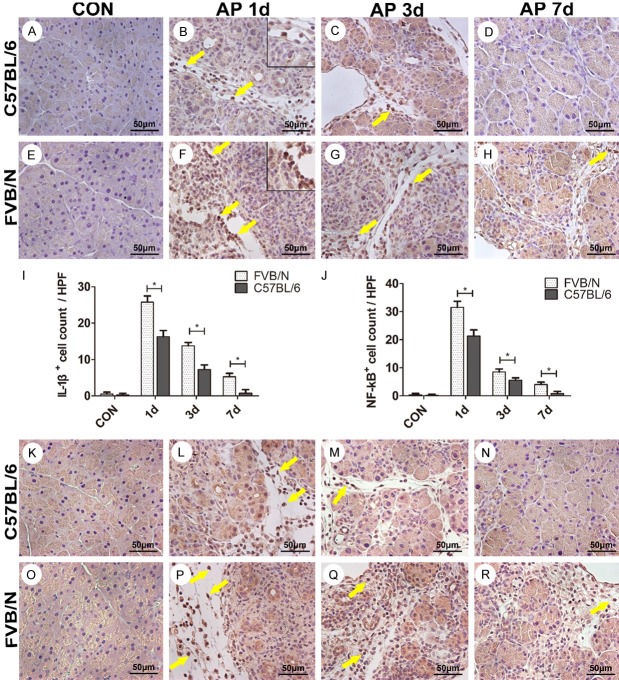
FVB/N mice showed more severe inflammatory response than C57BL/6 strain
Analysis of immunohistochemistry was used to evaluate the expression of pro-inflammatory cytokines in pancreatic tissues. As shown in Figure 3F, intense expression of pro-inflammatory cytokine IL-1β was observed in the FVB/N mouse pancreatic tissues on AP 1 d, whereas C57BL/6 strain exhibited a relatively lower expression of IL-1β at this time point (Figure 3B, 3F, P<0.05), thus indicating that FVB/N mice experienced more severe inflammatory response than C57BL/6 strain. On AP 3 d and AP 7 d, both strains showed less IL-1β+ cells, but there was still significant difference between the two strains (Figure 3C, 3D, 3G, 3H, P<0.05). Immunohistochemical analysis of nuclear expression of NF-κB further supported the results that FVB/N mice experienced more severe inflammation compared to C57BL/6 mice. As shown in Figure 3L, 3P, FVB/N mice exhibited intensive nuclear expression of NF-κB compared to C57BL/6 mice on AP 1 d. Both strains showed less nuclear NF-κB expression on AP 3 d and AP 7 d, respectively, but significant difference was observed between the two strains (Figure 3M, 3N, 3Q, 3R, P<0.05).
Figure 3.
FVB/N mice experienced more severe inflammatory response after cerulein treatment than C57BL/6 mice. A-H. IL-1β expression in control group and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; I. Statistical analysis of IL-1β+ cells of C57BL/6 and FVB/N mice; J. Statistical analysis of nuclear NF-κB+ cells of C57BL/6 and FVB/N strains. K-R. Expression of nuclear NF-κB in control group and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains. *P<0.05.
FVB/N mice showed more apoptotic cells than C57BL/6 strain
Caspase-3 plays an important role in cell apoptosis. In this study, little expression of activated caspase-3 by anti-cleaved caspase-3 antibody was observed in both control groups (Figure 4A, 4E), indicating rare apoptosis occurred in control groups, which was in consistent with our TUNEL assay that both control groups showed bare TUNEL+ cells (Figure 4I, 4M). On AP 1 d, FVB/N mice showed intense expression of activated caspase-3 in nucleus and cytoplasm of acinar cells and inflammatory cells (Figure 4F), and TUNEL+ cells increased significantly compared with C57BL/6 mice (Figure 4J, 4N, P<0.01). As shown in Figure 4C, 4G, 4K, 4O, on AP 3 d, FVB/N mice still showed higher level of the expression of activated caspase-3, as well as more TUNEL+ cells than C57BL/6 mice. On AP 7 d, both strains showed bare expression of activated caspase-3 (Figure 4D, 4H). TUNEL+ cells decreased in both strains on 7 days after cerulein treatment, but significant difference still existed between the two strains (Figure 4L, 4P, P<0.01).
Figure 4.
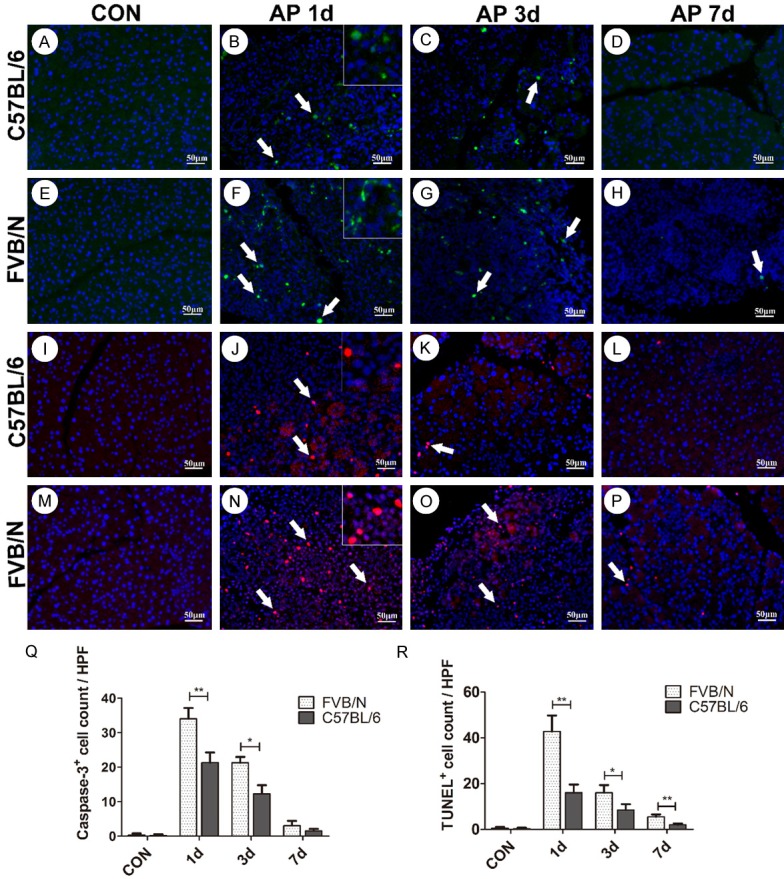
FVB/N mice showed more apoptosis after cerulein treatment than C57BL/6 mice. A-H. Activated caspase-3 expression in control and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; I-P. TUNEL levels in control and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; Q. Statistical analysis of activated caspase-3+ cells of C57BL/6 and FVB/N mice; R. Statistical analysis of TUNEL+ cells of C57BL/6 and FVB/N strains. **P<0.01, *P<0.05.
FVB/N mice exhibited more cellular proliferation compared to C57BL/6 strain
Ki67 is a nuclear protein and is used as a biomarker of cellular proliferation. In this study, both control groups showed little expression of Ki67 (Figure 5A, 5E), indicating rare cellular proliferation in control. On AP 1 d, both strains showed increased Ki67 expression compared to control groups, and FVB/N mice exhibited more Ki67+ cells compared to C57BL/6 mice (Figure 5B, 5F, P<0.05), suggesting increased cellular proliferation in treated FVB/N mice compared to C57BL/6 strain. On AP 3 d, both strains showed increased Ki67+ cells compared to AP 1 d groups, FVB/N mice exhibited more Ki67+ cells compared to C57BL/6 mice (Figure 5C, 5G, P<0.05). On AP 7 d, both strains showed decreased Ki67+ cells compared to AP 3 d groups, but there was still significant difference between the two strains (Figure 5D, 5H, P<0.05).
Figure 5.
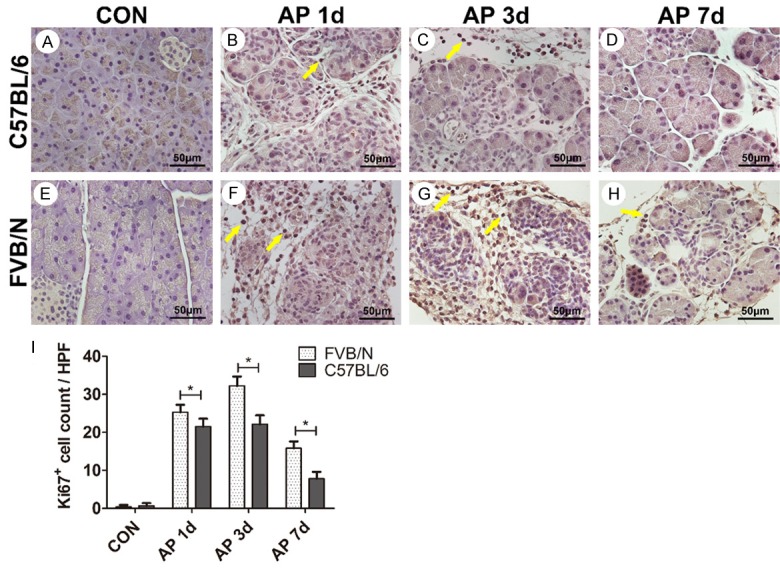
FVB/N mice showed more cellular proliferation than C57BL/6 mice. A-H. Ki67 expression in control and AP 1 d, 3 d, 7 d groups of C57BL/6 and FVB/N strains; I. Statistical analysis of Ki67+ cells of C57BL/6 and FVB/N strains. *P<0.05.
Discussion
Cerulein-induced pancreatic injury is an ideal model to study acute pancreatitis and the afterwards recovery process. Previous studies have tested different doses of cerulein and the time course of cerulein treatment. Besides these two factors, different strains of mice used in the experiments also exert much influence on the severity of pancreatitis. Wang et al. [21] demonstrated that mouse strains did influence the severity of pancreatitis induced by cerulein by using C57BL/6J, BALB/c, CBA/J, JF1 and C3H/HeJ mouse strains, but they did not include the FVB/N strain. C57BL/6 and FVB/N mice are two commonly used mouse strains in basic biology and preclinical study. We used C57BL/6 and FVB/N strains to establish a 4-days cerulein-induced AP model and intensively examined three time points i.e. 1 d, 3 d, 7 d-post injury, which allowed us to trace both the injury and recovery process after cerulein treatment. The important information will provide critical and solid base for further mechanistic study in the AP disease.
In this study, we induced AP by six i.p. injection of cerulein dissolved in 0.9% saline administered at hourly intervals on four consecutive days. The pathological analysis confirmed the successful establishment of cerulein-induced AP. In this work, compared to C57BL/6 mice, FVB/N strain showed more severe pancreatic injury after cerulein treatment. The difference of the pancreatic injury, which was further confirmed by pathological scoring, indicated that mouse strain is an important factor that influences the severity of AP.
During inflammation, neutrophils are recruited to sites of infection where they can recognize and eliminate pathogens through production of ROS (reactive oxygen species), releasing antimicrobial ingredients and formation of NET (neutrophil extracellular traps) [22]. MPO expressed in neutrophils is essential for the functions of neutrophils and plays an important role in the elimination of pathogens. However, excessive generation of MPO-derived oxidants may cause severe inflammation and subsequent tissue damage [23]. We observed FVB/N mice exhibited more MPO+ cell infiltration in the injured pancreatic areas compared to C57BL/6 mice, which is in consistent with our findings that FVB/N mice experienced more severe neutrophil infiltration than C57BL/6 strain based on the pathological analysis. These suggested that compared to C57BL/6 mice, FVB/N strain went through more severe inflammation and injury in our cerulein-induced pancreatitis model.
Nuclear factor κB (NF-κB) is an inducible dimeric transcription factor that belongs to NF-κB/Rel family [24]. It is an important intracellular messenger and regulates a large number of genes involved in cell growth, tissue development, and responses to stress and pathogen infection. There exists different opinions as to its role in inflammation [25]. Some suggested the protective role of NF-κB in acute inflammation [26], while the others considered its pro-inflammatory function. Yinon et al. [27] stated that although NF-κB is of much importance during stressful conditions and invasion of tissues by microorganisms, the overprotection may cause the host suffering. NF-κB has been proved to play an important role in the development of AP [28]. Huang et al. [29] used p65 transgenic mice and cerulein-induced pancreatitis model, and found that increased expression of NF-κB in acinar cells correlated with the severity of AP. Intra-ductal injection of RelA/p65 (Adp65) directly activated NF-κB and led to acinar cell injuries and neutrophil infiltration in pancreatic tissue [30]. Richard et al. [31] found that selective inhibition of NF-κB by NBD peptide which is a peptide that binds to the NF-κB essential modifier binding domain (NBD) attenuated the severity of cerulein-induced pancreatitis. In this work, immunohistochemical analysis of NF-κB demonstrated that FVB/N strain exhibited higher level of expression of nuclear NF-κB than C57BL/6 mice. Combined with the pathological analysis, it suggested a pro-inflammatory role of NF-κB in our cerulein-induced pancreatitis model.
IL-1β is a well-known pro-inflammatory cytokine and genes encoding IL-1β can be regulated by NF-κB [32]. At the same time IL-1β in turn plays critical role in the activation and regulation of canonical NF-κB pathway [33]. In this study, we observed the intense immunoactivity of IL-1β in the damaged areas of pancreatic tissues of FVB/N mice, which is much higher than that of C57BL/6 strain. These indicated more severe inflammation happened in FVB/N mice in our experimental conditions.
TUNEL staining has been considered generally as a standard method for the detection of DNA damage (DNA fragmentation or others). When it is combined with other specific methods of apoptotic assay, like activated caspase-3, it is more accurate for identifying apoptotic cells [34]. Caspase-3 is considered to play a central role in the induction of apoptosis [35]. In our AP model, FVB/N mice experienced more apoptosis compared to C57BL/6 strain, which is strongly supported by the data from both TUNEL and activated caspase-3 assays.
Antigen Ki-67 is a nuclear protein that is considered to be associated with cellular proliferation. Rankin et al. [36] demonstrated that in pancreatic duct ligation (PDL) induced pancreatitis, Ki67+ cells were significantly increased in tail pancreas at 7, 14, or 30 days compared to the controls. In our cerulein-induced AP model, FVB/N mice showed more cellular proliferation compared to C57BL/6 strain, indicating differential repair and regeneration between the two strains of mice.
Mouse model is an ideal tool to study cell and molecular mechanisms of human diseases. Accumulating clinical evidence suggested that the susceptibility and severity of pancreatic diseases including AP are associated with genetic background [37]. In this study, we characterized the difference of severity of AP and subsequent repair and recovery between C57BL/6 and FVB/N strains. We found that compared to C57BL/6 strain, FVB/N strain is more susceptible to AP under our experimental setup, and experienced a longer time to recover from the pancreatic injury, which suggested that FVB/N strain rather than C57BL/6, may be a better choice for us to study the injury and regeneration process after cerulein-induced pancreatitis.
Acknowledgements
This work was supported by NIH HL096007 and HL136706 and Herdricks foundation (to G.W.). X.Z. is as a joint graduate student between Shandong University and SUNY Upstate Medical University.
Disclosure of conflict of interest
None.
References
- 1.Kui B, Balla Z, Vasas B, Vegh ET, Pallagi P, Kormanyos ES, Venglovecz V, Ivanyi B, Takacs T, Hegyi P, Rakonczay ZJ. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS One. 2015;10:e0117588. doi: 10.1371/journal.pone.0117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 3.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afghani E, Pandol SJ, Shimosegawa T, Sutton R, Wu BU, Vege SS, Gorelick F, Hirota M, Windsor J, Lo SK, Freeman ML, Lerch MM, Tsuji Y, Melmed GY, Wassef W, Mayerle J. Acute pancreatitis-progress and challenges: a report on an international symposium. Pancreas. 2015;44:1195–1210. doi: 10.1097/MPA.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Liao K, Zhao K, Wang W, Zuo T, Deng W, Chen C, Yu J, Guo W, He X, Abliz A, Wang P, Zhao L. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators Inflamm. 2015;2015:685043. doi: 10.1155/2015/685043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Q, Chen C, Deng W, Wang P, Zuo T, Zhao L, Yu J, Zhao K, Mei F, Li C, Wang G, Wang W. Hydrogenrich saline attenuates acute hepatic injury in acute necrotizing pancreatitis by inhibiting inflammation and apoptosis, involving JNK and p38 mitogen-activated protein kinase-dependent reactive oxygen species. Pancreas. 2016;45:1424–1431. doi: 10.1097/MPA.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J. Animal models of pancreatitis: can it be translated to human pain study? World J Gastroenterol. 2013;19:7222–7230. doi: 10.3748/wjg.v19.i42.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SB, Bae GS, Jo IJ, Seo SH, Kim DG, Shin JY, Hong SH, Choi BM, Park SH, Song HJ, Park SJ. Protective effects of lithospermum erythrorhizon against cerulein-induced acute pancreatitis. Pancreas. 2015;44:31–40. doi: 10.1097/MPA.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, Takeda K, Hozawa S, Yoshimura A, Hibi T. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010–1020. doi: 10.1053/j.gastro.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 11.Sledzinski M, Borkowska A, Sielicka-Dudzin A, Halon M, Wozniak M, Spodnik JH, Antosiewicz AH, Antosiewicz J. Cerulein-induced acute pancreatitis is associated with c-Jun NH(2)-terminal kinase 1-dependent ferritin degradation and iron-dependent free radicals formation. Pancreas. 2013;42:1070–1077. doi: 10.1097/MPA.0b013e318287d097. [DOI] [PubMed] [Google Scholar]
- 12.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, Bartlett DL, Whitcomb DC, Chang EB, Zhu X, Wang H, Lu B, Tracey KJ, Cao L, Fan X, Lotze MT, Zeh HJ, Tang D. Intracellular Hmgb1 Inhibits Inflammatory Nucleosome Release and Limits Acute Pancreatitis in Mice. Gastroenterology. 2014;146:1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB. 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausmann S, Regel I, Steiger K, Wagner N, Thorwirth M, Schlitter AM, Esposito I, Michalski CW, Friess H, Kleeff J, Erkan M. Loss of periostin results in impaired regeneration and pancreatic atrophy after cerulein-induced pancreatitis. Am J Pathol. 2016;186:24–31. doi: 10.1016/j.ajpath.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Saponara E, Grabliauskaite K, Bombardo M, Buzzi R, Silva AB, Malagola E, Tian Y, Hehl AB, Schraner EM, Seleznik GM, Zabel A, Reding T, Sonda S, Graf R. Serotonin promotes acinar dedifferentiation following pancreatitis-induced regeneration in the adult pancreas. J Pathol. 2015;237:495–507. doi: 10.1002/path.4595. [DOI] [PubMed] [Google Scholar]
- 16.Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- 17.Moran MM, Virdi AS, Sena K, Mazzone SR, McNulty MA, Sumner DR. Intramembranous bone regeneration differs among common inbred mouse strains following marrow ablation. J Orthop Res. 2015;33:1374–1381. doi: 10.1002/jor.22901. [DOI] [PubMed] [Google Scholar]
- 18.Li TT, Larrucea S, Souza S, Leal SM, Lopez JA, Rubin EM, Nieswandt B, Bray PF. Genetic variation responsible for mouse strain differences in integrin alpha 2 expression is associated with altered platelet responses to collagen. Blood. 2004;103:3396–3402. doi: 10.1182/blood-2003-10-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Shi Q, Liu J, Abdel-Razek O, Xu Y, Cooney RN, Wang G. Innate immune molecule surfactant protein d attenuates sepsis-induced acute pancreatic injury through modulating apoptosis and NF-κB-mediated inflammation. Sci Rep. 2015;5:17798. doi: 10.1038/srep17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Ohmuraya M, Suyama K, Hirota M, Ozaki N, Baba H, Nakagata N, Araki K, Yamamura K. Relationship of strain-dependent susceptibility to experimentally induced acute pancreatitis with regulation of Prss1 and Spink3 expression. Lab Invest. 2010;90:654–664. doi: 10.1038/labinvest.2010.44. [DOI] [PubMed] [Google Scholar]
- 22.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 24.Bhanot UK, Moller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest. 2009;89:489–497. doi: 10.1038/labinvest.2009.19. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Liu Z. A special issue on NF-κB signaling and function. Cell Res. 2011;21:1–2. doi: 10.1038/cr.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Neriah Y, Schmitz ML. Of mice and men: meeting on the biology and pathology of NF-κB. EMBO Rep. 2004;5:668–673. doi: 10.1038/sj.embor.7400187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakonczay Z, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-κB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Liu Y, Daniluk J, Gaiser S, Chu J, Wang H, Li ZS, Logsdon CD, Ji B. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144:202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-κB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 31.Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Karin M. Missing pieces in the NFkappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loo DT. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Methods Mol Biol. 2011;682:3–13. doi: 10.1007/978-1-60327-409-8_1. [DOI] [PubMed] [Google Scholar]
- 35.Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- 36.Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. Beta-cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62:1634–1645. doi: 10.2337/db12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitcomb DC. Value of genetic testing in the management of pancreatitis. Gut. 2004;53:1710–1717. doi: 10.1136/gut.2003.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]