Abstract
The aim of this study was to determine whether vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) promoted the mobilization and recruitment of endothelial progenitor cells (EPCs) to protect kidneys from ischemia and reperfusion injury (IRI) in male rats. At 24 h and 72 h after reperfusion, serum samples were respectively collected for renal function. Besides, kidney tissues were harvested to observe renal morphology changes. Subsequently, VEGF, Ang-1 and angiopoietin-2 (Ang-2) expression levels in different groups were measured at the indicated time points after reperfusion. Compared with IRI-operated group, rats that were intervened with EPCs significantly reduced in the levels of blood urea nitrogen, serum creatinine at 24 hours and 72 hours, particularly in injecting EPCs suspension liquid transfected by VEGF165-adenovirus and Ang-1-adenovirus. At 72 hours after reperfusion, renal function and morphology were exhibited significant improvements in two EPCs-transfected VEGF165-adenovirus and Ang-1-adenovirus groups. In addition, expression levels of VEGF, Ang-1 and Ang-2 in the kidneys of EPCs-treated rats which were transfected by VEGF165-adenovirus and Ang-1-adenovirus were markedly increased compared to rats subjected to IRI. The present work suggested that VEGF and Ang-1 might play important roles in the protective effect of homing of EPCs on renal acute IRI.
Keywords: Endothelial progenitor cells, ischemia and reperfusion injury, vascular endothelial growth factor
Introduction
Ischemia and reperfusion injury (IRI) is a major cause of acute organ dysfunction, and IRI related acute kidney injury (AKI) is a common clinical problem with high mortality and morbidity [1,2]. In addition, the pathogenesis of renal IRI may be mediated by multiple mechanisms including endothelial dysfunction, systemic and local inflammatory responses, oxidative stress, apoptosis and necrosis [3,4]. However, the exact etiology and mechanism of renal IRI is still unclear. Despite the increased awareness of the pathogenesis mechanism of AKI, AKI remains without any effective treatment. Therefore, there is an appropriate and reasonable period of time to implement preventive measures.
Previous studies have confirmed that the concept of ischemic preconditioning (IPC), defined as a well-established phenomenon in which brief exposure to sublethal episodes of ischemia and reperfusion induces a tolerance to injurious effects of prolonged ischemia by exploiting intrinsic defense mechanisms [5], is an effective approach to minimize subsequent events of IRI [6,7]. In addition, reducing inflammation and enhancing the mobilization and recruitment of EPCs have been proven to have protective effects in kidney IRI [8-10]. This phenomenon has been initially described for the heart by Murry et al. [11] and Ambros et al. [12]. Although the renoproctective effects of IPC have been successfully validated, the underlying mechanisms remain unknown, which limited the clinical application of EPCs to a certain extent [13].
In the last decade, many articles have reported the therapeutic effects of EPCs on tissue wound, heart infarction, stroke, and IRI [14-17]. It has been suggested that the therapeutic effects of EPCs are likely affected by pathological and physiological conditions of the body [18,19]. Interestingly, mobilization of EPCs from their niches was associated with improvement in renal function following IRI, due to ischemic stress generating specific cell factors. Furthermore, EPCs can be efficiently transplanted to ischemic tissues, preserving or restoring organs by participating in enhanced repair of renal microvasculature, tubule epithelial cells and synthesis of high-levels of pro-angiogenic cytokines. Moreover, lack of EPCs is likely to accelerate vascular injury and eventually lead to renal insufficiency [20]. Therefore, the ability to sufficiently increase the number of EPCs might become a reasonable and important method to be applied for the treatment of renal IRI.
Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen and a secreted dimeric protein, and as such can induce angiogenesis in a variety of ways [21-23]. Of all the known angiogenic molecules, VEGF is probably the primary angiogenic growth factor and a best-characterized modulator in vessel patterning that responsible for angioblast differentiation and tube formation. Furthermore, the role of VEGF in angiogenesis is crucial for the development in ischemia and reperfusion of tissue, as well as for tumor formation [24-26]. In addition, angiopoietins do not belong to the VEGF family, but are also key mediators in angiogenesis. Meanwhile, these are oligmeric glycoproteins that bind to the endothelial cell-specific tyrosine kinase receptor, namely the tyrosine kinase with immunoglobulin-like and EGF-like domains TIE-2 receptor [27]. As a main ligand of TIE-2 receptors, angiopoietin-1 (Ang-1) is constitutively expressed on blood vessels and acts as a regulator of blood vessel maturation. Thus, Ang-1 and VEGF are thought to have a complementary effect on blood vessel growth [28].
A renal IRI model was used to investigate whether homing of EPCs enhanced the release of protective cytokines, including VEGF, Ang-1 and angiopoietin-2 (Ang-2) in ischemic kidney, with the goal of directly preserving the microcirculation in the IRI by incorporation of EPCs into vascular structures. Moreover, it appeared logical to determine whether EPCs could protect the remaining renal tissue following IRI through the mechanism described above. Therefore, the present study aimed to examine that VEGF and Ang-1 mediated the mobilization and recruitment of EPCs to protect renal function in an experimental model of IRI.
Materials and methods
Ethics statement and animals
All adult male Sprague-Dawley (SD) rats, weighing 200-240 g, were obtained from the Center for Experimental Animals at Southeast University Medical College (Nanjing, Jiangsu, China). Animals were maintained in our Experimental Animal Center with conventional housing conditions at 24±2°C and a controlled 12-hours light/dark cycle under a pathogen-free condition. Besides, drinking water and rodent chow diet were free intake during this experiment. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures conducted in experimental animals and the protocols were approved by the Committee on the Ethics of Animal Research in Animal Care Facility of Nanjing Medical University (Nanjing, Jiangsu, China).
Rat model and surgical procedure
All male SD rats were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg) and placed on a warming table to maintain a rectal temperature of 37°C. The surgical site was epilated, disinfected with betadine, and a midline laparotomy was made using micro-scissors. Besides, we removed the right kidney, and separated the left renal artery that was subjected to IRI after only 3 days.
These rats were randomly divided into five groups: (1) Sham-operated group (n=10), that only underwent the surgical procedure (without the clamping of the renal artery); (2) IRI-operated group (n=10), their arteries were occluded with a nontraumatic vascular clamp for 45 minutes when the kidney was kept warm and moist during the observation period; (3) EPCs-treated group (n=10), rats were injected EPCs suspension liquid (1×106) from the femoral vein of rats, after the establishment of renal IRI model; (4) EPCs-transfected VEGF165-adenovirus group (n=10), rats were injected EPCs suspension liquid (1×106) which was transfected by recombined adeno-associated viral vector encoding VEGF165 from the femoral vein of rats after the operation method of IRI-operated group; and (5) EPCs-transfected Ang-1-adenovirus group (n=10), same as EPCs-transfected VEGF165-adenovirus group but which was transfected by recombined Ad-Ang-1. Subsequently, the surgical incision was closed with 6-0 monofilament sutures and a synthetic absorbable surgical tissue adhesive (Tissuemend II SC; Veterinary Product Laboratory, Phoenix, AZ, USA), and analgesic buprenorphine (0.1 mg/kg) was subcutaneously administrated. These rats were monitored until recovery in a chamber on a heating pad. Besides, animals were maintained for 3 days on the normal chow diet following surgery.
Cell culture and characterization of EPCs
Male adult SD rats (weight ranges from 200 to 240 g) were sacrificed by injection of an overdose of sodium pentobarbital (100 mg/kg) into the peritoneal cavity in this experiment. EPCs were generated from bone marrow mononuclear cells (MNCs). In brief, bone marrow samples were flushed out with phosphate-buffered saline (PBS) (Gibco, Grand Island, USA) from tibias and femurs. MNCs were isolated by density gradient centrifuge method by 2500 rpm for 30 min. Next, MNCs isolated from rats were counted and plated on 6-well plates precoated with 0.2 mg/ml human plasma fibronectin (EMD Millipore Corporation, Billerica, USA) and maintained at 37°C under an atmosphere containing 5% CO2. Meanwhile, MNCs were grown in endothelial cell basal medium-2 (EBM-2) (Clonetics, Lonza, Walkersville, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, USA), containing EPCs growth cytokine cocktail (Lonza, Walkersville, USA). After 5-day culture in wells not coated with biomaterial membranes, non-adherent cells were removed by washing with PBS. Thereafter, culture medium was changed every 2-3 days. During culture, an inverted phase contrast microscope (IX70-81FZ, Olympus Corporation, Tokyo, Japan) was used to observe the EPCs morphology and growth in vitro. When the confluence of cells reached 70%-80%, they were treated with 0.25% trypsin-EDTA for subculturing, and seeded at a ratio of 1:2 with EGM-2. EPCs after one passage were used for this experiment.
To identify the identities of EPCs, the cells were incubated with Dil-acetylated-low density lipoprotein (10 mg/ml) (Dil-ac-LDL; Molecular Probes, Thermo Fisher Scientific, Inc., USA) for 6 h and then fixed with 4% paraformaldehyde for 15 min, incubated with fluorescein isothiocyanate (FITC)-Ulex europaeus agglutinin (UEA)-1 (10 mg/ml) (FITC-UEA-1; Sigma-Aldrich, USA) for 1 h and examined under a laser confocal scanning microscope (Leica, Wetzlar, Germany) for observation, differentiation and identification. In addition, cells that were double positive for Dil-ac-LDL and FITC-UEA-1 were identified as EPCs by dual staining for fluorescently. Approximately 85% of cells were positive for both markers.
Construction of VEGF165-adenovirus and Ang-1-adenovirus
After 10 days in cell culture, EPCs were transfected with a recombinant adenovirus encoding VEGF165 gene (Ad-VEGF165) and Ang-1-adenovirus (Ad-Ang-1). To establish the appropriate virus concentration for adenoviral gene transfer into EPCs, the effectiveness of different multiplicities of infection was evaluated in accordance with the instructions of the adenovirus manufacturer. According to previously published methods [29], EPCs were washed with PBS before transfection with Ad-VEGF165 and Ad-Ang-1 (at multiplicity of infection of 50) for 2 hours. After transfection, the viruses were removed, and the cells were washed with PBS. Besides, EPCs were incubated with fresh medium for another 72 h before subsequent experiments. The expression of the transfected genes in transfected EPCs were further confirmed by Western blotting.
Serum levels of BUN and Scr
Briefly, the blood drawn from fundus venous plexus after reperfusion for 24 hours and from the inferior vena cava at 72 hours after reperfusion were collected respectively, and centrifuged at 3,000×g for 10 minutes for equal volumes of supernate to be stored at -80°C to harvest the sera. In addition, the serum urea nitrogen (BUN), serum creatinine (Scr) levels were measured using clinical automated analysis (Hitachi 7020, Hitachi High-Technologies Corporation, Tokyo, Japan).
Histological examination
One half of each harvested renal tissue was fixed with 10% formaldehyde for 24 hours and embedded in paraffin blocks, sectioned at 4-5 μm thickness. After gradual deparaffinizing and hydration, the chosen transverse sections from each sample were examined using hematoxylineosin staining. All histomorphological analyses described below were performed in blinded fashion. Then the sections were examined under light microscopy (Olympus BX-51, Olympus, Tokyo, Japan) to evaluate structural changes in tubular necrosis, tubular dilatation and atrophy, vacuolization, inflammatory cell infiltration, MNCs infiltration, capillary dilatation, interstitial structural changes, renal corpuscle morphology, or cellular edema. We assessed histomorphological injury scoring using a previously described semiquantitative method based on a scale of 0 to 4 with higher values representing more severe damage as follows: 0, normal kidney; 1, minimal necrosis (25% involvement of the cortex or outermedulla); 2, mild necrosis (25%-50% involvement of the cortex or medulla); 3, moderate necrosis (50%-75% involvement of the cortex or medulla; and 4, severe necrosis (75% involvement of the cortex or medulla) [30,31].
Estimation of the recruitment of EPCs by immunohistochemical staining
Renal tissue sections were dewaxed, rehydrated, and washed 3 times for 5 minutes PBS. After slides were microwaved for 20 min, and allowed to cool for 1 h at room temperature, endogenous peroxidase activity was blocked in all sections by incubating the sections in 3% H2O2 for 15 minutes. The sections were incubated with rabbit anti-rat CD31 and CD34 antibody (Abbiotec, San Diego, USA) overnight at 4°C. Next day, the slides were washed and incubated with a horseradish peroxidase (HRP)-conjugated anti-goat secondary antibody (Abbiotec, San Diego, USA) at a 1:100 dilution for 1 hour. After stained by DAB, the sections were observed under light microscopy.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from rat kidney samples with the TRIZOL Reagent (Ambion, Austin, TX). RNA to cDNA from 1 μg of total RNA was reverse-transcribed in a final volume of 10 μL using random primers and a Reverse Transcription Kit (Takara Biotechnology Co. Ltd., Dalian, China). The reverse transcription was performed at 37°C for 15 min, then 85°C for 5 s. All operations were performed according to the manufacturer’s instructions. Real-time PCR (RT-PCR) analyses were used as template using a standard protocol from Power SYBR Green (Takara Biotechnology Co. Ltd., Dalian, China) on an ABI StepOne Plus instrument (Applied Biosystems, Carlsbad, CA, USA) and in a total reaction volume of 10 μL, including 5 μL of SYBR Premix (2×), 0.4 μL of PCR forward primer (10 μM), 0.4 μL of PCR reverse primer (10 μM), 0.2 μL ROX Reference Dye II (50×), 1 μL of cDNA, 3 μL of diethy pyrocarbonate. The qRT-PCR cycle profile was 95°C for 10 min, followed by 40 cycles of 15 s at 95°C with a denaturation temperature, 30 s at annealing temperatures of 60°C, and 10 s at 72°C for a final extension. In addition, each experiment was asked to repeat three times.
The PCR primers were as follows: VEGF: Forward: 5’-GTCCAATTGAGACCCTGGTG-3’, Reverse: 5’-CTATGTGCTGGCTTTGGTGA-3’; Ang-1: Forward: 5’-GGAGCATGTGATGGAAAATTA-3’, Reverse: 5’-TGTGTTTTCCCTCCATTTCTA-3’; Ang-2: Forward: 5’-AAAGAGTA CAAAGAGGGCTTC-3’, Reverse: 5’-TCCAGTAGTACCACTTGATAC-3’; β-actin: Forward: 5’-ACTGGAACGGTGAAGGTGAC-3’, Reverse: 5’-AGAGAAGTGGGGTGGCTTTT-3’.
Detection of VEGF, Ang-1 and Ang-2 by western blot analysis
The proteins of freshly obtained kidney tissues were extracted according to the manufacturer’s instructions. Protein extracts separated upon 7.5% SDS-PAGE were transferred to 0.45 μm PVDF membrane (Bio-Rad, California, Hercules, USA). The membranes were blocked with 5% low fat milk powder in TBST (20 mM Tris-HCL, pH 7.5, 150 mM NaCl, 0.1% Tween 20) before western blotting overnight at 4°C with rabbit polyclonal antibodies against mouse VEGF, Ang-1 and Ang-2. After 1 hour incubation with HRP-conjugated secondary antibody, immunoreactive bands were visualized with electrochemiluminescence reagent (Amersham, Uppsala, Sweden). Densitometric and ImageQuant analysis were subsequently performed using NIH Image software (Bethesda, Md, USA).
Statistical analysis
Statistical analyses were performed using analysis of variance (ANOVA), followed by the Student-Newman-Keuls test. All data were expressed as mean values standard deviation (SD). The differences were evaluated using SPSS 22.0 (Armonk, New York, USA). Each experiment was performed at least 3 times with significant differences accepted at P<0.05.
Results
Kidney and body weight
As was shown in Table 1, body weight and kidney weight of these rats didn’t differ between the five groups. The ratio of renal weight/body weight was increased in three EPCs transplantation groups compared to IRI-operated group, particularly in two EPCs-transfected VEGF165-adenovirus and Ang-1-adenovirus groups, but was not statistically significant. Gross morphology of the kidneys was normal.
Table 1.
Effect of EPCs transplantion on body weight and kidney weight in an IRI rat model
| Group | Body weight (g) | Kidney weight (mg) | Renal weight indices (mg/g) |
|---|---|---|---|
| Sham-operated group | 230±14.3 | 1065±29.48 | 4.64±0.30 |
| IRI-operated group | 242±13.5 | 1077±29.22 | 4.47±0.19 |
| EPCs-treated group | 236±15.1 | 1073±24.89 | 4.56±0.30 |
| EPCs-transfected VEGF165-adenovirus group | 233±11.2 | 1073±22.48 | 4.61±0.21 |
| EPCs-transfected Ang-1-adenovirus group | 234±11.2 | 1074±25.36 | 4.60±0.28 |
Notes: Data shown as mean ± standard deviation.
Renal function parameters
Compared with Sham-operated group, the blood serum BUN and Scr levels were significantly increased in other groups. In addition, the serum BUN and Scr levels were obviously decreased among using EPCs transplantation groups relative to IRI-operated group (Figure 1), particularly in EPCs-transfected VEGF165-adenovirus group and EPCs-transfected Ang-1-adenovirus group (P<0.05).
Figure 1.
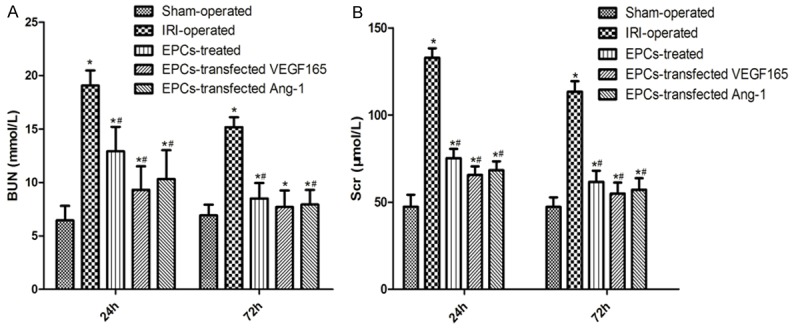
Effect of EPCs transplantation on renal function of different groups. A: Blood urine nitrogen (BUN, mmol/L). B: Serum creatinine (Scr, μmol/L). Columns represent mean ± SD. *Significant difference vs. Sham group (P<0.05); #Significant difference vs. IRI group (P<0.05).
Renal morphology
As was shown in Figure 2, EPCs transplantation groups significantly attenuated ischemic tubulointerstitial abnormalities and displayed moderate to severe ischemia with characteristic tubulointerstitial lesions at 72 hours following reperfusion (P<0.05). Light microscopic examination identified acute tubular necrosis in the IRI-operated group in the form of marked dilatation and atrophy, massive epithelial cells, atrophic epithelial lining, pyknotic nuclei, intraluminal necrotic debris, tubule cast formation, and congestion in the peritubular capillaries. However, EPCs-transfected VEGF165 group and EPCs-transfected Ang-1 group obviously improved the IRI-induced histological alterations as observed by number of tubule/unit area (P<0.05). Hence, our results indicated that VEGF and Ang-1 reduced renal inflammatory intensity, protecting the kidney from IRI to some extent. Meanwhile, at 72 hours after operation, VEGF and Ang-1 might promote mobilization and recruitment of EPCs, which obviously mitigated the ischemic damage.
Figure 2.
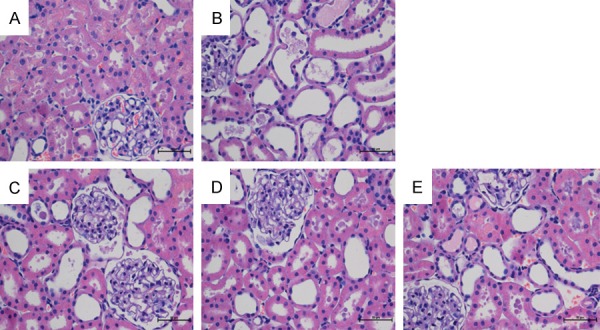
Effect of EPCs transplantation on renal morphology at 72 h following reperfusion. Renal sections were stained with hematoxylin and eosin and examined using light microscopy at a magnification ×400. A: Sham-operated rats exhibited minimal pathological changes in the kidneys. B: Following IRI, more severe lesions were observed in renal tubules, with tubular atrophy, dilatation, and intratubular casts, as well as congestion in the peritubular capillaries, massive epithelial cells and atrophic epithelial lining. C: EPCs suspension liquid caused a significant reduction in the severity of acute tubular necrosis. D: Degree of renal injury was clearly slighter than in the EPCs-treated group. E: Degree of renal injury was markedly slightest compared to rats subjected to IRI.
EPCs mobilization
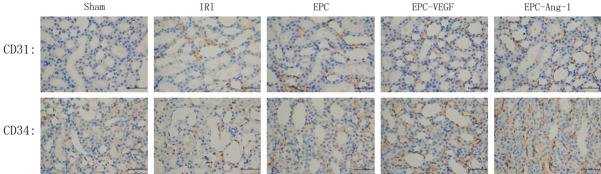
The expressions of CD31 and CD34 in the kidney were examined using immunohistochemical analysis. High levels of CD31 and CD34 expression were detected in three EPCs transplantation groups, whereas there were low levels in Sham-operated group. At 72 hours after the operation, the expression levels of CD31 and CD34 in these EPCs transplantation groups were significantly increased relative to IRI-operated group, especially in EPCs-transfected VEGF165-adenovirus and Ang-1-adenovirus groups (P<0.05; Figure 3). Moreover, positive staining of CD31 and CD34 cells, which primarily accumulated in the renal medullary interstitium, was distributed in the tubulointerstitium after injecting EPCs suspension liquid which was transfected by recombined adenoviral vector encoding VEGF165 and Ang-1. Collectively, these results suggested that VEGF and Ang-1 promoted the homing of CD31 and CD34 cells in the recruitment of EPCs on renal IRI rats.
Figure 3.
Immunohistochemical staining for CD31 and CD34 at 72 h after reperfusion (×400). CD31 and CD34 expression levels were decreased in IRI-operated group compared with the EPCs-treated group, EPCs-transfected VEGF165-adenovirus group and EPCs-transfected Ang-1-adenovirus group. Data are shown as mean ± SD. *Significant difference vs. Sham group (P<0.05); #Significant difference vs. IRI-operated group (P<0.05).
Expression of VEGF and Ang-1 in EPCs
To further address the functions of VEGF and Ang-1 in EPCs, we infected EPCs and selected stably infected cells. The over-expressed cell lines were respectively named as EPCs-VEGF and EPCs-Ang-1, while the matched control cell lines were named as EPCs-NC. Expression levels of VEGF and Ang-1 in EPCs were further examined by both Western blot (P<0.05; Figure 4A, 4C) and qRT-PCR (P<0.05; Figure 4B, 4D).
Figure 4.
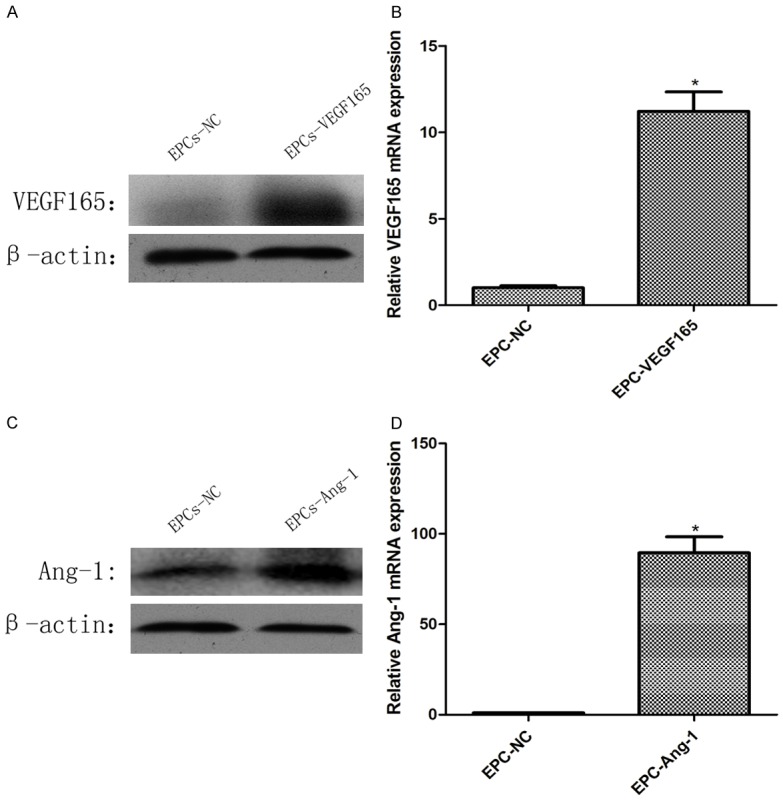
Expression of VEGF and Ang-1 in EPCs after transfection of recombinant adenovirus. VEGF expression levels in western blotting (A) and qRT-PCR (B), Ang-1 expression levels in western blotting (C) and qRT-PCR (D).
mRNA expression of angiogenic factors
qRT-PCR was used to investigate the levels of mRNA of angiogenic factors in the kidney. VEGF mRNA expression was significantly higher in three EPCs transplantation groups compared with the other groups following reperfusion (P<0.05). When investigating mRNA levels of Ang-1 and Ang-2, a significantly increased Ang-1 and Ang-2 expression was observed in EPCs transplantation groups at 72 h after reperfusion compared to the Sham-operated group and IRI-operated group (P<0.05). In particular, VEGF, Ang-1 and Ang-2 mRNA expression levels were more abundant in EPCs-transfected VEGF165-adenovirus group and EPCs-transfected Ang-1-adenovirus group, compared to EPCs-treated group at 72 h after reperfusion (P<0.05) (Figure 5).
Figure 5.
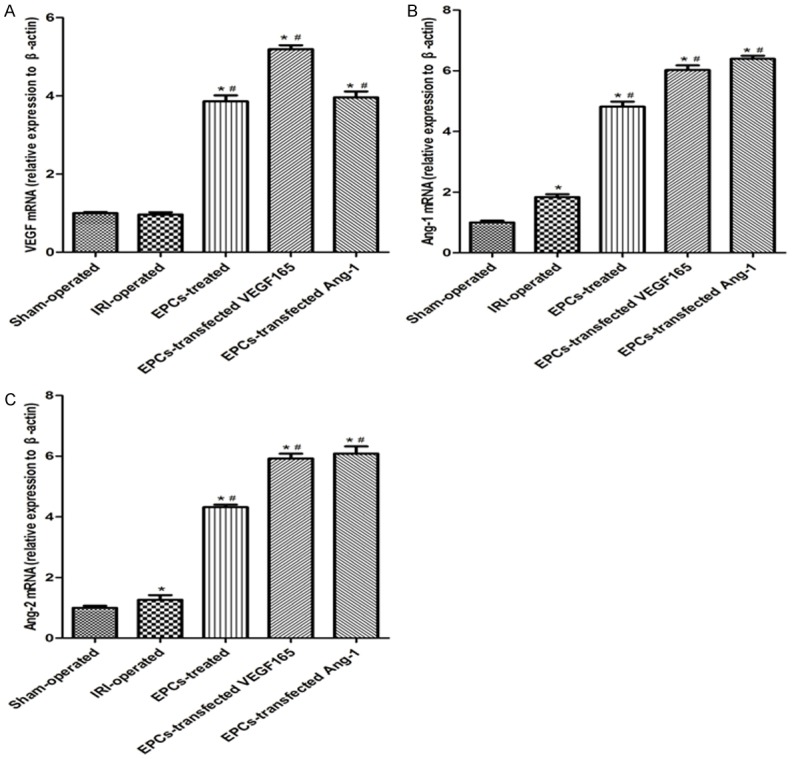
Effect of EPCs transplantation on expression of mRNA levels in different groups. A-C: mRNA expression levels of VEGF, Ang-1 and Ang-2 in different groups. Data are expressed as mean ± SD. *significant difference vs. Sham group (P<0.05); #significant difference vs. IRI group (P<0.05).
Angiogenic factor protein expression
Expression levels of VEGF, Ang-1 and Ang-2 proteins were analyzed using western blotting at 72 hours after the operation. As was shown in Figure 6, the expression levels of VEGF, Ang-1 and Ang-2 in the kidneys of two EPCs-transfected adenovirus groups were dramatically increased compared with IRI-operated group (P<0.05). In consequence, the results indicated that VEGF and Ang-1 might mediate the mobilization and recruitment of EPCs to protect kidneys from IRI.
Figure 6.
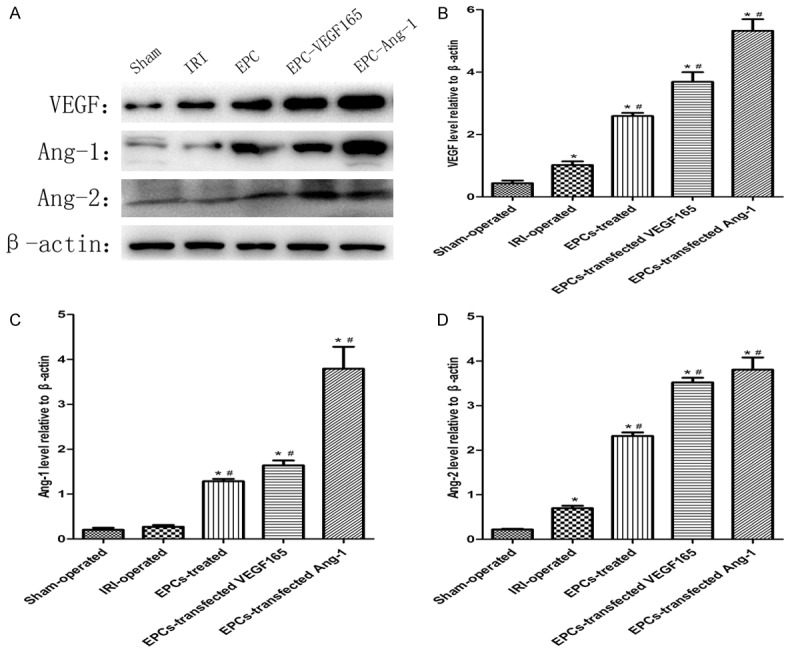
Effect of EPCs transplantation on protein expression in different groups. A: Protein expression levels of VEGF, Ang-1 and Ang-2 in different groups. β-actin was used as a protein control to normalize volume of protein expression. B-D: Protein levels were determined by densitometric analysis and normalized to the β-actin signal. Data are expressed as mean ± SD. *Significant difference vs. Sham group (P<0.05); #Significant difference vs. IRI group (P<0.05).
Discussion
Renal IRI is a complex pathophysiologic process that may occur during several clinical conditions, such as kidney transplantation, partial nephrectomy, cardiac arrest with recovery, and vascular surgery, which is a common cause of renal cell death, kidney failure, and delayed graft function [32,33]. The mechanisms underlying IRI to the kidneys are likely multifactorial and interdependent, involving hypoxia, oxidative stress injury, and inflammatory responses [34]. AKT produced by ischemia and reflow is a clinical and complex syndrome involving renal vasoconstriction, extensive tubular damage, tubular cell necrosis, glomerular filtration failure, glomerular injury, and signs of tubular obstruction with cell debris [35,36]. Studies at the level of animal model have revealed a number of protective factors that could protect the body against IRI associated with ischemic AKT.
Renal microcirculation dysfunction and impaired renal vascular activity that occurs after renal reperfusion are key factors to the development of renal IRI, which can decrease renal artery blood flow and hamper the full recovery of renal IRI [37,38]. Previous studies demonstrated that IPC participated in stem cell mobilization and the latter was closely related to ischemic repair [39,40]. These findings suggested that increased numbers of EPCs might provide a possible explanation for the observed protective effects of IPC. Multiple studies provided evidence that IPC played a protective role in a variety of organs including the kidneys [41,42]. In addition, IPC has been reported to attenuate microcirculation disturbance through EPCs homing [41,43,44]. In the previous study, IPC significantly increased the number of EPCs in the ischemic kidney and afforded partial renoprotection after kidney operation. These findings provided evidence for EPCs modulation by IPC, which attenuated IRI. Besides, these results were in agreement with the reports of Li et al. [10] who stated that acute myocardial ischemia might be alleviated by EPC recruitment during IPC. However, whether the regulation of the protective mechanisms of IPC by the recruitment and homing of EPCs in the IRI kidney remains far from complete [45,46]. Therefore, in the current study, VEGF and Ang-1 might play important roles in the protective effect of EPCs homing on renal acute IRI, involving promotion of cell proliferation and angiogenesis.
These mechanisms could be attributed to incorporation into the injured cells and paracrine effects [47-50]. Previous studies showed that only low numbers of EPCs could be identified as incorporating into the new capillaries following EPCs transplantation, suggesting that EPCs did not act via direct incorporation into the injured cells, but rather by a paracrine mechanism [51,52]. Besides, it was reported that EPCs had the ability to secrete angiogenic factors, playing different functions in tissue repair and reconstruction [53]. Interestingly, paracrine factors greatly increased EPC-mediated angiogenesis [54,55] and played an important role in mobilization, migration, homing, and differentiation of EPCs [56,57]. In the present study, we found that EPCs enhanced the release of protective cytokines, including VEGF, Ang-1 and Ang-2 in ischemic kidney, which might explain the kidney-protective functions through paracrine effects.
VEGF, as an important soluble angiogenic mitogen, can stimulate angiogenesis and improve tissue capillary density [58]. The high expression level of VEGF can be stimulated by hypoxia via mediation of hypoxia-inducible factor [59]. In human studies, there has not consistent conclusion regarding the VEGF levels in patients with IRI. Some studies indicated that VEGF was higher in IRI subjects when compared with health subjects, and IRI was correlated with the increased VEGF levels regardless of confounding factors [60,61]. In addition, early studies have deemed that angiogenesis might be controlled by spatially regulated endothelial endocytosis [62]. Moreover, the internalization process was often accompanied with distinct signaling pathways. Hence, we proposed that increased VEGF receptor endocytosis could change the downstream signaling pathway that regulated the angiogenesis of EPCs. What’s more, the VEGF receptor endocytosis could be a potential target to stimulate the revascularization. Therefore, VEGF was very likely to play a critical role in the development of homing of EPCs on renal IRI.
Both Ang-1 and Ang-2 are members of a family of secreted proteins that bind to TIE-2, an endothelium-specific receptor tyrosine kinase [27]. Although Ang-1 and Ang-2 bind to Tie-2 with similar affinity, they exert different functions [63]. Besides, Ang-1, as a very important factor, is secreted by endothelial cells, pericytes, mesenchyme and vascular smooth muscle cells of the developing vasculature, which is thought to stabilize the formation of newly formed blood vessels and promote endothelial cell survival by inhibiting apoptosis [64-66]. Therefore, in this study, VEGF and Ang-1 could improve renal function through EPCs homing in the model of renal IRI.
Although the results obtained had a positive role to the treatment of renal IRI, some limitations of our study should be taken into consideration. Firstly, a variety of factors could influence renal IRI, suggesting that might result from complex interactions. There are certainly several factors that can affect the capacity of EPCs homing in renal protection, and angiogenic factors are only one such factor. Exploring more new and potential mechanism was required by more researches in the future. Secondly, there was no long-term observation for only 72 hours after reperfusion in the effects of EPCs homing on renal IRI. In order to obtain the satisfactory outcomes, we should conduct long-term observation. Thus, further experiments need to be conducted to explore a causal mechanism and observe the protective effects of VEGF and Ang-1 on kidney IRI for longer time periods. What’s more, the detailed mechanisms that regulate VEGF and Ang-1 expression levels in homing of EPCs and the differential effects of VEGF and Ang-1 remain unknown and need to be studied in the following study. Accordingly, it was required that further studies could be performed to explore the method for increasing VEGF and Ang-1 expression in homing of EPCs to protect renal IRI.
Conclusion
In summary, adding the number of EPCs contributed to the improvement effect of BUN, Scr and morphological changes, thereby alleviating IRI-induced renal dysfunction and histological damage. Meanwhile, VEGF and Ang-1 might play important roles in the protective effect of the homing of EPCs on renal acute IRI, involving promotion of cell proliferation and angiogenesis. Further studies with different measuring methods and long-term follow-up will be warranted to validate such results.
Acknowledgements
This work has been supported by the National Natural Science Foundation of China (No. 81370853, 81570613).
Disclosure of conflict of interest
None.
References
- 1.Onorati F, Rubino AS, Nucera S, Foti D, Sica V, Santini F, Gulletta E, Renzulli A. Off-pump coronary artery bypass surgery versus standard linear or pulsatile cardiopulmonary bypass: endothelial activation and inflammatory response. Eur J Cardiothorac Surg. 2010;37:897–904. doi: 10.1016/j.ejcts.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502–516. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsui H, Sugiura T, Hayashi K, Ohkita M, Takaoka M, Yukimura T, Matsumura Y. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2009;603:73–78. doi: 10.1016/j.ejphar.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemiareperfusion injury. Anesthesiology. 2001;94:1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152–157. doi: 10.1097/00007890-199807270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 9.Blanco M, Lizasoain I, Sobrino T, Vivancos J, Castillo J. Ischemic preconditioning: a novel target for neuroprotective therapy. Cerebrovasc Dis. 2006;21(Suppl 2):38–47. doi: 10.1159/000091702. [DOI] [PubMed] [Google Scholar]
- 10.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 11.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 12.Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int. 2007;20:219–229. doi: 10.1111/j.1432-2277.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Poppel H. Efficacy and safety of nephronsparing surgery. Int J Urol. 2010;17:314–326. doi: 10.1111/j.1442-2042.2010.02482.x. [DOI] [PubMed] [Google Scholar]
- 14.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, Tervaert JW, Lodder J. Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke. 2008;39:2158–2165. doi: 10.1161/STROKEAHA.107.507251. [DOI] [PubMed] [Google Scholar]
- 16.Rozen N, Bick T, Bajayo A, Shamian B, Schrift-Tzadok M, Gabet Y, Yayon A, Bab I, Soudry M, Lewinson D. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone. 2009;45:918–924. doi: 10.1016/j.bone.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 17.Atesok K, Li R, Stewart DJ, Schemitsch EH. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res. 2010;28:1007–1014. doi: 10.1002/jor.21083. [DOI] [PubMed] [Google Scholar]
- 18.Roncalli JG, Tongers J, Renault MA, Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26:276–283. doi: 10.1016/j.tibtech.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Jiménez-Quevedo P, Silva GV, Sanz-Ruiz R, Oliveira EM, Fernandes MR, Angeli F, Willerson JT, Dohmann HF, Perin EC. Diabetic and nondiabetic patients respond differently to transendocardial injection of bone marrow mononuclear cells: findings from prospective clinical trials in “no-option” patients. Rev Esp Cardiol. 2008;61:635–639. [PubMed] [Google Scholar]
- 20.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 21.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzer LA, Cör A, Pfandlsteiner G, Holzer G. Expression of VEGF, its receptors, and HIF-1alpha in Dupuytren’s disease. Acta Orthop. 2013;84:420–425. doi: 10.3109/17453674.2013.814011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan R, Kreipke CW, Roberts G, Bagchi M, Rafols JA. Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol Res. 2007;29:375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 25.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 26.Costache MI, Ioana M, Iordache S, Ene D, Costache CA, Săftoiu A. VEGF expression in pancreatic cancer and other malignancies: a review of the literature. Rom J Intern Med. 2015;53:199–208. doi: 10.1515/rjim-2015-0027. [DOI] [PubMed] [Google Scholar]
- 27.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng QY, Li XQ, Yu XB, Lei FR, Jiang K, Li CY. Transplantation of VEGF165-gene-transfected endothelial progenitor cells in the treatment of chronic venous thrombosis in rats. Chin Med J (Engl) 2010;123:471–477. [PubMed] [Google Scholar]
- 30.Jia RP, Xie JJ, Luo FY, Zhu JG. Ischemic preconditioning improves rat kidney allograft function after ischemia/reperfusion injury: the role of tumor necrosis factor-alpha. Transplant Proc. 2008;40:3316–3320. doi: 10.1016/j.transproceed.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 31.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almond PS, Matas AJ, Gillingham K, Dunn DL, Payne WD, Gores P, Gruessner R, Najarian JS. Predictors of chronic rejection in renal transplant recipients. Transplant Proc. 1993;25:936. [PubMed] [Google Scholar]
- 33.Baker GL, Corry RJ, Autor AP. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg. 1985;202:628–641. doi: 10.1097/00000658-198511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med. 1994;42:632–639. [PubMed] [Google Scholar]
- 35.Bird JE, Milhoan K, Wilson CB, Young SG, Mundy CA, Parthasarathy S, Blantz RC. Ischemic acute renal failure and antioxidant therapy in the rat. The relation between glomerular and tubular dysfunction. J Clin Invest. 1988;81:1630–1638. doi: 10.1172/JCI113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn WF. Nephron heterogeneity in polyuric acute renal failure. J Lab Clin Med. 1981;98:21–29. [PubMed] [Google Scholar]
- 37.Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr Opin Nephrol Hypertens. 2012;21:33–38. doi: 10.1097/MNH.0b013e32834d085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1358–R1363. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czeiger D, Dukhno O, Douvdevani A, Porat Y, Shimoni D, Fulga V, Ament JD, Shaked G. Transient extremity ischemia augments CD34+ progenitor cell availability. Stem Cell Rev. 2011;7:639–645. doi: 10.1007/s12015-011-9234-x. [DOI] [PubMed] [Google Scholar]
- 40.Kamota T, Li TS, Morikage N, Murakami M, Ohshima M, Kubo M, Kobayashi T, Mikamo A, Ikeda Y, Matsuzaki M, Hamano K. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Wu R, Jia RP, Zhong B, Zhu JG, Yu P, Zhao Y, Ge YZ, Wu JP. Ischemic preconditioning increases endothelial progenitor cell number to attenuate partial nephrectomy-induced ischemia/reperfusion injury. PLoS One. 2013;8:e55389. doi: 10.1371/journal.pone.0055389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Liu X, Wan X, Wu Y, Chen Y, Cao C. Ischemic preconditioning attenuates renal ischemia-reperfusion injury by inhibiting activation of IKKbeta and inflammatory response. Am J Nephrol. 2009;30:287–294. doi: 10.1159/000225928. [DOI] [PubMed] [Google Scholar]
- 43.Bo CJ, Chen B, Jia RP, Zhu JG, Cao P, Liu H, Wu R, Ge YZ, Wu JP. Effects of ischemic preconditioning in the late phase on homing of endothelial progenitor cells in renal ischemia/reperfusion injury. Transplant Proc. 2013;45:511–516. doi: 10.1016/j.transproceed.2012.05.095. [DOI] [PubMed] [Google Scholar]
- 44.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa T, Mimura Y, Kaminishi M. Renal denervation abolishes the protective effects of ischaemic preconditioning on function and haemodynamics in ischaemia-reperfused rat kidneys. Acta Physiol Scand. 2002;174:291–297. doi: 10.1046/j.1365-201x.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa T, Mimura Y, Hiki N, Kanauchi H, Kaminishi M. Ischaemic preconditioning ameliorates functional disturbance and impaired renal perfusion in rat ischaemia-reperfused kidneys. Clin Exp Pharmacol Physiol. 2000;27:997–1001. doi: 10.1046/j.1440-1681.2000.03378.x. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Gao Y, Qin J, Kuang CY, Song MB, Yu SY, Cui B, Chen JF, Huang L. CCN1 promotes the differentiation of endothelial progenitor cells and reendothelialization in the early phase after vascular injury. Basic Res Cardiol. 2010;105:713–724. doi: 10.1007/s00395-010-0117-0. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Wu F, Xia WH, Zhang YY, Xu SY, Cheng F, Liu X, Zhang XY, Wang SM, Tao J. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res. 2010;88:462–470. doi: 10.1093/cvr/cvq207. [DOI] [PubMed] [Google Scholar]
- 49.Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T. Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion. J Histochem Cytochem. 2010;58:687–694. doi: 10.1369/jhc.2010.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 51.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 52.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S, Zhu J, Yu L, Fu G. Endothelial progenitor cells: current development of their paracrine factors in cardiovascular therapy. J Cardiovasc Pharmacol. 2012;59:387–396. doi: 10.1097/FJC.0b013e3182440338. [DOI] [PubMed] [Google Scholar]
- 54.Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM. Combination of stromalderived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 55.Yi C, Xia W, Zheng Y, Zhang L, Shu M, Liang J, Han Y, Guo S. Transplantation of endothelial progenitor cells transferred by vascular endothelial growth factor gene for vascular regeneration of ischemic flaps. J Surg Res. 2006;135:100–106. doi: 10.1016/j.jss.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormonemediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 57.Henrich D, Hahn P, Wahl M, Wilhelm K, Dernbach E, Dimmeler S, Marzi I. Serum derived from multiple trauma patients promotes the differentiation of endothelial progenitor cells in vitro: possible role of transforming growth factor-beta1 and vascular endothelial growth factor165. Shock. 2004;21:13–16. doi: 10.1097/01.shk.0000101669.49265.50. [DOI] [PubMed] [Google Scholar]
- 58.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 59.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 60.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25:59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 61.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 62.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad SA, Liu W, Jung YD, Fan F, Reinmuth N, Bucana CD, Ellis LM. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138–1143. doi: 10.1002/1097-0142(20010901)92:5<1138::aid-cncr1431>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 64.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 65.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 66.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]